Abstract
A 993-bp regulatory region upstream of the translation start codon of subtilisin-like serine protease gene was isolated from Gossypium barbadense. This (T/A)AAAG-rich region, GbSLSP, and its 5′- and 3′-truncated versions were transferred into tobacco and Arabidopsis after fusing with GUS or GFP. Histochemical and quantitative GUS analysis and confocal GFP fluorescence scanning in the transgenic plants showed that the GbSLSP-driven GUS and GFP expressed preferentially in guard cells, whereas driven by GbSLSPF2 to GbSLSPF4, the 5′-truncated GbSLSP versions with progressively reduced Dof1 elements, both GUS and GFP expressed exclusively in guard cells, and the expression strength declined with (T/A)AAAG copy decrement. Deletion of 5′-untranslated region from GbSLSP markedly weakened the activity of GUS and GFP, while deletion from the strongest guard cell-specific promoter, GbSLSPF2, not only significantly decreased the expression strength, but also completely abolished the guard cell specificity. These results suggested both guard cell specificity and expression strength of the promoters be coordinately controlled by 5′-untranslated region and a cluster of at least 3 (T/A)AAAG elements within a region of about 100 bp relative to transcription start site. Our guard cell-specific promoters will enrich tools to manipulate gene expression in guard cells for scientific research and crop improvement.
Introduction
Stomata are the specialized structure of plant epidermal cells, containing a pair of guard cells and a pore between them. They control gas exchange between plant and atmosphere, taking an important part in photosynthesis, respiration and transpiration. Their density and pore size greatly affect the gas exchange rate and loss of water. Under normal environmental conditions, a given plant can make balance between the CO2 uptake for photosynthesis and the loss of water for transpiration by changing stomatal pore size [1]–[3]. In other hand, the stomatal pore size is also regulated by both biotic stimuli and abiotic stimuli such as leaf excision [4], pathogens [5], [6], light [7], [8], CO2 [5], [1], [9], ozone (O3) [10], temperature [11], H2S [12], humidity [13], abscisic acid (ABA) and other plant hormones [14]–[16], and combination of abiotic factors In some case, the biotic and abiotic stimuli cross-regulated stomatal pore size [17]. However, no mater the stomatal pore size change is actively regulated by plant itself or passively regulated by biotic and/or abiotic stimuli, it is the guard cells that carry out these regular “orders” and make stomata movement appropriately. Consequently, the guard cells are key regulatory elements in the control of photosynthesis and transpiration [18]. Therefore, these “orders-receptor(s)” and “orders-executor(s)” must be guard cells-specific.
Search of these receptor(s) and/or executor, besides proteins involved in the growth and development of guard cells themselves, led to identification, isolation and functional analysis of a grand body of guard cells-specific and/or preferred genes and promoters [19], [20]. Akt1 [21], Kat1 [22], rha1 [23], dehydrins [24], CHX20 [25], MYB60 [26], SLAC1 [27], [28], ALMT12 [29], TPC1 [30] and ROP11 [31] from Arabidopsis and Kst1 [32] from potato are of the representative genes. As for the promoter, Kat1 [33], Kst1 [34], [35]) Abh1 [36], Chl1 [37], Rac1 [38], Osm1 [39], Ost1 [40], MYB60 [26], [41], [42], AtCHX20 [25], [43], SLAC1 [27], [28], PDR3 [26] and ROP11 [31] are in the list. Unfortunately, a grand majority of promoters elucidated are not strictly guard cell-specific, but guard cell-preferred, strongly or modestly. Recently, three promoters from Arabidopsis, pGC1 [44], CYP86A2 [45] and MYB60 [42], [46], were reported to drive guard cell-exclusive expression of genes in transgenic plants. Interestingly, although most native promoters from guard cell-preferred genes were not strictly guard cell-specific, some truncated promoters from these genes and even from genes not guard cells-preferred were strictly guard cell-specific. Muller-Rober et al [32] demonstrated that a fragment of ca. 300 bp left by 5′-deleting an ADP-glucose pyrophosphorylase promoter from potato could drive GUS reporter gene to express exclusively in the guard cells of transgenic potato and tobacco plants. This truncated promoter was used to drive strictly guard cell-specific expression of AtALMT12 in Arabidopsis successfully [29]. Guard cell-specific gene expression was found to be controlled principally by Dof1 protein-targeted cis-acting element, 5′-(T/A)AAAG-3′, in particular TAAAG, proximal to TATA-box in potato KST1 promoter [35]. This element, (T/A)AAAG, was later successfully used to construct and express a “tailor-made” drought-inducible guard cell-specific promoter DGP1 [47]. In this inspiration, we scanned DNA databases available with (T/A)AAAG as probe to identify and then clone guard cell promoters for further use in molecular engineering of guard cells and hence increasing the adaptation of crop plants to environment stress.
Our scanning with the probe (T/A)AAAG found large numbers of promoter candidates (Unpublished). Among them, the promoters of subtilisin-like serine protease (subtilase) genes attracted us most, because of some them involved in epidermal surface formation such as AtALE1 [48] and guard cell development including stomatal density and distribution such as AtSDD1 [49]–[51]. We targeted the promoters of cotton subtilisin-like serine protease genes and cloned a 5′-flanking fragment of 993 bp upstream of the translation start codon “ATG” from sea island cotton (Gossypium barbadense) [52]. Here we show that this (T/A)AAAG-rich fragment, GbSLSP, directed high level of guard cell-preferred expression of both GUS and GFP reporter genes in transgenic tobacco and Arabidopsis. We demonstrate that several 5′-end truncated versions of GbSLSP could drive the reporter genes to express exclusively and strongly in the guard cells. Finally, we reveal that the guard cell specificity of 5′-truncated GbSLSP is coordinately controlled by 5′-untranslated region (5′-UTR) and a cluster of at least 3 cis-acting elements (T/A)AAAG within a region of about 100 bp relative to transcription start site. Our results will provide an additional tool in getting strictly guard cell-specific promoters and thus in the improvement of crops adaptation to environment via gene engineering of guard cells.
Materials and Methods
Plant Material and Growth Conditions
Seeds of sea island cotton (Gossypium barbadense L. cv. SHZ2-214) were kindly provided by Dr. J.B. Zhu of University of Shehezi, China. Cotton and tobacco (Nicotiana tabacum cv. NC89) plants were grown in a greenhouse at 25±2°C, and Arabidopsis thaliana ecotype “Columbia” at 22±1°C under 16-h light/8-h dark cycle in a culture room.
Promoter Isolation and Plant Expression Vector Construction
Sea island cotton genomic DNA was extracted from fresh young leaves with improved CTAB method [53]. The 5′-flanking region of about 1000 bp upstream of the translation start codon “ATG” of a cotton subtilisin-like serine protease gene [54], [55] was isolated by using polymerase chain reaction (PCR) with primer pair 5′-AAGCTTACAACTTTTCTCTACCAATCA-3′/5′-GGATCCGCTAGAGAAAAATGGGAAGGTGAG-3′ (Hin dIII and Bam HI restriction sites added were underlined respectively). The PCR products were ligated in pBS-T vector (Qiagen, China), and then sequenced to check the identity after size verification by Hin dIII-Bam HI digestion. The expected fragment, named “GbSLSP” or simply “F1”, was designed as full length promoter in this study. From this full-length promoter, sets of progressive 5′-deletion and 3′-deletion fragments were generated by PCR using specific primers (Table 1). All fragments obtained were cloned into pBS-T vector and sequenced as described above.
Table 1. Oligonucleotide primers used for PCR cloning and deletion of GbSLSP promoter.
| Primer name | Primer sequence (5′ to 3′)* |
| Forward | |
| SLSPFW1 | AAGCTT ACAACTTTTCTCTACCAATCA |
| SLSPFW2 | CAATATGAAAAAGCTTGAGTGC |
| SLSPFW3 | AAGCTT ATTTTGGAAGATGAC |
| SLSPFW4 | AAGCTT CTTTACATGCATCATGTGATCG |
| SLSPFW5 | AAGCTT ATCGTGGGGGACCCGAAACTTGGCATAC |
| Reverse | |
| SLSPRW1 | GGATCC GCTAGAGAAAAATGGGAAGGTGAG |
| SLSPRW2 | GGATCC GTGG TTGGATGAGACT |
Underlined are Hin dIII and Bam HI recognizing sites added at the forward and reverse primers, respectively. The bold italic is the native Hin dIII recognizing site.
The sequencing-verified “promoter” fragments were isolated from their correspondent pBS-T with Hin dIII-Bam HI and individually cloned into a binary vector pBI121 (Clontech) to replace CaMV 35S (35S) promoter, which gave rise to pGbSLSPn-GUS vectors (here n = F1 to F2-sh). To construct pGbSLSPn-GFP vectors, the GUS coding sequence in the pGbSLSPn-GUS was replaced by GFP coding sequence PCR-amplified from pCAMBIA1300. During PCR amplification, Bam HI and Sac I restriction sites added to the 5′ and 3′ ends of the GFP.
Plant Transformation and Growth Conditions
All constructs described in the previous section were transferred to Agrobacterium tumefaciens strains GV3101 and LBA4404 for transformation of Arabidopsis and tobacco, respectively. Agrobacterium-mediated transformation of Arabidopsis (ecotype “Columbia”) and tobacco (N. tabacum cv. “NC89”) was conducted by using methods of floral-dip [56] and leaf disc co-culture [57], respectively.
Tobacco transformants were selection on MS medium containing 50 mg/L of kanamycine (Kan) and 500 mg/L of cefotaxime under 16-h light/8-h dark cycle at 24°C ±1°C in a culture room. The Kan-resistant shoots were rooted in MS containing 100 mg/L of Kan, and resulting plantlets were then transplanted in pots in a greenhouse. For selection of Arabidopsis transformants, the seeds of floral dip-transformed plants were surface-sterilized in dilute bleach (0.5% NaClO) for 10 min and then with 75% ethanol for 30 s, rinsed five times with sterile distilled water. The sterilized seeds were then germinated on MS medium containing 50 mg/L of Kan, stratified for 2 d at 4°C and then placed under 16-h light/8-h dark cycle at 22°C ±1°C in a culture room. The Kan-resistant seedlings were transplanted in pot and grew in the culture room.
Analysis of GUS and GFP Expression
Histochemical staining and quantitative analysis for GUS activity in the transgenic plants were performed as described by Jefferson et al. [58]. Briefly, for GUS staining, samples were incubated in GUS staining solution (50 mM phosphate buffer, pH6.7, 1 mM EDTA pH8.0, 0.2% (V/V) Triton-100, 1 mM K3FeCN6, 1 mM K4FeCN6, 0.5 mg/mL 5-bromo-4-chloro-3-indoxyl-D-glucuronic acid (X-gluc)) at 37°C for 12 to 16 h. After staining, the samples were cleared with 70% ethanol for more than 1 h at room temperature, and then photographed by using an Olympus SZX16-DP72 stereomicroscope. For quantitative GUS activity assay, the samples were prepared as previously described [59], and the enzymatic reaction was carried out in a reaction volume of 500 µl and at 37°C. At zero time, an aliquot of 50 µl reaction solution was taken out and added to 450 µl 0.2 M Na2CO3 and the same manipulation was performed at subsequent times 10, 20, 30, 45, 60 min. The GUS activity was detected in HITACHI F-4500 spectrofluorometer with excitation at 365 nm and emission at 455 nm, and expressed as nmol of 4-methylumbelliferone (MU) produced per mg protein per min.
Detection of GFP fluorescence in the leaves of transgenic plants was carried out using Carl Zeiss LSM510 laser scanning confocal imaging system at 488 nm excitation, and emission band width of 505–530 nm. For chlorophyll detection, the excitation was at 543 nm and emission at LP650 nm.
Results
GbSLSP has Multiple Copies of Guard Cell-specific cis-element TAAAG and Alike Elements
A 993-bp promoter region upstream of the translation start codon “ATG” of a subtilisin-like serine protease gene from sea island cotton was PCR-amplified by using primer pair SLSPFW1/SLSPRW1 (Table 1), and this region consisted of a regulatory fragment of 624 bp and a 5′-UTR of 369 bp based on online promoter prediction and comparison with reported GhSCFP (54, 55). Online analysis using SoftBerry (http://linux1.softberry.com) and PLACE (http://www.dna.affrc.go.jp/htdocs/PLACE/) [60] of the regulatory fragment revealed the presence of 1 TATA box (−31) and 10 Dof protein-targeted cis-acting elements “(T/A)AAAG” (Fig. 1). Among the cis-elements, three were guard cell-specific ones (TAAAG) as defined by Plesch et al. [35], one in sense strand (−229) and two in antisense one (−114, −483), and the rest were TAAAG-like element, “AAAAG”, 6 in sense strand and 1 in the antisense (Fig. 1). This promoter region was designated as “GbSLSP”, simply called “F1” and used as full length promoter for coming experiments.
Figure 1. Nucleotide sequence of the 5′-flanking region of GbSLSP gene.
Nucleotides are numbered on the left with the transcription start site designated as +1 which is white-boxed. The 5′-UTR is in lower case letters. The TATA-box is in italic letters and white-boxed. The DOF1-binding sites AAAAG are grey-boxed, and TAAAG, grey-boxed and underlined. The deletion positions are indicated with arrowheads behind the short name of forward (F2 to F5) and reverse (R2) primers.
GbSLSP Directed Strong Guard Cell-preferred Expression of GUS and GFP Reporter Genes in Transgenic Tobacco Plants
In order to investigate the driving pattern and strength of GbSLSP, we first constructed GbSLSP::GUS cassette (pGbSLSP-GUS) by cloning the GbSLSP into binary vector pBI121 to replace CaMV 35S promoter and obtained more than 30 independent transgenic tobacco plants via Agrobacterium-mediation transformation. Ten plants with expected strong and sharp PCR-amplified band (Figure not shown) were used for GUS expression analysis.
Histochemical GUS staining of T0 GbSLSP::GUS-transgenic tobacco showed that GUS gene was expressed very strongly in guard cells, strongly in mesophyll cells adjacent to the guard cells, less-strongly in veins and trichomes of the leaves, moderately in ovary wall and slightly in sepal, stigma and in some anthers (Fig. 2A: F1). The overall strength of GUS expression driven by GbSLSP in the leaf was approximately 70% of that driven by CaMV 35S (data not shown). In the Kan-resistant T1 seedlings at 3–4-leaf stage, GUS expression pattern and strength were similar to their parental lines in leaves (Figs. 3A & 3B: F1), but in guard cell-specific manner in cotyledons (Fig. 3C: F1). Very weak GUS staining also appeared in the lower part of the root vascular system (Fig. 3A: F1).
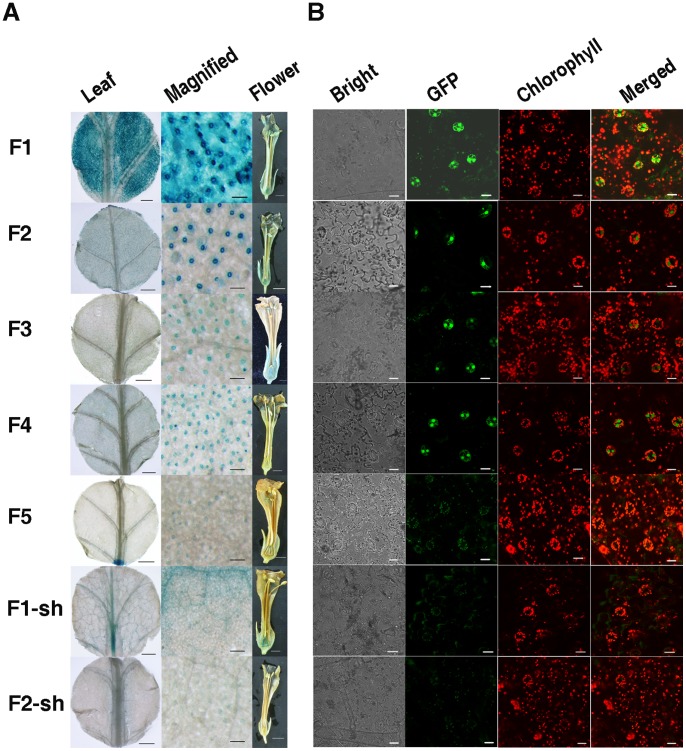
Figure 2. Histochemical GUS staining and Confocal GFP fluorescence scanning of transgenic tobacco T0 plants transformed with the full length promoter GbSLSP and its 5′- and 3′-truncated versions.
A, Histochemical GUS staining of young leaves and flowers. B, Confocal GFP fluorescence scanning of young leaves. F1: Transformed with full-length GbSLSP(F)::GUS/GFP. F2–F5: Transformed with GbSLSPFn:: GUS/GFP, where n = 2 to 5. F-sh: Transformed with full-length GbSLSP(F)-sh::GUS/GFP. F2-sh: Transformed with full-length GbSLSPF2-sh::GUS/GFP.
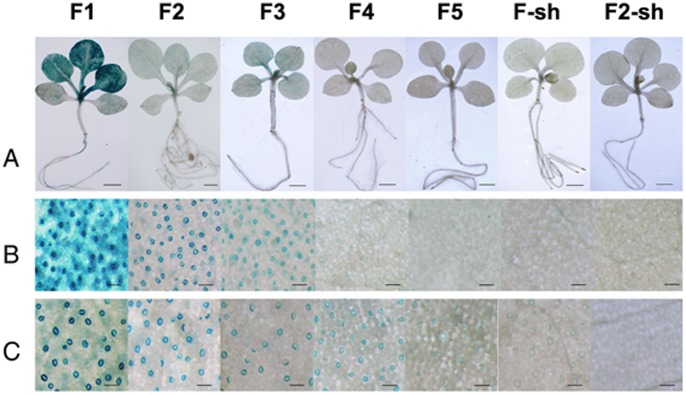
Figure 3. Histochemical GUS staining of T1 seedling of tobacco transformed with the full length promoter GbSLSP and its 5′- and 3′-truncated versions.
A, Whole seedlings, scale bars = 0.2 mm. B, Rosette leaf zones, scale bars = 0.1 mm. C, Cotyledon zones, scale bars = 0.1 mm.
In order to verify whether the expression patterns of GbSLSP observed using GUS as reporter were an accurate representation of the GbSLSP, we replaced the GUS with GFP in the vector pGbSLSP-GUS and generated more than 20 independent GbSLSP::GFP-transgenic tobacco plants also by Agrobacterium-mediation transformation. Ten independent T0 GbSLSP::GFP transformants at 5–6-leaf stage were selected for analysis of GFP expression pattern in the young leaves. As showed in Figure 2B:F1, the GFP expression pattern looked like the GUS expression as showed in Fig. 2A:F1 and the transgenic lines displayed strong GFP signals in guard cells and much weaker GFP signals in the cells adjacent to the guard cells in the leaves.
5′-truncated Versions of GbSLSP Drove GUS and GFP Reporter Genes to Express Specifically in Guard Cells of Transgenic Tobacco Plants
As analyzed in the previous section, the GbSLSP contained several copies of guard cell-specific cis-element TAAAG and TAAAG-like element, the targeted sites of Dof1 protein. To gain insight into the functional role of (T/A) AAAG elements in the expression pattern of the promoter, we made a progressive 5′-deletions of GbSLSP by PCR using specific primer pairs SLSPF2-SLSPFW5/SLSPRW1 (Table 1) with consideration of progressively reducing the copy number of (T/A)AAAG elements. The set of 5′-deletion generated four 5′-truncated promoters (Fig. 4A: F2 to F5). The F5 (−96 to +369) contained 2 (T/A)AAAG elements in sense strand, and from F5 to F3, one more (T/A)AAAG element in sense or antisense strand was presented near 5′-end. For F2, it contained 3 more (T/A)AAAG elements than F3, 2 in sense strand and 1 in antisense strand.
Figure 4. Schematic presentation of the GbSLSP promoter 5′-and 3′-deletion series.
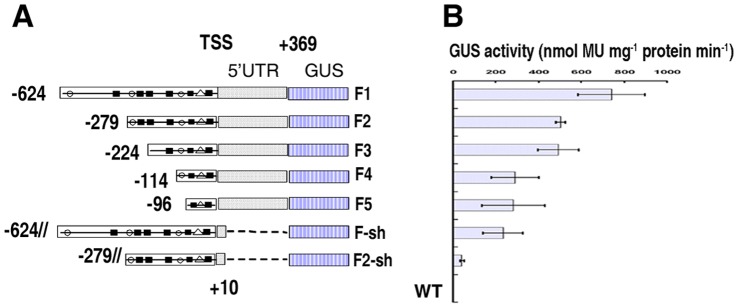
A, Schematic diagrams of deletion series constructs. Solid round (•), the (T/A)AAAG elements on sense strand (5′ to 3′); Solid square (▪), (T/A)AAAG elements on antisense strand (3′ to 5′); White triangle (△), TATA-box. B, Quantitative analysis of GUS activity from the deletion series in T0 transgenic tobacco leaves. Values represent mean and standard error.
Binary vectors GbSLSPFn-GUS and GbSLSPFn-GFP (n = 2 to 5) (Fig. 4A) were constructed and GbSLSPFn::GUS- and GbSLSPFn::GFP-transgenic tobacco plants (each construct with more than 30 independent transformants) were generated with the same methods used for the full length promoter GbSLSP as described above.
In the leaves of tobacco plants transformed by 5′-truncated GbSLSP promoter F2, F3 or F4, the GUS expression pattern was similar and GUS staining was observed exclusively in guard cells, although the staining strength varied with copy number of (T/A)AAAG motif and/or length of the promoter (Fig. 2A: F2 to F4). The F2 which contained 7 copies of Dof motif had the strongest GUS staining, whereas the F4 that possessed only 3 copies of Dof motif, had much weaker one. In F5-transgenic tobacco leaves, less than half of plants showed very week GUS staining exclusively in some guard cells, and the rest displayed very week and diffused GUS staining in the cells other than guard cells (Fig. 2A: F5). This GUS staining strength was confirmed by GUS quantitative assay (Fig. 4B). In comparing with full length promoter GbSLSP (F1), the 5′-truncated ones gained guard cell-specificity, but lost part of driving power, even F2 (Fig. 2A: F2–F5 vs. F1; Fig. 4B).
In the flowers of 5′-truncated GbSLSP-transformants, the GUS expression pattern was varied depending on the length or (T/A)AAAG copy number of the promoter. The F2 retained the expression pattern and strength of the full-length promoter, whereas F4 and F5, only very week GUS staining could be detected in ovary wall, top of style and stigma (Fig. 2A: F2 to F5).
As for the full-length promoter, T1 seedlings of 5′-truncated GbSLSP-transgenic lines were GUS stained. As showed in Fig. 3, the seedlings of F2 and F3 had stronger GUS staining than those of F4 and F5 (Fig. 3A). The GUS staining was guard cell-specific in both young leaves and cotyledons of the seedling from F2 and F3 transformants, whereas for F4, this specificity was clearly visible only in the cotyledons (Figs. 3B & 3C). In the T1 seedlings of F5 transformants, no guard cell-specific but a very week blade cell-diffused GUS staining was observed in both young leaves and cotyledons (Figs. 3B & 3C). The GUS staining strength in the T1 seedlings of all 5′-tructated GbSLSPs was similar to their parents, and weaker than that from the full-length GbSLSP.
GFP expression pattern in the young leaves of T0 transformants of all 5′-truncated GbSLSPs was similar to that of GUS staining except for F4, in which the GFP signal was clearly guard cell-specific whereas the guard cell-specificity of GUS staining was not very net (Fig. 2B: F2 to F5).
5′-UTR Plays an Important Role in the Determination of Tissue/organ-specificity and Strength of the Promoters in Transgenic Tobacco
Both full-length GbSLSP promoter and its 5′-truncated versions contained a 5′-UTR of 369 bp (from TSS to just upstream of the translation start codon “ATG”, Figs. 1 & 4A). In order to investigate the possible involvement of the 5′-UTR in the determination of tissue/organ-specificity and strength of promoter, we conducted PCR 3′-deletion of the full-length GbSLSP and one of its 5′-tructated versions, F2, the strongest guard cell-specific promoter, leaving only 9 bp of 5′-UTR just downstream of the TSS and generated promoters F1-sh and F2-sh, respectively (Fig. 4A). As for functional analysis of the full-length GbSLSP, we constructed F1-sh::GUS/GFP and F2-sh::GUS/GFP vectors and generated transgenic tobacco plants.
GUS staining of young leaves of T0 transformants showed that deletion of 5′-UTR from the full-length promoter GbSLSP not only decreased significantly the GUS activity, but also almost completely abolished its guard cell-preference in leaves (Fig. 2A: F1-sh ). In comparing with intact full-length GbSLSP, theF1-sh had weak and diffused GUS blue in the veins and blade cells (Fig. 2A: F1-sh vs. F1). Differently, deletion of 5′-UTR from F2 did not clearly altered GUS expression pattern, retaining the guard cell-specificity, although the GUS activity was greatly reduced in the leaves (Fig. 2A:F2-sh). In the flowers of T0 transformant, F2-sh had only some anther slightly GUS-stained (Fig. 2A: F2-sh), whereas for F1-sh, strong GUS staining were present in the sepal, ovary wall and style top (Fig. 2A: F1-sh).
The GUS staining pattern of the T1 seedlings of 3′-deleted GbSLSPs transformants was overall similar to their parent lines, and the guard cell-specific staining was only seen in some of the seedlings from F2-sh, but not in those from F1-sh (Fig. 3A), both in young leaves (Fig. 3B) and cotyledons (Fig. 3C).
The GFP florescence detection of the young leaves of T0 transgenic tobacco showed that no clear GFP signal was detected in F1-sh transformants, but few guard cells had very weak GFP signal in F2-sh transgenic lines (Fig. 2B:F1-sh & F2-sh).
The Guard Cell-specificity of GbSLSPF2, a 5′-trucated Version of GbSLSP Promoter, was Confirmed in Transgenic Arabidopsis
To study whether or not that the expression patterns of GbSFLP and its 5′-deleted versions seen in transgenic tobacco are reproducible in different plant taxa, we generated transgenic Arabidopsis T1 and T2 plants with 3 representative constructs, GbSLSP(F1)::GUS, GbSLSPF2 (F2)::GUS and GbSLSPF5 (F5)::GUS.
As what seen in transgenic tobacco, the full-length GbSLSP drove GUS gene to express strongly in the developing and fully expanded rosette leaves (young leaves), inflorescence shoots, flower pedicles, sepals, stamen, and styles in Arabidopsis T1 transformants, and the expression was more pronounced in guard cells than other epidermic cells (Fig. 5A: F1). The F2::GUS also expressed in above organs, but exclusively in their guard cells (Fig. 5A: F2). In F5::GUS-transformants, GUS blue was almost absent in the sepals, stamen, but present moderately in inflorescence shoot, flower pedicles and styles, weakly in young leaves (Fig. 5A: F5). The GUS expression pattern of F5 in Arabidopsis T1 transformants was similar to that in transgenic tobacco, but the expression was stronger, and in particular in the inflorescence shoot, style and rosette leaves.
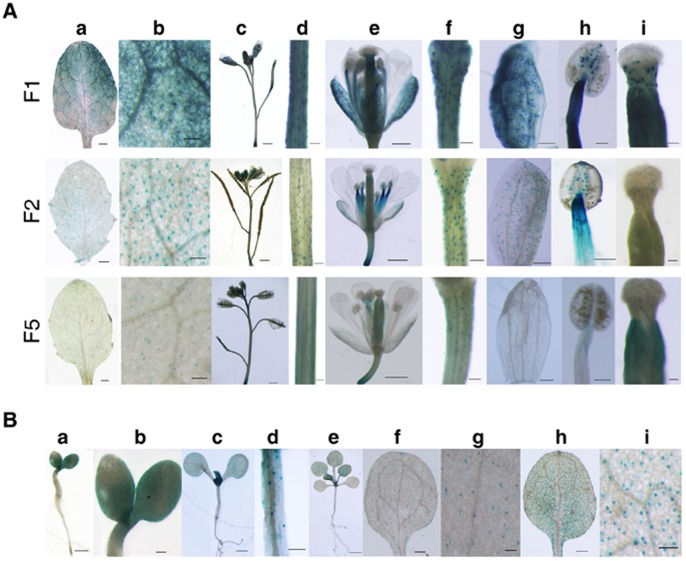
Figure 5. Histochemical GUS staining of transgenic Arabidopsis plants.
A, GUS staining in different organs of the T1 transgenic plants. a, Rosette leaves, scale bars = 0.5 mm. b, Magnified view of a, scale bars = 0.1 mm. c, Inflorescence, scale bar = 1.0 mm. d, Inflorescence shoots, scale bars = 0.1 mm. e, Flowers, scale bars = 0.5 mm. f, Flower pedicles, scale bars = 0.1 mm. g, Sepals, scale bars = 0.1 mm. h, Stamen, scale bars = 0.1 mm. i, Styles, scale bars = 0.1 mm. B, GUS staining in different developmental stages of T2 seedlings of the plants transformed by GbSLSPF2. a, 3-day old seedling, scale bar = 0.5 mm. b, Magnified view of a, scale bar = 0.2 mm. c, 7-day old seedling, scale bar = 1.0 mm. d, Hypocotyl of c, scale bar = 0.2 mm. e, 15-day old seedling, scale bar = 1.5 mm. f, Cotyledon of e, scale bar = 0.3 mm. g, Magnified view of f, scale bar = 0.1 mm. h, Rosette leaf of e, scale bar = 0.3 mm. i, Magnified view of h, scale bar = 0.1 mm.
GUS staining of Arabidopsis T2 seedlings of F2 transformants showed that the guard cell-specificity conferred by F2 was retained, and the expression seemly regulated by developmental stages (Fig. 5B). In 3-d seedlings, a strong GUS blue appeared in cotyledons and up-part of hypocotyl adjacent to cotyledon with a guard cell-preferred manner (Fig. 5B: a & b). However, In 7-d (Fig. 5B: c & d) and older seedlings (Fig. 5B: e to i), the GUS expression became guard cell-specific in the hypocotyl, cotyledon and young leaf.
Discussion
Mining for Gene and its Major Regulatory Element(s) of a Specific Interest
Rapid increasing DNA and mRNA sequence databases provide very rich resources for mining genes and their regulatory element(s) of a specific interest [59], [61]. In order to isolate guard cell-specific promoter for further use in stomata study and in the improvement of crops adaptation to environments, we scanned available DNA and mRNA databases with two criteria: A, presence of the guard cell-specific cis-acting element “(T/A)AAAG” [35] approximate to the transcription start site (TSS) in the regulatory region of a gene. B, the protein deduced coded by the gene is involved in stomatal density, distribution, development and/or movement. This scanning led to target an up-land cotton gene “GhSCFP” which was cloned and named by Hou et al. [54], [55]. We separately online analyzed in detail the regulatory region and deduced protein of GhSCFP by using SoftBerry (http://linux1.softberry.com), PLACE [60] and Blast (NCBI), respectively. The analysis of the regulatory region disclosed that there existed more than one “(T/A)AAAG” elements near TSS (date not shown), which meets our first selection criteria. Blasting of the deduced protein revealed its sharing more than 85% homology with subtilisin-like serine proteases (subtilases) from Arabidopsis, potato and rice (date not shown). Thus, the “SCFP” was renamed “SLSP”. In Arabidopsis, SDD1, one of 56 copies of subtilases [62], was contributed to stomatal development, density and distribution [50], and thus satisfies our second selection criteria. Therefore, we cloned the regulatory region upstream of the translation start point of SLSP from sea island cotton (Gossypium barbadense) [52]. As predicated, our cloned promoter region of GbSLSP had the TATA-Box, TSS and their around sequences almost identical to those of GhSCFP (date not shown), and contained 10 copies of Dof1 elements (grey-boxed in Fig. 1), including 3 copies of guard cell-specific cis-elements, TAAAG, approximate to TSS, 1 in sense strand (−229) and 2 in antisense one (−110, −479) (grey-boxed and underlined in Fig. 1). The full-length GbSLSP indeed directed strong guard cell-preferred expression of GUS and GFP reporter genes in both transgenic tobacco (Fig. 2: F1;Fig. 3: F1) and Arabidopsis (Fig. 5: F1). These results suggest that it would be easy to mine available DNA and mRNA sequence databases for genes and their major regulatory element(s) of a specific interest if the “probe” and probing criteria are appropriate. The results suggest also that the cis-element (T/A)AAAG approximate to TSS identified by Plesch and colleagues [35] and used in this experiment is an appropriate probe for guard cell-preferred and/or -specific promoter mining.
Relationship between cis-acting Element (T/A)AAAG and Guard Cell-specific Expression
Cis-acting element (T/A)AAAG of promoters is well-known as the target site of Dof1 zinc finger transcription factors [63] and the TAAAG in potato Kst1 promoter was found to play a critical role in guard cell specific gene expression [35]. However, a grand body of promoters contain (T/A)AAAG elements, usually in more than one copy, but they are not guard cell-specific, even not guard cell-preferred [44], such as, Bnfs, Bofs, Bpfs, Bcfs [59], ATA7 [61] and even the full length of potato AGPase promoter [32] from which the guard cell-specific element TAAAG was identified [35]. Thus the relationship between (T/A)AAAG elements and guard cell specific gene expression is beyond the simplicity.
Müller-Rober et al [32] reported that the full length of potato AGPase promoter which contained 10 (T/A)AAAG elements didn’t drive guard cell-specific expression of the GUS reporter gene, but its 300 bp 5′-truncated version which retained only 5 elements could specifically expressed in the guard cells. They postulated that the (T/A) AAAG elements far away from the TSS might not work for the guard cell specificity. This position effect was also observed in CYP86A2 promoter in which the presence of 2 more (T/A) AAAG elements at −805/−883 abolished the guard cell specificity [26]. Our results showed that the full-length promoter GbSLSP (F1) contained 3 more (T/A)AAAG elements (including 1 guard cell-specific one at −479) at 5′ distal than F2, (Figs. 1 & 4A) and directed only guard cell-preferred expression of GUS and GFP reporter gene in both transgenic tobacco (Fig. 2: F1; Fig. 3: F1) and Arabidopsis (Fig. 5A: F1), whereas the F2, 5′-truncated version of GbSLSP, did confer the guard cell-specific expression of the reporter genes (Fig. 2: F2; Figs. 3 & 5: F2). This suggests that the (T/A)AAAG elements, especially TAAAG, proximal to TSS might determine guard cell specific expression of the gene, whereas those far away from the TSS might not only do not work for, but also impede the guard cell specificity. Neininger et al [64] and Dorbe et al [65] observed that in spinach and tobacco NIR gene promoters, the sequences close to their TSS were sufficient to confer nitrate-responsive increases in reporter enzyme activity. Besides the position effect of (T/A)AAAG relative to the TSS, the distance in (T/A)AAAG clusters and/or the distance between clusters and coding region may affect guard cell specific expression, for which the Kat1 [33] is exampled. Galbiati and co-workers [26] suggested that a cluster of at least 3 copies of (T/A)AAAG elements located on the same strand within a region of 100 bp of AtMYB60 be decisive to guard cell-specific expression of the promoter. In our experiment, the F4 which contains a cluster of 3 (T/A)AAAG elements located on the different strands (2 in sense strand and 1 in antisense one) in a region of ca. 100 bp relative to TSS (Fig. 1) was “true” guard cell-specific (Fig. 2: F4; Fig. 3: F4), and removal of one distal TAAAG element from the cluster (Figs. 1 & 4A) resulted in complete abolishment of the guard cell specificity (Fig. 2:F5; Figs. 3 & 5:F5). Thus, if the cluster were decisive to guard cell-specific expression of the promoter, the distal copy of element in the cluster would play an essential role without the necessity of same strand location of the (T/A) AAAG elements in the cluster.
In addition to their position and/or distance effects, the (T/A)AAAG element copy number may have some effects on the guard cell-specific expression. Yang and colleagues [44] observed that AtMYB61 which contains 29 (T/A)AAAG elements had lower expression in guard cell than AtACT7 which has only 23 (T/A) AAAG elements, and block mutagenesis of the central TAAAG motif on the sense strand in the 8 TAAAG motifs-containing region (−861 bp to −224 bp) of GC1 promoter did not affect reporter expression in guard cells. Thus they thought that it was not the number or mutation of several (T/A) AAAG elements that could affect the expressive activity in guard cells. However, in our experiment, progressively reducing the number of (T/A) AAAG elements proximal to the TSS, i.e. from F2 (containing 7 copies of (T/A) AAAG elements) to F5 (containing only 2 copy), greatly decreased the expressive activity of both GUS and GFP reporter genes in the guard cells of transgenic tobacco (Fig. 2:F2 to F5) and Arabidopsis (Fig. 5A). Thus, the Dof elements in the strict guard cell-specific promoters seemly have an additive effect on the gene expression strength in guard cells, which was also observed by Cominelli and colleagues in a “true guard cell-specific promoter”, AtMYB60 [42]. This different effects of (T/A) AAAG copy number may be contributed to much larger distance of the Dof elements relative to TSS in GC1 promoter (−861 bp to −224 bp) than in our F2 to F5 (−262 bp to −44 bp) and than in AtMYB60 minimal promoter region (−196 bp), because the Dof elements far away from the TSS may enhance the guard cell expression activity, but decreased the guard cell specificity as discussed above. Of course, the (T/A)AAAG element alone may not completely explain why guard cell-specific promoters exhibited guard cell-specific expression, as discussed by Yang and colleagues [44], demonstrated by Cominelli et al [42] and revealed by our 3′-deletion of the GbSLSP which will be discussed in the following.
Roles Played by 5′-UTR in the Determination of the Guard Cell Expression Activity and Specificity
It is well known that the 5′-untranslated region (5′-UTR) takes an important part in regulating gene expression at transcriptional and post-transcriptional levels [66], [67]. This regulation was mostly reported concentrated on gene expression strength, i.e. increasing or decreasing downstream gene’s expression. For example, the 5′-UTRs of ntp303 [68], OsADH [69] and OsGluc [70] enhanced markedly endogenous gene and/or GUS reporter gene expression, whereas the 5′-UTR of LAT59 greatly decreased mRNA yields [71]. In our experiment, the 5′-UTR of GbSLSP promoter affected not only the gene expression strength, but also the gene expression specificity. Removal of 359 bp out 369 bp 5′-UTR from full-length GbSLSP by 3′-deletion (Fig. 4A), significantly decreased the expression strength of both GUS and GFP reporter genes in transgenic tobacco (in Fig. 2: F1-sh vs. F1; Fig. 3: F1-sh vs. F1; Fig. 4B), and the same 3′-deletion in the strong guard cell-specific promoter F2 (Fig. 4A), not only reduced the expression activity (Fig. 4B), but also completely abolished the guard cell-specificity of reporter genes (Fig. 2: F2-sh vs. F2; Fig. 3: F2-sh vs. F2). From these comparisons (F1 vs. F1-sh, F2 vs. F2-sh) and comparisons in the previous section (F1 vs. F2 to F5), we can see that the 5′-UTR in the GbSLSP(s) acts as an enhancer in one hand, and takes part in guard cell-specific expression of the reporter genes in the other hand.
In summary, we isolated a 993-bp promoter region upstream of the translation start point of subtilisin-like serine protease (subtilase) gene from sea island cotton, and demonstrated that 5′-end truncated versions of the promoter, F2 to F4, could drive GUS and GFP reporter genes to express exclusively and strongly in the guard cells of both transgenic tobacco and Arabidopsis plants, while the full-length GbSLSP directed high level guard cell-preferred expression. We revealed that the guard cell specificity and expression strength of the promoters were coordinately controlled by 5′-untranslated region (5′-UTR) and a cluster of at least 3 copies of (T/A)AAAG elements within a region of about 100 bp relative to transcription start site (TSS). We are aware that in order to better use these new “true” guard cell-specific promoters to manipulate gene expression in guard cells for physiological and biochemical studies and for biotechnological improvement of crop plants, further work is needed to investigate whether the guard cell specificity and strength of these new promoters are regulatable, and if yes, what is the major regulator(s).
Funding Statement
This work was supported by grants for XXG from Transgenic Plant R&D Key Program, the Ministry of Agriculture of China (Grant No. 2011ZX08002-001-07) and from National Nature Science Foundation of China (Grant No. 31070268). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mott KA, Sibbernsen ED, Shope JC (2008) The role of the mesophyll in stomatal responses to light and CO2 . Plant Cell Environ 31: 1299–1306. [DOI] [PubMed] [Google Scholar]
- 2. Lawson T (2009) Guard cell photosynthesis and stomatal function. New Phytol 181: 13–34. [DOI] [PubMed] [Google Scholar]
- 3. Antunes WC, Provart NJ, Williams TCR, Loureiro ME (2012) Changes in stomatal function and water use efficiency in potato plants with altered sucrolytic activity. Plant Cell Environ 35: 747–759. [DOI] [PubMed] [Google Scholar]
- 4. Powles JE, Buckley TN, Nicotra AB, Farquhar GD (2006) Dynamics of stomatal water relations following leaf excision. Plant Cell Environ 29: 981–992. [DOI] [PubMed] [Google Scholar]
- 5. Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980. [DOI] [PubMed] [Google Scholar]
- 6. Koers S, Guzel-Deger A, Marten I, Roelfsema MRG (2011) Barley mildew and its elicitor chitosan promote closed stomata by stimulating guard-cell S-type anion channels. Plant J 68: 670–680. [DOI] [PubMed] [Google Scholar]
- 7. Shimazaki KI, Doi M, Assmann SM, Kinoshita T (2007) Light Regulation of Stomatal Movement. Annu Rev Plant Biol 58: 219–247. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Noguchi K, Terashima I (2008) Distinct light responses of the adaxial and abaxial stomata in intact leaves of Helianthus annuus L. Plant Cell Environ 31, 1307–1316. [DOI] [PubMed]
- 9. Messinger SM, Buckley TN, Mott KA (2006) Evidence for Involvement of Photosynthetic Processes in the Stomatal Response to CO2 . Plant Physiol 140: 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wittig VE, Ainsworth EA, Long SP (2007) To what extent do current and projected increases in surface ozone affect photosynthesis and stomatal conductance of trees? A meta-analytic review of the last 3 decades of experiments. Plant Cell Environ 30: 1150–1162. [DOI] [PubMed] [Google Scholar]
- 11. Peak D, Mott KA (2011) A new, vapour-phase mechanism for stomatal responses to humidity and temperature. Plant Cell Environ 34: 162–178. [DOI] [PubMed] [Google Scholar]
- 12. Garcia-Mata C, Lamattina L (2010) Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol 188: 977–984. [DOI] [PubMed] [Google Scholar]
- 13. Shope JC, Peak D, Mott KA (2008) Stomatal responses to humidity in isolated epidermes. Plant Cell Environ 31: 1290–1298. [DOI] [PubMed] [Google Scholar]
- 14. Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424: 901–908. [DOI] [PubMed] [Google Scholar]
- 15. Ribeiro DM, Desikan R, Bright J, Confraria A, Harrison J, et al. (2009) Differential requirement for NO during ABA-induced stomatal closure in turgid and wilted leaves. Plant Cell Environ 32: 46–57. [DOI] [PubMed] [Google Scholar]
- 16. Jiang K, Sorefan K, Deeks MJ, Bevan MW, Hussey PJ, et al. (2012) the ARP2/3 complex mediates guard cell actin reorganization and stomatal movement in Arabidopsis. Plant Cell 24: 2031–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee SC, Luan S (2012) ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ 35: 53–60. [DOI] [PubMed] [Google Scholar]
- 18. Mott KA (2009) Opinion: Stomatal responses to light and CO2 depend on the mesophyll. Plant Cell Environ 32: 1479–1486. [DOI] [PubMed] [Google Scholar]
- 19. Kopka J, Provart NJ, Muller-Rober B (1997) Potato guard cells respond to drying soil by a complex change in the expression of genes related to carbon metabolism and turgor regulation. Plant J 11: 871–882. [DOI] [PubMed] [Google Scholar]
- 20. Gray JE, Holroyd GH, van der Lee FM, Bahrami AR, Sijmons PC, et al. (2000) The HIC signalling pathway links CO2 perception to stomatal development. Nature 408: 713–716. [DOI] [PubMed] [Google Scholar]
- 21. Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon JM, et al. (1992) Cloning and expression in yeast of a plant potassium ion transport system. Science 256(5057): 663–665. [DOI] [PubMed] [Google Scholar]
- 22. Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF (1992) Functional expression of a probable Arabidopsis thaliana potassium channel in Sacchromyces cerevisiae . Proc Natl Acad Sci U S A 89: 3736–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Terryn N, Arias MB, Engler G, Tire C, Villarroel R, et al. (1993) rha1, a gene encoding a small GTP binding protein from Arabidopsis, is expressed primarily in developing guard cells. Plant Cell 5: 1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nylander M, Svensson J, Palva ET, Welin BV (2001) Stress-induced accumulation and tissue-specific localization of dehydrins in Arabidopsis thaliana . Plant Mol Biol 45: 263–279. [DOI] [PubMed] [Google Scholar]
- 25. Padmanaban S, Chanroj S, Kwak JM, Li X, Ward JM, et al. (2007) Participation of Endomembrane Cation/H+ Exchanger AtCHX20 in Osmoregulation of Guard Cells. Plant Physiol 144: 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galbiati M, Simoni L, Pavesi G, Cominelli E, Francia1 P, et al (2008) Gene trap lines identify Arabidopsis genes expressed in stomatal guard cells. Plant J 53: 750–762. [DOI] [PubMed] [Google Scholar]
- 27. Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, et al. (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486. [DOI] [PubMed] [Google Scholar]
- 28. Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, et al. (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meyer S, Mumm P, Imes D, Endler A, Weder B, et al. (2010) AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J 63: 1054–1062. [DOI] [PubMed] [Google Scholar]
- 30. Rienmuller F, Beyhl D, Lautner S, Fromm J, Al-Rasheid KAS, et al. (2010) Guard cell-specific calcium sensitivity of high Density and Activity SV/TPC1 Channels. Plant Cell Physiol 51(9): 1548–1554. [DOI] [PubMed] [Google Scholar]
- 31. Li Z, Kang J, Sui N, Liu D (2012) ROP11 GTPase is a negative regulator of multiple ABA responses in Arabidopsis. J Integr Plant Biol 54 (3): 169–179. [DOI] [PubMed] [Google Scholar]
- 32. Müller-Rober B, Cognata UL, Sonnewald U, Willmitzer L (1994) A truncated version of an ADP-glucose pyrophosphorylase promoter from potato specifies guard cell-selective expression in transgenic plants. Plant Cell 6: 601–612. [PMC free article] [PubMed] [Google Scholar]
- 33. Nakamura RL, McKendree WL, Hirsch RE, Sedbrook JC, Caber RF, et al. (1995) Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol 109: 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plesch G, Kamann E, Mueller-Roeber B (2000) Cloning of regulatory sequences mediating guard-cell-specific gene expression. Gene 249: 83–89. [DOI] [PubMed] [Google Scholar]
- 35. Plesch G, Ehrhardt T, Mueller-Roeber B (2001) Involvement of TAAAG elements suggests a role for Doftranscription factors in guard cell specific gene expression. Plant J 28: 455–464. [DOI] [PubMed] [Google Scholar]
- 36. Hugouvieux V, Kwak JM, Schroeder JI (2001) An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transdution in Arabidopsis . Cell 106: 477–487. [DOI] [PubMed] [Google Scholar]
- 37. Guo FQ, Wang R, Chen M, Crawford NM (2001) The Arabidopsis dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is activated and functions in nascent organ development during vegetative and reproductive growth. Plant Cell 13: 1761–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lemichez E, Wu Y, Sanchez JP, Mettouchi A, Mathur J, et al. (2001) Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev 15(14): 1808–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu J, Gong Z, Zhang C, Song CP, Damsz B, et al. (2002) OSM1/SYP61: A syntaxin protein in Arabidopsis controls abscisic acid-mediated and non-abscisic acid-mediated responses to abiotic stress. Plant Cell 14(12): 3009–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Girandat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cominelli E, Galbiati M, Vavasseur A, Conti L, Sala T, et al. (2005) A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance. Curr Biol 15: 1196–1200. [DOI] [PubMed] [Google Scholar]
- 42. Cominelli E, Galbiati M, Albertini A, Fornara F, Conti L, et al. (2011) DOF-binding sites additively contribute to guard cell-specificity of AtMYB60 promoter. BMC Plant Biol 11: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sze H, Padmanaban S, Kwak J (2012) Guard cell-specific toll for molecular manipulation of draught avoidance/water loss in plants. Patent, US7993926B2.
- 44. Yang YZ, Costa A, Leonhardt N, Siegel RS, Schroeder JI (2008) Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods 4: 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Francia P, Simoni L, Cominelli E, Tonelli C, Galbiati M, et al. (2008) Gene trap-based identification of a guard cell promoter in Arabidopsis. Plant Signal Behav. 3(9): 684–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tonelli C., Galbiati M (2010) Arabidopsis-stomatal-specific promoter and a genetic construct containing the promoter for expression of nucleic acids in plants. Patent US7662947.
- 47. Li J, Gong XM, Lin HQ, Song QB, Chen J, et al. (2005) DGP1, a drought-induced guard cell-specific promoter and its function analysis in tobacco plants. Sci China Ser. C 48(2): 181–186. [DOI] [PubMed] [Google Scholar]
- 48. Tanaka H, Onouchi H, Kondo M, Nishimura IH, Nishimura M, et al. (2001) A subtilisin-like serine protease is required for epidermal surface formation in Arabidopsis embryos and juvenile plants. Development 128: 4681–4689. [DOI] [PubMed] [Google Scholar]
- 49. Siezen RJ, Leunissen JA (1997) Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci 6: 501–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Berger D, Altmann T (2000) A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana . Genes & Dev 14: 1119–1131. [PMC free article] [PubMed] [Google Scholar]
- 51. Groll UV, Berger D, Altmann T (2002) The Subtilisin-like serine protease SDD1 mediates cell-to-cell signaling during Arabidopsis stomatal development. Plant Cell 14: 1527–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao XG, Han L (2012) Plant guard cell-specific promoter and use thereof. Patent CN102367439A.
- 53. Paterson AH, Brubaker CL, Wendel JF (1993) A rapid method for extraction of cotton (Gossypium spp.) genomic DNA suitable for RFLP or PCR analysis. Plant Mol Biol Rep 11(2): 122–127. [Google Scholar]
- 54.Hou L, Pei Y, Luo M, Xiao YH, Li DM, et al.. (2006) Cotton fiber-specific promoter and its application. Patent CN100471954C.
- 55. Hou L, Liu H, Li JB, Yang X, Xiao YH, et al. (2008) SCFP, a novel fiber-specific promoter in cotton. Chinese Sci Bull 53: 2639–2645. [Google Scholar]
- 56. Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . Plant J 16(6): 735–743. [DOI] [PubMed] [Google Scholar]
- 57. Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, et al. (1985) A simple and general method for transferring genes into plants. Science 227(4691): 1229–1231. [DOI] [PubMed] [Google Scholar]
- 58. Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6(13): 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Geng AQ, Zhao ZJ, Nie XL, Xiao XG (2009) Expression analysis of four flower-specific promoters of Brassica spp. in the heterogeneous host tobacco. Afr J Biotech 8(20): 5193–5200. [Google Scholar]
- 60. Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 27(1): 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nie XL, Zhu JW, Geng AQ, Xiao XG (2010) Cloning and functional analysis in transgenic tobacco of a tapetum-specific promoter from Arabidopsis. . Afr J Biotech 9(41): 6826–6834. [Google Scholar]
- 62. Rautengarten C, Steinhauser D, Bussis D, Stintzi A, Schaller A, et al. (2005) Inferring hypotheses on functional relationships of genes: analysis of the Arabidopsis thaliana subtilase gene family. PLoS Comput Biol 1(4): 297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yanagisawa S, Schmidt RJ (1999) Diversity and similarity among recognition sequences of Dof transcription factors. Plant J 17(2): 209–214. [DOI] [PubMed] [Google Scholar]
- 64. Neininger A, Back E, Bichler J, Schneiderbauer A, Mohr H (1994) Deletion analysis of a nitrite-reductase promoter from spinach in transgenic tobacco. Planta 194: 186–192. [Google Scholar]
- 65. Dorbe MF, Truong HN, Crété P, Vedele FD (1998) Deletion analysis of the tobacco Niilpromoter in Arabidopsis thaliana . Plant Sci 139: 17. [Google Scholar]
- 66. Bate N, Spurr C, Foster GD, Twell D (1996) Maturation-specific translational enhancement mediated by the 5′-UTR of a late pollen transcript. Plant J 10: 613–623. [DOI] [PubMed] [Google Scholar]
- 67. Hua XJ, Cotte BBVD, Montagu M, Verbruggen N (2001) The 5′-untranslated region of At-P5R gene is involved in both transcriptional and post-transcriptional regulation. Plant J 26: 157–169. [DOI] [PubMed] [Google Scholar]
- 68. Hulzink RJM, Groot PFMD, Croes AF, Quaedvlieg W, Twell D (2002) The 5′-untranslated region of the ntp303 gene strongly enhances translation during pollen tube growth, but not during pollen maturation. Plant Physiol 129: 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sugio T, Satoh J, Matsuura H, Shinmyo A, Kato K (2008) The 5′-untranslated region of the Oryza sativa alcohol dehydrogenase gene functions as a translational enhancer in monocotyledonous plant cells. J Biosci Bioeng 105: 300–302. [DOI] [PubMed] [Google Scholar]
- 70. Liu WX, Liu HL, Chai ZJ, Xu XP, Song YR, et al. (2010) Evaluation of seed storage-protein gene 5′- untranslated regions in enhancing gene expression in transgenic rice seed. Theor Appl Genet 121: 1267–1274. [DOI] [PubMed] [Google Scholar]
- 71. Curie C, McCormick SA (1997) Strong inhibitor of gene expression in the 5′-untranslated region of the pollen-specific LAT59 gene to tomato. Plant Cell 9: 2025–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]