Standfirst
Novel findings draw attention to the perverse effect epithelial cell's neoplastic transformation has on intestinal barrier function and cancer progression. The study shows that impaired intestinal barrier facilitate microbial products translocation, a process causing production of immune cell-derived cytokines which subsequently promote tumour progression in mice. These findings could open new therapeutic strategies for patient with early stage of CRC.
The inflammatory microenvironment surrounding tumours has long been identified as a powerful modifier of cancer development. A new study greatly contributes to our understanding of the intricate interplay between the intestinal milieu, host inflammatory responses and colorectal cancer (CRC).1 Grivennikov and colleagues1 demonstrate in mice that defective intestinal barrier function at tumour sites facilitates the translocation of bacterial products, leading to activation of the myeloid-cell-derived IL-23–IL-17 cytokine network and promotion of tumour growth.
From the mouth to the rectum, the gastrointestinal tract is delineated by a single layer of epithelial cells that maintains intestinal homeostasis by two main mechanisms: by absorbing, secreting and transporting water, nutrients and macromolecules; and by forming a physical separation between the mucosal immune system and the luminal compartment containing a complex microbiota estimated at a total of 1014 microorganisms. Inappropriate translocation of bacteria and bacterial-derived products (for example, lipopolysaccharide [LPS], nucleic acids and flagellin) into the mucosal compartment could have pathological consequences for the host. In an elegant study by Grivennikov and co-workers,1 we learn that defective barrier function is observed early in neoplastic progression, a phenomenon leading to increased LPS translocation into the mucosal compartment and increased production of myeloid-cell-derived IL-23p19 and downstream effector cytokines IL-6 and IL-17A. This bacteria-induced local mucosal inflammation favours tumour growth and cancer progression in a murine model of CRC (Figure 1).
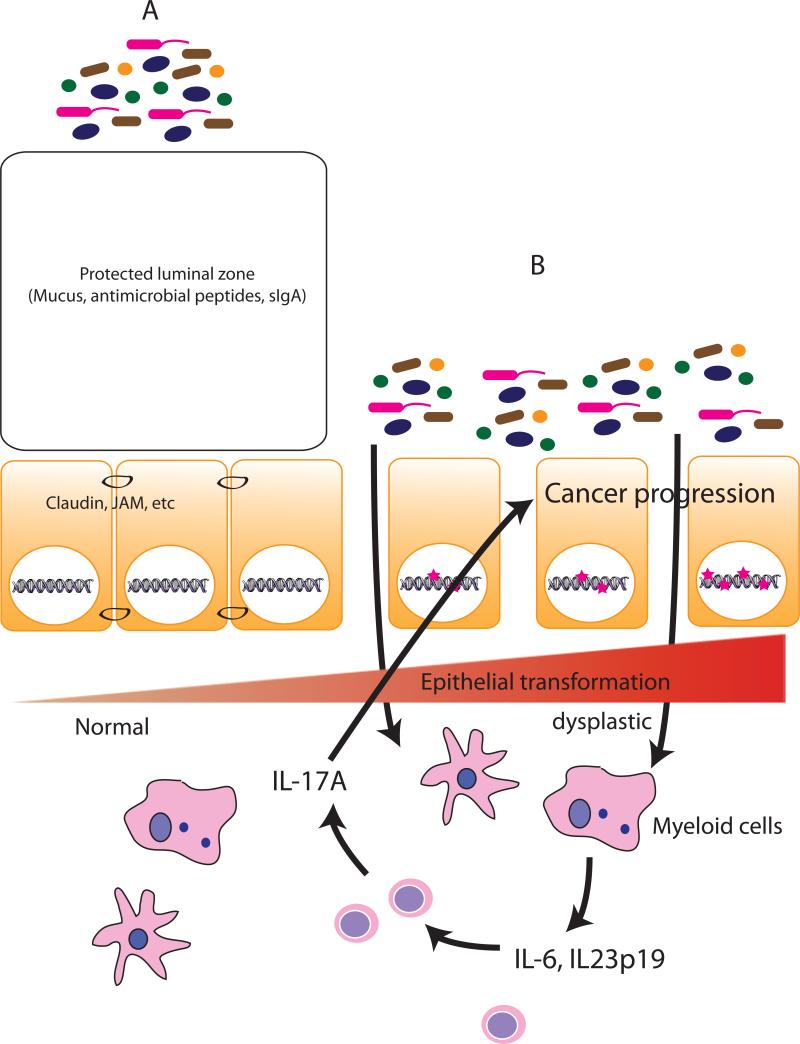
Fig. 1.
A healthy intestinal barrier prevents uncontrolled bacterial access to the immune cell compartment and dysregulated immune response (A). Following neoplastic changes (B), epithelial barrier loosen junctional contact (claudin, JAM, etc) and barrier function (mucin, goblet cells). This process facilitates microbial products access to the mucosal myeloid immune cells, which then increased production of IL-23p19 and downstream target IL-17A. This inflammatory microenvironment fosters progression of colorectal cancer.
Grivennikov and colleagues1 investigated the interplay between the intestinal barrier function, cytokine production and tumour progression. Using a spontaneous model of CRC in which the adenomatous polyposis coli (Apc) gene is selectively deleted by a Cdx2-driven cre recombinase (CPC-APC mice), the authors observed abundant production of Il23p19 and Il17A in tumour sites compared with adjacent normal ‘healthy’ tissue. A similar increase of IL-23p19 and Il17A expression was noted in human CRC tumours compared with control tissue samples. Genetic ablation of Il23p19 strongly attenuated tumour development in CPC-APC mice, which correlated with decreased production of Il17A. The authors next investigated the role of innate immune signalling proteins in driving this unique inflammatory environment. Using bone marrow from mice with defective innate immune signalling—Myd88–/–, Tlr2–/–, Tlr4–/– and Tlr9–/– mice—they demonstrated that CPC-APC mice whose immune systems were reconstituted with this bone marrow displayed reduced Il23p19 mRNA production. Interestingly, epithelial-specific Myd88 deletion led to compromised barrier function and increased mucosal bacteria adherence, showing the complexity of MyD88 signalling in intestinal homeostasis 2. CPC-APC mice transplanted with bone marrow from Myd88–/– mice exhibited a strong reduction in tumour development, which correlated with decreased IL-17A colonic tissue production. Antibiotic-mediated bacterial depletion markedly attenuated Il23p19–Il17A expression and CRC development in CPC-APC mice, thus the authors concluded that microbial products must be responsible for this pathology.
A key function of the epithelial barrier is to isolate the host from the potential harmful microbial population inhabiting the intestinal lumen. From Grivennikov's data, it seems that this task is not adequately performed by the epithelium of CPC-APC mice. To characterize barrier function in CPC-APC mice, Grivennikov and colleagues1 performed permeability assays using fluorescein isothyocyanate (FITC)-labelled-dextran and Alexa488-labelled LPS. The authors observed an increased translocation of both FITC-dextran and Alexa-labelled LPS in tumour lesions of CPC-APC mice, but not in normal ‘healthy’ adjacent tissues. Furthermore, they observed a reduction in goblet cells number and reduced levels of the barrier protecting mucin-2 protein in tumour lesions. At the molecular cellular level, the authors noticed decreased expression of junctional adhesion molecule A and mislocalization of claudin-4 in epithelial cells from tumour lesions compared with normal tissues. Finally, using a tamoxifen-inducible gene deletion system, they demonstrated that Apc loss parallels epithelial cell transformation, decreased mucin production and increased IL-23 expression, suggesting that early neoplastic changes set in motion a breakdown of barrier function and dysregulated intestinal immune responses.
The study by Grivennikov and colleagues1 brings into light the intricate interplay between the intestinal barrier, luminal microbial products and mucosal immune compartment in the progression of CRC. Although illuminating, the study raises a number of intriguing questions that must be addressed before this new paradigm can be fully understood. The most pressing question relates to the luminal bacterial entities or products driving immune cell activation and cancer progression. As alluded to by Grivennikov et al.1, Toll-like receptor 4 (Tlr4) (and presumably LPS) acts as tumour promoter,3 whereas the innate sensor nucleotide-binding oligomerization domain-containing protein 1 (Nod1) confers protection in mice against the same pathology.4 As Tlr4 and Nod1 detect different microbial products (LPS and peptidoglycan, respectively), it will be important to define the nature of the microbial antigen that influences CRC development in the CPC-APC model, and verify whether the findings are reproduced in other experimental model of CRC. Genetic ablation of Nod1, Nod2 or other Nod-like receptors will help define the differential contribution of bacterial products in CRC. Nod2 is of particular interest as this innate sensor has been linked to CRC development by findings from a meta-analysis.5 Consequently, the relevance of the study to human pathology would need to be confirmed.
Another important aspect requiring further investigation is the relationship between microbial composition and CRC susceptibility. Probably, the defective barrier function observed in CPC-APC mice would not only permit enhanced translocation of bacteria and bacterial products, but also increased colonization of adherent bacteria at neoplastic sites. Recent studies have shown differences in microbial community composition and richness in healthy rectal tissue from patients with adenomas versus healthy individuals controls 6-9. Therefore, it will be important to longitudinally examine the microbial community of normal tissue and tumour lesions of CPC-APC mice, and then apply gnotobiotic technology to determine cause–effect relationships between dysbiotic microbiota and the development of CRC.
The findings by Grivennikov and colleagues imply that defective barrier function is an early biological event in colorectal tumorigenesis and represents a critical step for activation of underlining immune cells by microbial products. It will be necessary to translate these findings to humans and determine whether early neoplastic changes are accompanied by increased permeability defects and bacterial translocation. Of note, patients with IBD—a population at high risk of developing CRC—exhibit increased intestinal permeability that precedes clinical inflammatory symptoms.10 Improving intestinal barrier function is already a priority for clinicians managing patients with IBD, and the work of Grivennikov et al.1 demonstrates that targeting intestinal permeability should be evaluated as a potential therapeutic avenue for individuals at high-risk of developing CRC.
Acknowledgements
C. Jobin would like to acknowledge grant support from NIH grants DK047700 and DK073338.
Biography
Dr. Christian Jobin is a PhD graduate in Immunology/Microbiology from Université Laval (Quebec, Canada), and is currently an Associate Professor of Medicine, Pharmacology and Immunology/Microbiology at the University of North Carolina at Chapel Hill. His research program focuses on bacteria/host interaction and ensuing innate/immunological responses during health and diseases. He utilized gnotobiotic conditions, microbiome approaches (next-generation sequencing, microbial gene mutations and RNA-seq) to study the differential contribution of bacteria in gut physiology.
Footnotes
Competing interests
The author declares no competing interests.
References
- 1.Grivennikov SI, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. doi: 10.1038/nature11465. http://dx.doi.org/10.1038/nature11465. [DOI] [PMC free article] [PubMed]
- 2.Frantz AL, et al. Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin-2, and antibacterial peptides. Mucosal Immunol. 2012;5:501–512. doi: 10.1038/mi.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukata M, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen GY, Shaw MH, Redondo G, Nunez G. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 2008;68:10060–10067. doi: 10.1158/0008-5472.CAN-08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian Y, et al. Differential effects of NOD2 polymorphisms on colorectal cancer risk: a meta-analysis. Int. J. Colorectal Dis. 2009;25:161–168. doi: 10.1007/s00384-009-0809-9. [DOI] [PubMed] [Google Scholar]
- 6.Castellarin M, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostic AD, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen XJ, et al. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes. 2010;1:138–147. doi: 10.4161/gmic.1.3.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanapareddy N, et al. Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 2012;6:1858–1868. doi: 10.1038/ismej.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedin CR, Stagg AJ, Whelan K, Lindsay JO. Family studies in Crohn's disease: new horizons in understanding disease pathogenesis, risk and prevention. Gut. 2012;61:311–318. doi: 10.1136/gut.2011.238568. [DOI] [PubMed] [Google Scholar]