In the current healthcare environment, it is critical that emerging medical technologies rapidly demonstrate clear and successful clinical outcomes. Because of keen economic competition and rapid loss of enthusiasm for medical technologies without a clearly demonstrated path for clinical adoption, there is little tolerance for early translational failures. However, the medical physics community can mitigate the prospect of translational failure by developing and specifying performance standards for emerging technology platforms.
For example, fluorescence molecular imaging is a new combinational technology platform with significant clinical promise, but currently is without performance standards for translating its devices and associated, molecularly targeted imaging agents (drugs). The appealing prospect of illuminating surface tissues with nonionizing, dim light, and sensitively collecting the fluorescence emanating from a molecularly targeted imaging agent has technological foundations in nuclear medicine. Yet unlike nuclear imaging, there are no current performance standards for quantifying sensitivity and for qualifying a device for “first-in-humans,” fluorescence molecular imaging agents.
The lack of task-specific, device specifications relevant for medical imaging jeopardizes the translation and adoption of fluorescence molecular imaging. For example, the failure to image contrasted diseased tissues could be interpreted as a failure of the imaging agent, when in fact the unqualified device was simply not sensitive enough for its detection. In the absence of standards that can qualify and predict performance in the clinical setting, we and others have used indocyanine green (ICG), a dye that has an established safety record in humans for over 50 years to directly visualize tissues when administered in mg doses. Indocyanine green possesses poor near-infrared (NIR) fluorescent properties, is notoriously unstable, and cannot be conjugated to targeting moieties, obviating its use for molecular imaging as well as for robust device qualification. Nonetheless, our FDA CDER approved studies were conducted to qualify our imaging devices with trace administration of ICG before conducting “first-in-humans” molecular imaging agent (drug) trials.
More recently, we developed a certifiable, solid phantom to compare the sensitivity of our investigational NIR fluorescence device qualified in humans to image trace (10–400 μg) doses of ICG with that of a commercial, market-approved device for NIR fluorescence imaging following i.v. administration of 5–25 mg ICG. The solid phantom consists of polymethylmethacrylate polymer cured with TiO2 particles to create the worse-case condition of backscattered light and with varying quantities of NIR excitable quantum dots to assess limit of fluorescence detection and dynamic range in the presence of scattering. The images of low and high concentrations of quantum dot phantoms acquired from both commercial, market-approved and investigational devices are shown in Fig. 1 with their respective intensity profiles. Whether the phantom provides an appropriate or complete measurement of imaging device performance in the clinical setting depends not only upon the specific imaging task but also on yet to-be-adopted standards. Nevertheless, the phantom images demonstrate dramatic differences in sensitivity and detection limits between the two devices. Indeed, investigators who use a device of unknown or unqualified performance in “first-in-humans” fluorescence molecular imaging agent studies could erroneously underestimate the sensitivity and targeting specificity of their investigational agent. First, the device may not be able to detect the targeted agent at trace tissue concentrations, leading to the erroneous conclusion of poor agent sensitivity. Second, the device may detect the agent only after administration of high doses sufficient for device detection, but not after agent clearance from normal tissues as is crucial to imaging contrast (leading to the erroneous conclusion of poor agent specificity).
Figure 1.
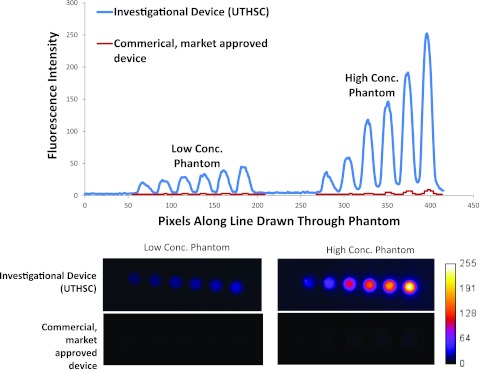
(Top) Intensity values read from images of low and high concentration as acquired from the investigational device (upper curve) at optimized settings for clinical imaging (converted to 8 bit to enable comparison) and from the commercial, market-approved device (lower curve) at factory settings for clinical imaging. (Bottom) Unprocessed image files for low concentration (pM) and high concentration (nM) quantum dots captured by investigational and commercial, market-approved devices from which intensity values in plot above are drawn. The SNR for the phantom with the highest concentration of quantum dots was 36 for the investigational device and 11 for the commercial, market-approved device. These comparisons are not made to claim superiority of one device over another, but rather to point out the variability of detection limits and sensitivity that could reflect differences in device performance in the clinical setting. Specifications of the commercial, market-approved device were not available to understand the causes of the differences in task-specific, imaging performance. We measured the incident excitation light fluence at the operational tissue surface of the commercial, market-approved device was approximately 12 mW/cm2, while that of the investigational device was less than 1/10 of that value.
Interestingly and perhaps alarmingly, there is also a lack of industry-standards for established small animal fluorescence imaging systems, leading some device manufacturers to specifically discourage users from employing competitor's imaging agents with their animal imaging devices. Other imaging agent manufacturers likewise discourage the use of their imaging agents on unauthorized small animal imaging devices due to the nonstandard (i.e., unknown) instrumentation response and sensitivities. In contrast, preclinical and clinical nuclear, CT, and MR imaging modalities are platform-based technologies that have utility with different manufacturer's contrast agents and maximal use for basic research and patient care.
Developing industry-standards that are judiciously based upon the physics of fluorescence generation and propagation in tissues will dramatically impact clinical translation and adoption. Standards and industry-wide instrument specifications could enable comparison of devices, determination of whether device performance is adequate/inadequate for “first-in-humans” imaging agent studies, and permit approval of future NIR imaging agents for an entire platform of devices, rather than a single imaging device. The lack of standards and specifications encourages the current track of device-specific, “first-in-humans” fluorescence molecular imaging agents which is costly, inefficient, and will inevitably limit or even prevent the clinical adoption of this potentially impactful medical technology. Standardized procedures for evaluating a platform of fluorescent medical imaging devices urgently need to be developed and validated by our community in order to maximize the success of “first-in-humans” fluorescent imaging agent studies and to accelerate clinical adoption of the technology. As industry-wide standards have been set for preclinical and clinical PET [National Electrical Manufacturer Association (NEMA) NU-2-2007 and NU-4-2008], so too are they needed for fluorescence imaging devices.
Recently, a workshop sponsored by the National Institute of Standards and Technology highlighted the need to develop industry-wide working standards for optical medical imaging techniques (http://www.opticsinfobase.org/boe/issue.cfm?volume=3&issue=6). Until certified standards and specifications are developed and used to qualify fluorescence imaging devices, the road for translating “first-in-humans” molecular fluorescent imaging agents may be “bumpy” with detours that lead away from intended clinical destinations.


