Abstract
Background
The progesterone (P4 ) metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP), acts in the midbrain ventral tegmental area (VTA) to modulate the intensity and duration of lordosis. 3α,5α-THP can also have anti-anxiety and anti-stress effects in part through actions in the hippocampus. Separate reports indicate that manipulating 3α,5α-THP levels in the VTA or hippocampus respectively can influence lordosis and affective behavior. 3α,5α-THP levels can also be altered by behavioral experiences, such as mating or swim stress. Whether endogenous levels of 3α,5α-THP modulate and/or are increased in response to affective and/or reproductively-relevant behaviors was investigated.
Methods
In Experiment 1, rats in behavioral estrus or diestrus were individually tested sequentially in the open field, elevated plus maze, partner preference, social interaction, and paced mating tasks and levels of 17 β-estradiol (E2), P4, dihydroprogesterone (DHP), and 3α,5α-THP in serum, midbrain, hippocampus, diencephalon, and cortex were examined. In Experiments 2 and 3, rats in behavioral estrus or diestrus, were individually tested in the battery indicated above, with, or without, paced mating and tissues were collected immediately after testing for later assessment of endocrine measures.
Results
In Experiment 1, behavioral estrous, compared to diestrous, rats demonstrated more exploratory, anti-anxiety, social, and reproductive behaviors, and had higher levels of E2 and progestins in serum, midbrain, hippocampus, diencephalon, and cortex. In Experiment 2, in midbrain and hippocampus, levels of 3α,5α-THP and its precursor DHP were increased among rats in behavioral estrus that were mated. In diencephalon, and cortex, DHP levels were increased by mating. In Experiment 3, in midbrain, levels of 3α,5α-THP and its precursor DHP were increased among diestrous rats that were tested in the behavioral battery with mating as compared to those tested in the behavioral battery without mating.
Conclusions
Increased levels of 3α,5α-THP in behavioral estrus versus diestrous rats are associated with enhanced exploratory, anti-anxiety, social, and reproductive behaviors. Rats in behavioral estrus that are mated have further increases in 3α,5α-THP and/or DHP levels in midbrain, hippocampus, diencephalon, and cortex than do non-mated rats in behavioral estrus, whereas diestrous rats only show 3α,5α-THP increases in midbrain in response to behavioral testing that included mating.
Keywords: Progesterone; 3α,5α-THP; Lordosis; GABAA receptor; Ventral tegmental area; Social behavior, affect
Introduction
Increases in ovarian hormones, 17β-estradiol (E2) and progesterone (P4 ), which occur during behavioral estrus of rodents, modulate sexual behavior of female rodents, typically operationally defined by the occurrence of lordosis, a stereotypical posture that enables mating to occur. Ovariectomy (ovx) of rodents produces decrements in lordosis, E2, and progestin levels. Systemic administration of E2 and P4 to ovx rats, in a regimen analogous to ovarian secretion, reinstates lordosis responses of female rodents to sexually-relevant stimuli. Actions of P4 at intracellular progestin receptors in the ventromedial hypothalamus (VMH) are required to initiate lordosis of E2-primed rats [1–3]. Subsequent actions of P4 in the ventral tegmental area (VTA) mediate the onset and duration of lordosis through formation of P4’s 5α-reduced metabolite, mα-pregnan-3α-ol-20-one (3α,5α-THP) [4–6]. Although P4 and 3α,5α-THP’s actions in the VTA to mediate lordosis are well understood [7], the effects and mechanisms by which progestins may mediate other behavioral processes (such as avoidance, aggression, approach, and/or proceptivity) that may culminate in mating, are not.
Progestins may mediate behaviors other than lordosis that contribute to mating. Elevated concentrations of P4 and 3α,5α-THP in plasma and hippocampus that occur on behavioral estrus, compared to diestrus, coincide with increases in exploratory and anti-anxiety behavior of intact rats [8, 9]. Ovx decreases exploration and anti-anxiety behavior that are seen during behavioral estrus [10, 11]. Systemic [7, 10–12] or central administration of P4 or 3α,5α-THP to the hippocampus [13] or amygdala [12, 14] of ovx rodents results in exploration, approach, and/or anti-anxiety behavior that are analogous to that seen among receptive rodents, unless P4 ’s metabolism to 3α,5α-THP is inhibited by genetic deletion or systemic and/or intrahippocampal administration of inhibitors of metabolism enzymes [11, 15–18]. These findings suggest that changes in 3α,5α-THP over the cycle may influence approach and/or avoidance behaviors that may influence mating.
Multiple sources of 3α,5α-THP may underlie its functional effects. For example, 3α,5α-THP is secreted by the ovaries and adrenals in a manner similar to P4 [19, 20]. 3α,5α-THP is also formed centrally when ovarian and/or adrenal P4 is metabolized in brain [21, 22] and it is also a neurosteroid [23, 24]. Neurosteroids are produced de novo in brain independent of peripheral gland secretion, they have paracrine effects, which typically occur at non-traditional steroid substrates, and rapid changes in levels of neurosteroids have been demonstrated in response to environmental and/or behavioral stimuli [25–32]. Neurosteroids are synthesized from cholesterol that binds mitochondrial benzodiazepine receptors, which facilitates transport to the inner mitochondrial membrane and initiates neurosteroid biosynthesis [33]. In contrast to some steroids that exhibit traditional actions via intracellular steroid receptors, most neurosteroids have actions via modulatory effects on neurotransmitter receptor activity, most notably at GABAA receptors [5, 34]. Exposure to extreme stimuli such as cold-water swim, restraint, footshock, carbon dioxide inhalation, ether exposure, or loud noise enhances neurosteroidogenesis [25–32]. As well, mating stimuli can also increase 3α,5α-THP levels to those which can produce agonist-like actions at GABAA receptors [35]. Together, these findings have contributed to the notion that 3α,5α-THP may be an important neuroendocrine modulator of homeostasis [36].
Actions of 3α,5α-THP in the VTA for lordosis may involve neurosteroidogenesis. First, inhibiting or increasing biosynthesis in the VTA by administration of agents which inhibit or activate mitochondrial benzodiazepine inhibitors disrupts or enhances, respectively, lordosis of intact and ovx, hormone-primed rats and hamsters [37–39]. Second, 3α,5α-THP enhances lordosis via actions at GABAA, NMDA, and/or D1, rather than progestin, receptors in the VTA [5, 7, 40–42], as demonstrated by studies which pharmacologically target these substrates in the VTA and produce commensurate effects on progestin-facilitated lordosis. Third, mating enhances 3α,5α-THP levels in the midbrain VTA independent of peripheral gland secretion of progestins. Engaging in mating behaviors increases 3α,5α-THP levels in midbrain of E2-primed rats that are lacking peripheral sources of progestins (ovaries and adrenals) [5]. Together, these data suggest that 3α,5α-THP’s effects in the VTA to mediate lordosis may involve neurosteroidogenesis.
There is also evidence that neurosteroidogenesis in the hippocampus is involved in modulating behavioral processes. In support, increasing levels of 3α,5α-THP in hippocampus via manipulations of mitochondrial benzodiazepine receptors reduces fear and anxiety behaviors in the shock-probe burying test and the elevated plus maze in male rats, an effect which is abrogated by co-administration of GABAA receptor antagonists [15]. Blocking actions at GABAA receptors attenuates P4 ’s anti-anxiety effects in male mice [43]. Effects of manipulating neurosteroidogenesis and actions at non-traditional substrates on anxiety behavior of female rodents have not been investigated as systematically as is needed. Further, whether 3α,5α-THP is increased in hippocampus in response to behavioral processes that may involve anxiety is not well known. Thus, although data suggest that neurosteroidogenesis in hippocampus may influence anxiety behaviors, further investigation is necessary.
The present studies were designed to address two questions. First, whether differences in 3α,5α-THP in midbrain VTA over the estrous cycle are related to changes in exploration, anxiety, other social and approach behaviors, and mating was investigated in Experiment 1. This question was investigated by comparing behavior of rats with high (behavioral estrus) and low (diestrous) endogenous 3α,5α-THP levels. We expected that behavioral estrous, compared to diestrous, rats would exhibit enhanced exploration, anti-anxiety, social, and reproductive behaviors concomitant with increased levels of E2, P4, DHP, and 3α,5α-THP. Second, whether engaging in these behaviors may alter E2, P4, DHP, 3α,5α-THP levels was examined in Experiments 2 and 3. To investigate this, concentrations of E2, P4, DHP, and 3α,5α-THP in serum, midbrain, hippocampus, diencephalon, and cortex of behavioral estrous (Experiment 2) or diestrous (Experiment 3) rats that were individually behaviorally tested through a battery of exploratory, anxiety, and social tasks, with or without paced mating, was investigated. It was expected that 3α,5α-THP levels in midbrain would be increased by paced mating and that 3α,5α-THP in other brain areas might be altered by other reproductively-relevant behaviors.
Materials and Methods
These methods were pre-approved by The Institutional Care and Use Committee at The University at Albany-SUNY and studies were conducted in compliance with ethical guidelines defined by The National Institute of Mental Health and the Society for Neuroscience.
Animals and Housing
Adult, intact, female Long-Evans rats (n = 34) were utilized in the present study. Rats were group-housed in the Laboratory Animal Care Facility in the Social Sciences Building at The University at Albany-SUNY, in a temperature- and humidity-controlled room with ad libitum access to water and rat chow in their cages. Rats were on a reversed, 12:12 h light cycle (lights off at 08: 00 h) and all behavioral testing was conducted between 08: 00 and 13: 00 h.
Determination of Estrous Cycle Phase
Vaginal epithelium was examined daily, between 07: 00 and 08: 00 h, to determine the phase of the estrous cycle, as previously reported [8, 44]. Rats were cycled through two normal estrous cycles (4- to 5-day cycle) and then randomly assigned for testing during behavioral estrous or diestrous phases of the estrous cycle.
Stimulus Males
Male stimulus rats in the present studies were breeders used to maintain our rat colony. As such, these sexually-responsive males were screened with a sexually-receptive female every other day, to ascertain that consistent, high levels of sexual responsiveness were exhibited. To further ensure that all experimental females received analogous stimulation, immediately prior to engaging in paced mating, stimulus males were paired with a stimulus female that is optimally receptive due to exogenous hormone priming. Only stimulus males that reliably and consistently make sexual contacts in both evaluations were utilized.
Behavioral Testing
Behavioral data were collected by 1 of 2 observers who were blind to the experimental condition of rats and/or the hypothesized outcome of the study. Each rat was individually tested sequentially in the behavioral battery of tasks described below, with no time between tasks, other than that necessary to transfer rats from one apparatus to another. The behavioral battery requires about 40 min for each rat to complete. The order of the tasks was the same for each rat. Prior reports from our laboratory have demonstrated that similar behavioral and endocrine (5α-reduced androgens) outcomes are observed irrespective of whether rats are tested sequentially through the battery of tasks or are tested in individual tasks only [45].
Multiple measures were obtained in each of the tasks utilized in the present study. Measures used as indices of exploration, anxiety, social, and/or sexual behaviors were of primary interest in the current study. Other, secondary measures, which typically represent performance parameters, such as motor effects, were collected and used as control measures to ascertain the specificity of behavioral effects in all tasks utilized. Only data from primary measures that do not appear to be attributable to differences in performance parameters (secondary measures) are reported.
Open Field
The open field (76 × 57 × 35 cm) has a 48-square grid floor (6 × 8 squares, 9.5 cm/side), with the 24 central squares illuminated from above. As previously reported, rats were placed in the open field and the number of peripheral and central squares entered was recorded during a 5-min test period [8, 46, 47]. Increased central square entries are an indication of increased exploration and anti-anxiety behavior.
Elevated Plus Maze
The elevated plus maze is 50 cm off the ground and has four arms (49 cm long and 10 cm wide), two of which were enclosed by walls (30 cm high) and two of which were open. The number of entries into, and the amount of time spent on, the open and closed arms were recorded in a 5-min test [8, 48]. More time on the open arms is considered indicative of increased exploration and anti-anxiety behavior.
Partner Preference Test
Stimulus rats (1 ovx female, 1 intact male) were confined in opposite corners of an open field (76 × 57 × 35 cm) by clear plexiglass compartments with small holes drilled in the bottom. This allows experimental rats to receive both visual and olfactory stimuli, but not physical contact, from stimulus rats. Experimental rats were placed in the center of the open field and the amount of time spent in close proximity (with-in a body’s length) to either of the stimulus rats, was recorded in a 5-min test [49]. Increased time spent with one stimulus rat over another is considered a partner preference.
Social Interaction
An experimental rat and an ovx female conspecific were placed in opposite corners of an open field (76 × 57 × 35 cm) and the total duration of time that the experimental rat engaged the stimulus rat in crawling over and under partner, sniffing of partner, following with contact, genital investigation of partner, tumbling, boxing and grooming was recorded during a 5-min test [8, 50]. Increased social interaction indicates decreased anxiety behavior.
Paced Mating
Some rats were tested in the paced mating task, per previous methods [51, 52]. Allowing females to pace the timing of sexual contacts facilitates fertility and fecundity and is believed to be more ethologically relevant [52]. Experimental females were placed in a chamber (37.5 × 75 × 30 cm), which was equally divided by a partition that had a small (5 cm in diameter) hole in the bottom center, to allow females free access to both sides of the chamber, but prevented the stimulus male from moving between sides. Females were observed in the box for an entire ejaculatory series. The frequency of mounts and intromissions that preceded an ejaculation was recorded. As well, the frequency (lordosis quotient) and intensity (lordosis rating) of lordosis, quantified by rating of dorsiflexion on a scale of 0–3 [53], was recorded. The percentage of sexual contacts that were preceded by proceptive (i.e. hopping, darting, ear wiggling, PQ = number of proceptive behaviors/number of total contacts) or aggressive (i.e. vocalizations, defensive postures, AQ = number of aggressive behaviors/number of total contacts) behaviors was recorded. Pacing measures included the percentage of times the female rat left the compartment containing the male rat after receiving a particular copulatory stimulus (% exits after mounts, intromissions, and ejaculations) and latencies in seconds to return to the male compartment after these stimuli. The normal pattern of pacing behavior for percent exits and return latencies to be longer after more intensive stimulation (ejaculations > intromissions > mounts) was observed in the present study.
Tissue Collection
Immediately following the completion of the test battery, each rat had trunk blood and whole brains collected for later measurement of circulating and central E2, P4, DHP, and 3α,5α-THP by radioimmunoassay (described below). Serum and brains were stored at –80 ° C for approximately 2 months until radioimmunoassay. Immediately prior to measurement of steroids, midbrain, hippocampus, diencephalon, and cortex were grossly dissected. Remaining subcortical tissue in close proximity to midbrain was also dissected and used as a control site (interbrain). The brain was initially positioned ventral side up for dissection. The anterior and posterior borders for gross dissection of midbrain were the optic chiasm and pontine regions, respectively. The lateral borders were ~1.5 mm from midline and the dorsal aspect was just ventral to the cerebral aqueduct. For diencephalon, anterior/posterior boundaries for dissection were the accumbens and optic chiasm, the lateral aspects were ~1.5 mm from midline, and the dorsal/ventral aspects were between the hypothalamus and thalamus and core and shell of the accumbens. For dissection of hippocampus and cortex, brains were turned over. Intact bilateral hippocampus, including amygdala, were separated out from cortex. Bilateral anterior cortical tissues (~2 mm dorsal to olfactory bulbs) were dissected and used for cortex measurements. Interbrain tissue was the remaining subcortical tissue above the previously dissected midbrain area, which included the central gray. Wet weight of dissected regions was obtained to account for tissue mass used for radioimmunoassay.
Measurement of Steroid Hormones
E2 and progestins were measured using radioimmunoassay per previous methods [54]. Serum E2 and progestins were extracted using diethyl ether. For extraction of E2 and progestins from the midbrain, hippocampus, striatum, and prefrontal cortex, tissues were homogenized with a glass/glass homogenizer in 50% MeOH, 1% acetic acid. Tissues were then centrifuged at 3,000 g and the supernatant was chromatographed using Sepak cartridges and increasing concentrations of MeOH. Solvents were removed using a speed drier and samples were reconstituted in assay buffer (pH = 7.4). Radioisotopes for assays were as follows: [3 H]E2 (NET-317, 51.3 Ci/mmol), [3 H]P4 (NET-208: specific activity = 47.5 Ci/mmol), and [ 3 H]3α,5α-THP (NET-1047: specific activity = 65.0 Ci/mmol), from PerkinElmer (Boston, Mass., USA). The E2 antibody (E#244, Dr. G.D. Niswender, Colorado State University, Fort Collins, Colo., USA) was used in a 1: 40,000 dilution and bound between 40 and 60% of [3 H]E2. The P4 antibody (P#337 from Dr. G.D. Niswender, Colorado State University) was used in a 1: 30,000 dilution and bound between 30 and 50% of [3H]P. The DHP (X-947) and 3α,5α-THP antibodies (purchased from Dr. Robert Purdy, Veterans Medical Affairs, La Jolla, Calif., USA) were used in a 1: 5,000 dilution, which binds between 40 and 60% of [3 H]DHP and [3 H]3α,5α-THP. Standard curves were prepared in duplicate for a range of nine concentrations. The range of the standard curve for E2 was 12.5–1,000 pg and for progestins 50–4,000 pg. Standards, the appropriate antibody, and [3 H]steroid were added to assay buffer. The total assay volumes for E2, P4, DHP, and 3α,5α-THP were 800, 950, 950, and 1,200 μl, respectively. All assays were incubated overnight at 4 ° C, except E2, which incubated at room temperature for 50 min. The rapid addition of dextran-coated charcoal resulted in separation of bound and free steroid. Following incubation with charcoal, samples were centrifuged at 3,000 g and the supernatant was pipetted into a glass scintillation vial with scintillation cocktail. Sample tube concentrations were calculated using the logit-log method of Rodbard and Hutt [55], interpolation of the standards, and correction for recovery. The intra- and interassay percentages of variance for each assay were: E2 9 and 10%, P4 10 and 11%, DHP 9 and 11%, and 3α,5α-THP 12 and 13%.
Procedure
In Experiment 1, behavior (in the test battery described above) and endocrine measures of behavioral estrous (n = 8) and diestrous (n = 9) rats were compared. Tissues were collected immediately following the completion of testing for later radioimmunoassay to compare levels of E2, P4, DHP, and 3α,5α-THP in serum, midbrain, hippocampus, diencephalon, and cortex. One-way ANOVAs were used to examine effects of estrous cycle (behavioral estrus, diestrus) on behavior and endocrine outcomes.
In Experiment 2, endocrine parameters of rats in behavioral estrus (n = 8) that were tested in the behavioral battery without mating were compared to that of rats in behavioral estrus (n = 8) that were exposed to the entire battery, including mating. Tissues were collected immediately after completion of testing for endocrine measures. Effects of paced mating (paced mating, no paced mating) on endocrine measures of rats in behavioral estrus were analyzed using one-way ANOVAs.
In Experiment 3, endocrine parameters of diestrous rats (n = 9) that were tested in the behavioral battery without mating were compared to that of diestrous rats (n = 9) that were exposed to the entire battery, including mating. Immediately following testing, tissues were collected for later hormone measurement. One-way ANOVAs were used to examine effects of paced mating (paced mating, no paced mating) on endocrine measures of diestrous rats.
Statistical Analyses
One-way ANOVAs were utilized to analyze data in each experiment. Correlational analyses were used to examine the relationship between hormone levels in specific brain areas and behaviors examined. α level for statistical significance was p ≤ 0.05.
Results
Experiment 1
Behavioral Measures
As expected, and previously demonstrated in investigations of individual behaviors [8, 9, 17, 56, 57], behavioral estrous, compared to diestrous, rats exhibited more exploratory, anti-anxiety, social, and sexual behavior. Behavioral estrous, compared to diestrous, rats entered significantly more central squares in the open field, spent significantly more time on the open arms of the elevated plus maze, more time in close proximity to a stimulus male and in social interaction with a conspecific. Rats in behavioral estrus also demonstrated enhanced lordosis quotients and ratings, percentage of exits, and proceptivity and aggression quotients than did diestrous rats ( table 1, A, B).
Table 1.
Performance in open field (OF), elevated plus maze (EPM), partner preference (PP), social interaction (SI), and pacing tasks and associated levels of E2, P4, DHP, and 3α,5α-THP in serum, midbrain, hippocampus, diencephalon, and cortex
| Diestrus | Behavioral estrus |
F(1,15) | p | r | |
|---|---|---|---|---|---|
| A Exploration/anxiety/social behaviors | |||||
| Central entries | 22±3 | 46±5* | 12.88 | 0.002 | |
| Open arm time | 12±4 | 83±32* | 5.33 | 0.03 | |
| Proximity to male | 81±13 | 203±18* | 28.95 | 0.001 | |
| Proximity to female | 78±13 | 21±5* | 13.24 | 0.002 | |
| Social interaction | 47±7 | 103±11* | 17.64 | 0.001 | |
|
| |||||
| B Sexual behaviors | |||||
| LQ, % | 10±4 | 95±2* | 29.59 | 0.001 | |
| Lordosis ratings | 0.5±0.1 | 2.7±0.1* | 21.75 | 0.001 | |
| % of exits | 1±1 | 71±10* | 25.86 | 0.001 | |
| PQ, % | 0±0 | 88−5* | 267.00 | 0.0001 | |
| AQ, % | 5±2 | 34±5* | 40.76 | 0.00001 | |
|
| |||||
| C E2 | |||||
| Plasma, ng/ml | 2.8±0.7 | 21.8±2.0* | 8.02 | 0.01 | |
| Midbrain, ng/g | 1.4±0.1 | 2.2±0.3* | 7.72 | 0.01 | |
| Hippocampus, ng/g | 1.9±0.1 | 2.7±0.2* | 8.82 | 0.01 | |
| Diencephalon, ng/g | 1.5±0.1 | 2.1±0.1* | 4.59 | 0.04 | |
| Cortex, ng/g | 1.8±0.2 | 2.4±0.1* | 5.61 | 0.03 | |
| Interbrain, ng/g | 1.1±0.2 | 2.1±0.3* | 3.57 | 0.05 | |
|
| |||||
| D P4 | |||||
| Plasma, ng/ml | 1.7±0.2 | 18.8±1.7* | 14.78 | 0.001 | |
| Midbrain, ng/g | 1.4±0.1 | 2.1±0.2* | 4.55 | 0.04 | |
| Hippocampus, ng/g | 1.4±0.1 | 2.2±0.3* | 9.29 | 0.01 | |
| Diencephalon, ng/g | 0.9±0.1 | 1.5±0.1* | 9.64 | 0.01 | |
| Cortex, ng/g | 1.1±0.1 | 1.6±0.1* | 6.37 | 0.02 | |
| Interbrain, ng/g | 1.2±0.1 | 2.0±0.2* | 7.21 | 0.04 | |
|
| |||||
| E DHP | |||||
| Plasma, ng/ml | 5.9±1.8 | 23.2±3.9* | 4.32 | 0.05 | |
| Midbrain, ng/g | 5.0±1.0 | 17.3±0.4* | 9.95 | 0.006 | |
| Hippocampus, ng/g | 5.2±1.1 | 12.4±2.4* | 4.56 | 0.05 | |
| Diencephalon, ng/g | 1.9±0.2 | 4.6±0.6* | 4.53 | 0.05 | |
| Cortex, ng/g | 2.0±0.5 | 4.0±0.2* | 4.88 | 0.04 | |
| Interbrain, ng/g | 0.9±0.1 | 2.1±0.1* | 4.11 | 0.05 | |
|
| |||||
| F 3α,5α-THP | |||||
| Plasma, ng/ml | 4.2±0.2 | 14.8±3.1* | 5.73 | 0.04 | NA |
| Midbrain, ng/g | 2.5±0.3 | 5.0±0.6* | 7.71 | 0.01 | OF 0.51, PP 0.46, LQ 59, LR 55, % exits 0.48, PQ 0.65 |
| Hippocampus, ng/g | 3.6±0.3 | 5.4±0.6* | 5.79 | 0.03 | OF 0.46, EPM 69, PP 0.31, LQ 0.48 |
| Diencephalon, ng/g | 2.3±0.3 | 3.3±0.3* | 4.56 | 0.04 | OF 0.51, PP 0.51, SI 0.54, LR 0.47, % exits 0.53, PQ 0.51 |
| Cortex, ng/g | 2.3±0.3 | 3.2±0.2* | 4.86 | 0.04 | OF 0.64, PP 0.47 |
| Interbrain, ng/g | 1.9±0.1 | 2.8±0.2* | 3.99 | 0.04 | NA |
All correlations shown are significant at p ≤ 0.05.
Endocrine Measures
As expected and previously observed [35], behavioral estrous, compared to diestrous, rats had significantly higher concentrations of E2, P4, DHP, and 3α,5α-THP in serum, midbrain, hippocampus, diencephalon, cortex, and interbrain ( table 1, C–F).
Behavior/Endocrine Correlations
There were a number of significant correlations between behaviors examined and 3α,5α-THP (but not other endocrine factors) in midbrain, hippocampus, diencephalon, cortex, but not interbrain ( table 1, F). Increased central square entries in the open field were associated with higher levels of 3α,5α-THP in midbrain, hippocampus, diencephalon, and cortex. More time spent on the open arms of the elevated plus maze were associated with higher levels of 3α,5α-THP in hippocampus. Time spent in close proximity to a stimulus male was positively associated with higher levels of 3α,5α-THP in midbrain, hippocampus, diencephalon, and cortex. Longer durations of social interaction were associated with higher levels of 3α,5α-THP in diencephalon. Greater lordosis quotients were positively correlated with 3α,5α-THP levels in midbrain and hippocampus. Higher lordosis ratings, percentage of exits, and proceptivity quotients were associated with higher levels of 3α,5α-THP in midbrain and diencephalon.
Experiment 2
E2 and P4 Levels
There was no effect of mating to increase levels of E2 or P4 in any brain area examined of rats in behavioral estrus ( table 2 ).
Table 2.
E2 and P4 concentrations of rats in behavioral estrus either exposed to paced mating, or not following behavioral testing
| Paced mating |
No paced mating |
F(1,14) | p | |
|---|---|---|---|---|
| E2 | ||||
| Serum, pg/ml | 21.8±2.0 | 20.0±1.9 | 0.42 | 0.52 |
| Midbrain, pg/g | 2.2±0.3 | 2.0±0.2 | 0.52 | 0.47 |
| Hippocampus, pg/g | 2.8±0.2 | 3.0±0.4 | 0.21 | 0.64 |
| Diencephalon, pg/g | 2.1±0.1 | 2.3±0.2 | 0.98 | 0.33 |
| Cortex, pg/g | 2.4±0.2 | 3.0±0.5 | 1.31 | 0.27 |
| Interbrain, pg/g | 2.1±0.3 | 2.1±0.2 | 1.76 | 0.98 |
|
| ||||
| P4 | ||||
| Serum, ng/ml | 18.9±1.8 | 20.1±1.4 | 0.52 | 0.48 |
| Midbrain, ng/g | 1.9±0.2 | 2.2±0.1 | 0.57 | 0.46 |
| Hippocampus, ng/g | 2.2±0.1 | 2.2±0.6 | 0.01 | 0.90 |
| Diencephalon, ng/g | 1.6±0.1 | 1.5±0.1 | 0.52 | 0.47 |
| Cortex, ng/g | 1.8±0.2 | 1.6±0.2 | 0.21 | 0.65 |
| Interbrain, ng/g | 2.0±0.2 | 1.9±0.3 | 0.39 | 0.53 |
DHP Levels
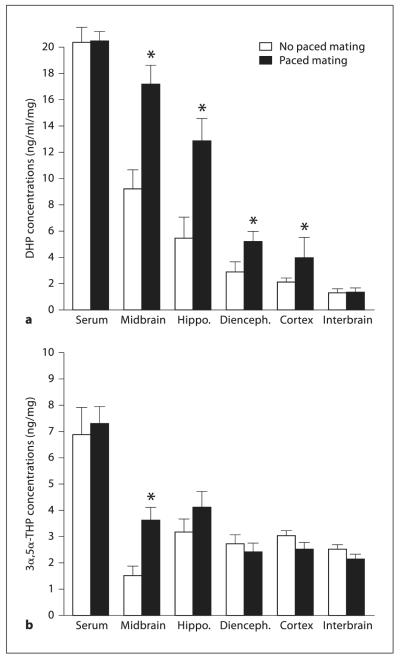
Behavioral estrous rats that were mated, compared to rats in behavioral estrus that were exposed to all of the behavioral tasks, except mating, had higher levels of DHP in midbrain (F(1,14) = 9.74, p = 0.007), hippocampus (F(1,14) = 6.49, p = 0.02), diencephalon (F(1,14) = 8.47, p = 0.01), and cortex (F(1,14) = 12.75, p = 0.003), but neither interbrain (F(1,14) = 0.02, p = 0.87) nor serum (F(1,14) = 0.64, p = 0.43) ( fig. 1 a).
Fig. 1.
Experiment 2: DHP (a) and 3α,5α-THP (b) levels in serum, midbrain, hippocampus, diencephalons, cortex and interbrain of rats that did (black bars) or did not (white bars) engage in paced mating. * p ≤ 0.05.
3α,5α-THP Levels
Levels of 3α,5α-THP in midbrain (F(1,14) = 8.64, p = 0.01), but neither hippocampus (F(1,14) = 4.13, p = 0.06), diencephalon (F(1,14) = 0.96, p = 0.34), cortex (F(1,14) = 0.11, p = 0.74), interbrain (F(1,14) = 0.76, p = 0.39), nor serum (F(1,14) = 0.45, p = 0.50) were increased in behaviorally tested rats in behavioral estrus that were mated compared to behavioral estrous rats that were not mated (fig. 1 b).
Experiment 3
E2 and P4 Levels
There was no effect of mating to increase E2 or P4 in any brain area examined of diestrous rats (table 3).
Table 3.
E2 and P4 concentrations of diestrous rats either exposed to paced mating, or not following behavioral testing
| Paced mating |
No paced mating |
F(1,16) | p | |
|---|---|---|---|---|
| E2 | ||||
| Plasma, pg/ml | 11.5±2.9 | 17.9±0.5 | 0.01 | 0.93 |
| Midbrain, pg/g | 1.4±0.1 | 1.4±0.1 | 0.30 | 0.59 |
| Hippocampus, pg/g | 1.9±0.2 | 1.7±0.2 | 0.53 | 0.47 |
| Diencephalon, pg/g | 1.6±0.2 | 1.5±0.2 | 0.01 | 0.91 |
| Cortex, pg/g | 1.8±0.2 | 1.5±0.1 | 1.44 | 0.24 |
| Interbrain, pg/g | 1.1±0.2 | 1.0±0.1 | 0.43 | 0.56 |
|
| ||||
| P4 | ||||
| Plasma, ng/ml | 4.5±1.2 | 2.0±0.1 | 1.07 | 0.31 |
| Midbrain, ng/g | 1.3±0.2 | 1.3±0.1 | 0.05 | 0.81 |
| Hippocampus, ng/g | 1.4±0.2 | 1.6±0.1 | 0.96 | 0.34 |
| Diencephalon, ng/g | 0.9±0.2 | 0.9±0.2 | 0.01 | 0.91 |
| Cortex, ng/g | 1.0±0.2 | 1.2±0.2 | 0.20 | 0.65 |
| Interbrain, ng/g | 1.2±0.1 | 0.9±0.1 | 0.25 | 0.62 |
DHP Levels
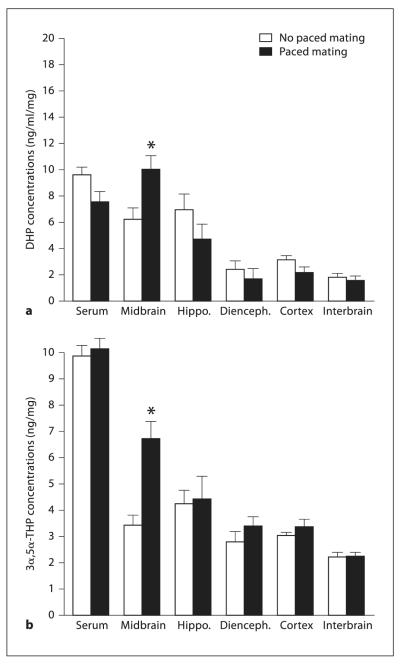
Levels of DHP were increased in midbrain (F(1,16) = 4.34, p = 0.05), but neither hippocampus (F(1,16) = 0.69, p = 0.41), diencephalon (F(1,16) = 0.32, p = 0.57), cortex (F(1,16) = 2.37, p = 0.14), interbrain (F(1,16) = 0.44, p = 0.86), nor serum (F(1,16) = 0.12, p = 0.72), of diestrous rats that were exposed to the behavioral test battery, including mating, compared to rats that were behaviorally tested, but not mated (fig. 2 a).
Fig. 2.
Experiment 3: DHP (a) and 3α,5α-THP (b) levels in serum, midbrain, hippocampus, diencephalons, cortex and interbrain of rats that did (black bars) or did not (white bars) engage in paced mating. * p ≤ 0.05.
3α,5α-THP Levels
Similar to DHP, levels of 3α,5α-THP were increased in midbrain (F(1,16) = 10.60, p = 0.005), but not hippocampus (F(1,16) = 3.18, p = 0.09), diencephalon (F(1,16) = 1.69, p = 0.21), cortex (F(1,16) = 0.36, p = 0.55), interbrain (F(1,16) = 0.37, p = 0.77), or serum (F(1,16) = 0.06, p = 0.79), of diestrous rats that were exposed to the behavioral test battery, including mating, compared to rats that were behaviorally tested, but not mated (fig. 2 b).
Discussion
The present data supported our hypotheses. First, behavioral estrous, compared to diestrous, rats demonstrated concomitant increases in exploration, anti-anxiety, social, approach, anti-conflict, and sexual behavior and had increased circulating and central levels of E2, P4, DHP, and 3α,5α-THP. 3α,5α-THP concentrations in midbrain, hippocampus, diencephalon, and cortex were positively correlated with enhanced anti-anxiety, social, and reproductive behaviors. Second, mating increased levels of 3α,5α-THP in midbrain and hippocampus of rats in behavioral estrus that were behaviorally tested and engaged in paced mating. 3α,5α-THP’s immediate precursor, DHP, was also increased in midbrain, hippocampus, diencephalon, and cortex (but not interbrain or serum) following paced mating. Among diestrous rats that were behaviorally tested with paced mating, 3α,5α-THP and DHP concentrations were increased only in midbrain. Together, these data suggest that higher levels of 3α,5α-THP in hippocampus, diencephalon, and cortex are associated with enhanced exploratory, anti-anxiety, social, and reproductive behaviors. Further, exposure to paced mating has site-specific effects to increase 3α,5α-THP and/or its precursor, DHP, in midbrain, hippocampus, diencephalon, and cortex of rats in behavioral estrus and midbrain of diestrous, rats.
These data confirm and extend previous reports that behavioral estrous, compared to diestrous, rats have enhanced sexual responsiveness. Prior reports indicate that rats in behavioral estrus, with higher levels of E2 and progestins, show greater lordosis responses than do diestrous rats, with lower E2 and progestin levels [41, 58]. In the present study, rats in behavioral estrus had greater lordosis responses than did diestrous rats. Behavioral estrous rats also exhibited more proceptive and less aggressive behaviors than did diestrous rats. Enhanced pacing behavior, as indicated by greater number of exits following sexual contacts, was also exhibited by rats in behavioral estrus compared to diestrous rats. Together, these data suggest that increased levels of E2 and progestins are associated with enhanced lordosis, as well as behaviors that may enhance (greater proceptive and pacing behaviors) and/or be permissive of (reduced aggression) mating.
In addition to estrous cycle differences in sexual behavior, the present data confirm and extend prior reports of changes in other behaviors across the estrous cycle. Separate reports have shown that rats in behavioral estrus spend more time on the open arms of the elevated plus maze and in social interaction, concomitant with increased E2 and progestin levels, compared to diestrous rats, with lower levels of E2 and progestins [8, 9, 41, 54]. In the present study, when tested in a sequence of tasks, behavioral estrous, compared to diestrous, rats entered more central squares in the open field, spent more time on the open arms of the elevated plus maze and in close proximity to a stimulus male, and had longer durations of social interaction with a conspecific. Behavioral estrous rats also had increased levels of E2, P4, DHP, and 3α,5α-THP in serum, midbrain, hippocampus, diencephalon, cortex, and interbrain. These data suggest that variations in E2 and progestins across the estrous cycle are associated with changes in exploratory, anti-anxiety, and social behaviors, which like proceptive and aggressive behaviors, may influence the probability of direct sexual contacts.
One question of interest is which hormone is modulating these behaviors and what are the brain areas involved? Data from our laboratory has previously demonstrated that behavioral estrous, compared to diestrous, rats have elevated concentrations of E2, P4, DHP, and 3α,5α-THP in midbrain, hypothalamus, hippocampus, and amygdala [54]. Here we confirm these effects and extend them to demonstrate that behavioral estrous rats also have higher E2, P4, DHP, and 3α,5α-THP concentrations in interbrain, which encompasses the central gray, an area involved in mediating sexual, aggression, and anxiety behaviors [47]. As well, in previous studies we did not examine correlations between endocrine measures and behavior. In the present studies, there is a robust pattern for 3α,5α-THP to be correlated with exploratory, anti-anxiety, social, and reproductive behaviors. In the present studies, enhanced anti-anxiety, social, and reproductive behaviors were correlated with concentrations of 3α,5α-THP, rather than E2, P4 or DHP. Increased levels of 3α,5α-THP in midbrain were associated with enhanced lordosis, proceptivity, and pacing behaviors, as would be expected. However, greater levels of 3α,5α-THP in midbrain were also associated with more central square entries in the open field and more time spent in proximity to a stimulus male. Likewise, 3α,5α-THP concentrations in hippocampus and diencephalon were associated with enhanced behaviors directly (lordosis, proceptivity, and/or pacing) and perhaps, indirectly (exploratory, anti-anxiety, and social), related to reproductive behavior, whereas 3α,5α-THP levels in cortex were associated with only exploratory, anti-anxiety, and social measures. There were no significant correlations between 3α,5α-THP levels in interbrain and any of the behaviors examined. Although we cannot make causal attributions about the effects of 3α,5α-THP on exploratory, anti-anxiety, social, and reproductive behaviors based upon these data, they do suggest that 3α,5α-THP levels in midbrain, hippocampus, and diencephalon may influence both sexual and exploratory, anti-anxiety, and social behaviors, while 3α,5α-THP in cortex may be more involved in the latter behavioral processes.
The present results also confirm and extend prior reports that mating increases 3α,5α-THP concentrations in the brain. In a standard mating paradigm, E2-primed rats and hamsters, which exhibited some mating behavior, had 3α,5α-THP concentrations and numbers of 3α,5α-THP-immunoreactive cells in midbrain that were similar to levels of E2- and P4-primed animals and significantly higher than vehicle-primed animals [41]. When progestin concentrations are examined following paced mating, we have seen significant increases in 3α,5α-THP, or its immediate precursor, DHP, in hippocampus, diencephalon, and cortex of ovx, hormone-primed rats [59, 60]. In the present study, DHP and 3α,5α-THP were increased in midbrain, hippocampus, diencephalon, and cortex, but not interbrain, of behavioral estrous rats after engaging in paced mating. Comparing data across studies, we might infer that paced mating is more effective at increasing DHP and 3α,5α-THP concentrations; however, this idea needs to be systematically examined. Thus, paced mating can induce increases in 3α,5α-THP and/or its precursor, DHP, in midbrain, as well as areas not traditionally associated with mating behavior and these effects can occur in intact and ovx, hormone-primed rats.
Interestingly, in the present studies, both 3α,5α-THP and DHP were increased following paced mating. Prior work in our laboratory has demonstrated that hamsters in behavioral estrus that are mated have increased DHP, but not 3α,5α-THP, in midbrain, cortex, and hypothalamus [unpubl. data]. We have also seen increases in plasma P4 of rats following mating [61]. Whether mating-induced increases in P4, DHP, and/or 3α,5α-THP are observed may depend, in large part, upon the timing of exposure to mating and subsequent tissue collection. Neurosteroidogenesis occurs in a very rapid timeframe and progestins are very labile [21, 62]. Standard mating in rats can require as little as 5 min to complete, which allows behavior and tissue collection to occur in a very short period of time. However, in the present studies, behavioral analyses required ~40 min (and hamsters require ~15 min). As such, increases in DHP in our prior work with hamsters and in the present studies may be a result of sufficient time for 3α,5α-THP to be increased and then back-converted to DHP [21]. Thus, the present data suggest that progestins can be increased in response to mating; however, the timeframe associated with mating may influence which progestin is increased.
As noted above, mating-induced increases in 3α,5α-THP and DHP were observed in brain areas not typically associated with sexual behavior. Among rats in behavioral estrus, 3α,5α-THP was increased in midbrain and hippocampus and DHP was increased in midbrain, hippocampus, diencephalon, and cortex, with no increases in 3α,5α-THP or DHP in interbrain. Diestrous rats had mating-induced increases in 3α,5α-THP and DHP only in midbrain. The hippocampus, diencephalon, and cortex are all progestin-sensitive and involved in mediating exploration, anti-anxiety, social behaviors. One possible function for increases in 3α,5α-THP and DHP in hippocampus, diencephalon, and cortex may be to facilitate behaviors necessary for successful mating. Mating among female rodents requires that some normally adaptive behaviors, such as aggression toward males, limited exploratory behavior, and restricted social interactions, be reversed [63]. The hippocampus, diencephalon, and cortex are involved in mediating aggressive, exploratory/anti-anxiety, and/or social behaviors [64–66]. Thus, increases in 3α,5α-THP and/or DHP in these areas may influence aggressive, exploratory, anti-anxiety, and social behaviors, which may enhance the probability of a successful mating encounter.
In the present studies, diestrous rats exhibited low levels of lordosis during the paced mating tasks. These data are consistent with prior reports that lordosis can occur on every day of the estrous cycle, albeit these previous data were obtained using manual palpation, rather than male mounting [67]. Diestrous rats showed much lower levels of reproductive behavior than did rats in behavioral estrus and demonstrated increased levels of 3α,5α-THP and DHP in midbrain, but not hippocampus, diencephalon, or cortex, as was seen by rats in behavioral estrus. One possible reason that increases were seen in midbrain of diestrous rats, but not these other areas that were seen among behavioral rats, may be due to the lower level of stimulation received during paced mating of diestrous compared to behavioral estrous rats. The midbrain, compared to all other brain areas examined, has the highest activity levels of enzymes necessary for the production of 3α,5α-THP [5, 68]. Hence, the level of stimulation received by diestrous rats may have been sufficient to induce increases in midbrain, but not hippocampus, diencephalon, cortex, or interbrain with lower enzyme activity. As well, lower levels of E2 among diestrous, compared to behavioral estrous, rats may also underlie differences in mating-induced 3α,5α-THP increases. E2 stimulates de novo synthesis of P4 in hypothalamus [69] and enhances 3α,5α-THP production in hippocampus by enhancing activity of metabolism enzymes [70, 71]. Hence, the less widespread increases in 3α,5α-THP and DHP among diestrous, compared to behavioral estrous, rats may be a function of low levels of E2, which are less likely to produce robust increases in P4 biosynthesis and/or metabolism to 3α,5α-THP. Thus, the hormonal milieu in which mating occurs may influence the extent to which mating-induced increases in progestins are observed.
These are intriguing data that extend our knowledge about the relationship between progestins and reproductively-relevant behaviors; however, the following caveats need to be considered. First, these studies did not reveal the stimulus or combination of stimuli necessary for altering 3α,5α-THP concentrations in these brain areas. Mating contact appears to play a critical role; however, which components of mating may be important is not yet known. One obvious possibility is vaginocervical stimulation (VCS), which is integral for mating. However, in our previous studies, VCS was neither necessary, nor sufficient, to increase 3α,5α-THP levels. E2-primed rats and hamsters that received low levels of VCS had increased concentrations of 3α,5α-THP in midbrain, which were comparable to levels of E2- and P4-primed rodents that received significantly more VCS [35]. It is possible that there is something unique about paced mating that may facilitate increases in progestins. Second, neurosteroidogenesis was not directly manipulated or measured in the present studies. Although 3α,5α-THP and DHP were increased following paced mating in behavioral estrous and diestrous rats, the present data did not elucidate whether these increases were due to central metabolism from peripheral sources or de novo biosynthesis. Future studies will investigate this question by pharmacologically blocking metabolism and/or neurosteroidogenesis in midbrain, hippocampus, diencephalon, or cortex and examining effects on exploratory, anti-anxiety, social, and sexual behaviors of rats lacking ovaries and/or adrenals.
In summary, these data support the idea that 3α,5α-THP may have actions in midbrain, hippocampus, diencephalon, and cortex to modulate reproductively-relevant behaviors beyond lordosis. Behavioral estrous rats with high endogenous 3α,5α-THP concentrations exhibited more exploratory, anti-anxiety, social, and reproductive behaviors than did diestrous rats, with low endogenous 3α,5α-THP levels. Further, paced mating can induce increases in progestin concentrations in brain areas, such as midbrain, hippocampus, diencephalons, and cortex, which are not typically associated with reproductive behavior. Engaging in mating behaviors increased progestin concentrations in midbrain, hippocampus, diencephalon, and cortex of behavioral estrous rats, and in midbrain of diestrous rats. These are intriguing data that suggest 3α,5α-THP may be an important neuroendocrine factor for regulating these reproductively-relevant behaviors. However, the mechanisms for such effects are not known and are the subject of our ongoing investigations.
Acknowledgment
This research was supported by a grant from the National Institute of Mental Health (MH06769801). The technical assistance of John Roberts is appreciated.
References
- 1.Etgen AM. Progestin receptors and the activation of female reproductive behavior: a critical review. Horm Behav. 1984;18:411–430. doi: 10.1016/0018-506x(84)90027-8. [DOI] [PubMed] [Google Scholar]
- 2.Rubin BS, Barfield RJ. Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrinology. 1983;37:218–224. doi: 10.1159/000123546. [DOI] [PubMed] [Google Scholar]
- 3.Rubin BS, Barfield RJ. Progesterone in the ventromedial hypothalamus facilitates estrous behavior in ovariectomized, estrogenprimed rats. Endocrinology. 1983;113:797–804. doi: 10.1210/endo-113-2-797. [DOI] [PubMed] [Google Scholar]
- 4.Pleim ET, Lisciotto CA, DeBold JF. Facilitation of sexual receptivity in hamsters by simultaneous progesterone implants into the VMH and ventral mesencephalon. Horm Behav. 1990;24:139–151. doi: 10.1016/0018-506x(90)90001-e. [DOI] [PubMed] [Google Scholar]
- 5.Frye CA. The role of neurosteroids and nongenomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Brain Res Rev. 2001;37:201–222. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- 6.Frye CA, Petralia SM. 3α,5α-THP’s actions in the ventral tegmental area for lordosis: A model system for defining mechanisms and function of progestins. In: Smith SS, editor. Neurosteroid Effects in the Central Nervous System: The Role of GABA A Receptor. CRC Press; Boca Raton: 2003. pp. 265–289. [Google Scholar]
- 7.Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3α-Hydroxy-5α-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006;138:1007–1014. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 9.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plusmaze test. Physiol Behav. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 10.Bitran D, Purdy RH, Kellogg CK. Anxiolytic effect of progesterone is associated with increases in cortical allopregnanolone and GABA A receptor function. Pharmacol Biochem Behav. 1993;45:423–428. doi: 10.1016/0091-3057(93)90260-z. [DOI] [PubMed] [Google Scholar]
- 11.Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABA A receptors. J Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 12.Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and painreducing effects in ovariectomized rats. Behav Neurosci. 2004;118:306–313. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- 13.Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3α-OH-5β-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- 14.Akwa Y, Purdy RH, Koob GF, Britton KT. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–125. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 15.Bitran D, Foley M, Audette D, Leslie N, Frye CA. Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology (Berl) 2000;151:64–71. doi: 10.1007/s002130000471. [DOI] [PubMed] [Google Scholar]
- 16.Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5α-reductase. Brain Res. 2004;1004:116–124. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- 18.Rhodes ME, Frye CA. Inhibiting progesterone metabolism in the hippocampus of rats in behavioral estrus decreases anxiolytic behaviors and enhances exploratory and antinociceptive behaviors. Cogn Affect Behav Neurosci. 2001;1:287–296. doi: 10.3758/cabn.1.3.287. [DOI] [PubMed] [Google Scholar]
- 19.Holzbauer M. Physiological variations in the ovarian production of 5α-pregnane derivatives with sedative properties in the rat. J Steroid Biochem. 1975;6:1307–1310. doi: 10.1016/0022-4731(75)90357-x. [DOI] [PubMed] [Google Scholar]
- 20.Holzbauer M, Godden U. Variations in the progesterone content of the rat adrenal gland during the oestrous cycle. J Steroid Biochem. 1974;5:109–111. doi: 10.1016/0022-4731(74)90115-0. [DOI] [PubMed] [Google Scholar]
- 21.Karavolas HJ, Hodges DR. Neuroendocrine metabolism of progesterone and related progestins. Ciba Found Symp. 1990;153:22–44. doi: 10.1002/9780470513989.ch3. [DOI] [PubMed] [Google Scholar]
- 22.Karavolas HJ, Hodges D, Normand N, O’Brien D. Conversion of 17α-hydroxyprogesterone to 5α-, 3α-, and 20α-reduced metabolites by female rat anterior pituitary and hypothalamus. Steroids. 1988;51:527–541. doi: 10.1016/0039-128x(88)90049-9. [DOI] [PubMed] [Google Scholar]
- 23.Baulieu EE, Schumacher M, Koenig H, Jung-Testas I, Akwa Y. Progesterone as a neurosteroid: actions within the nervous system. Cell Mol Neurobiol. 1996;16:143–154. doi: 10.1007/BF02088173. [DOI] [PubMed] [Google Scholar]
- 24.Jung-Testas I, Do Thi A, Koenig H, Desarnaud F, Shazand K, Schumacher M, Baulieu E. Progesterone as a neurosteroid: synthesis and actions in rat glial cells. J Steroid Biochem Mol Biol. 1999;69:97–107. doi: 10.1016/s0960-0760(98)00149-6. [DOI] [PubMed] [Google Scholar]
- 25.Baulieu EE, Robel P, Schumacher M. Neurosteroids: beginning of the story. Int Rev Neurobiol. 2001;46:1–32. doi: 10.1016/s0074-7742(01)46057-0. [DOI] [PubMed] [Google Scholar]
- 26.Barbaccia ML, Roscetti G, Trabucchi M, Cuccheddu T, Concas A, Biggio G. Neurosteroids in the brain of handling-habituated and naive rats: effect of CO2 inhalation. Eur J Pharmacol. 1994;261:317–320. doi: 10.1016/0014-2999(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 27.Barbaccia ML, Roscetti G, Bolacchi F, Concas A, Mostallino MC, Purdy RH, Biggio G. Stress-induced increase in brain neuroactive steroids: antagonism by abecarnil. Pharmacol Biochem Behav. 1996;54:205–210. doi: 10.1016/0091-3057(95)02133-7. [DOI] [PubMed] [Google Scholar]
- 28.Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABA A receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- 29.Drugan RC, Park R, Kaufman L, Holmes PV. Etiology of the sexual dimorphism in renal peripheral benzodiazepine receptor response to stress in rats. Horm Behav. 1993;27:348–365. doi: 10.1006/hbeh.1993.1026. [DOI] [PubMed] [Google Scholar]
- 30.Erskine MS, Kornberg E. Stress and ACTH increase circulating concentrations of 3α-androstanediol in female rats. Life Sci. 1992;51:2065–2071. doi: 10.1016/0024-3205(92)90157-k. [DOI] [PubMed] [Google Scholar]
- 31.Higashi T, Takido N, Shimada K. Studies on neurosteroids XVII. Analysis of stress-induced changes in neurosteroid levels in rat brains using liquid chromatography-electron capture atmospheric pressure chemical ionization-mass spectrometry. Steroids. 2005;70:1–11. doi: 10.1016/j.steroids.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience. 2006;138:749–756. doi: 10.1016/j.neuroscience.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 34.Lan NC, Bolger MB, Gee KW. Identification and characterization of a pregnane steroid recognition site that is functionally coupled to an expressed GABA A receptor. Neurochem Res. 1991;16:347–356. doi: 10.1007/BF00966098. [DOI] [PubMed] [Google Scholar]
- 35.Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THP and 3α-diol. J Neuroendocrinol. 1999;11:839–847. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- 36.Engel SR, Grant KA. Neurosteroids and behavior. Int Rev Neurobiol. 2001;46:321–348. doi: 10.1016/s0074-7742(01)46067-3. [DOI] [PubMed] [Google Scholar]
- 37.Frye CA, Petralia SM. Lordosis of rats is modified by neurosteroidogenic effects of membrane benzodiazepine receptors in the ventral tegmental area. Neuroendocrinology. 2003;77:71–82. doi: 10.1159/000068338. [DOI] [PubMed] [Google Scholar]
- 38.Frye CA, Vongher JM. Ventral tegmental area infusions of inhibitors of the biosynthesis and metabolism of 3α,5α-THP attenuate lordosis of hormone-primed and behavioural oestrous rats and hamsters. J Neuroendocrinol. 2001;13:1076–1086. doi: 10.1046/j.1365-2826.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- 39.Petralia SM, Jahagirdar V, Frye CA. Inhibiting biosynthesis and/or metabolism of progestins in the ventral tegmental area attenuates lordosis of rats in behavioural oestrus. J Neuroendocrinol. 2005;17:545–552. doi: 10.1111/j.1365-2826.2005.01342.x. [DOI] [PubMed] [Google Scholar]
- 40.Frye CA. The role of neurosteroids and nongenomic effects of progestins in the ventral tegmental area in mediating sexual receptivity of rodents. Horm Behav. 2001;40:226–233. doi: 10.1006/hbeh.2001.1674. [DOI] [PubMed] [Google Scholar]
- 41.Frye CA, Vongher JM. GABAA, D1, and D5, but not progestin receptor, antagonist and anti-sense oligonucleotide infusions to the ventral tegmental area of cycling rats and hamsters attenuate lordosis. Behav Brain Res. 1999;103:23–34. doi: 10.1016/s0166-4328(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 42.Frye CA, Walf AA, Sumida K. Progestins’ actions in the VTA to facilitate lordosis involve dopamine-like type 1 and 2 receptors. Pharmacol Biochem Behav. 2004;78:405–418. doi: 10.1016/j.pbb.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Reddy DS, Kulkarni SK. Role of GABAA and mitochondrial diazepam binding inhibitor receptors in the anti-stress activity of neurosteroids in mice. Psychopharmacology (Berl) 1996;128:280–292. doi: 10.1007/s002130050136. [DOI] [PubMed] [Google Scholar]
- 44.Long JA, Evans HM. Oestrous cycle in the rat and its associated phenomena. Mem Univ Calif. 1922;6:1–146. [Google Scholar]
- 45.Edinger KL, Frye CA. Testosterone’s analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5α-reduced metabolites in the hippocampus. Behav Neurosci. 2004;118:1352–1364. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- 46.Blizard DA, Lippman HR, Chen JJ. Sex differences in open-field behavior in the rat: the inductive and activational role of gonadal hormones. Physiol Behav. 1975;14:601–608. doi: 10.1016/0031-9384(75)90188-2. [DOI] [PubMed] [Google Scholar]
- 47.McCarthy MM, Felzenberg E, Robbins A, Pfaff DW, Schwartz-Giblin S. Infusions of diazepam and allopregnanolone into the midbrain central gray facilitate open-field behavior and sexual receptivity in female rats. Horm Behav. 1995;29:279–295. doi: 10.1006/hbeh.1995.1020. [DOI] [PubMed] [Google Scholar]
- 48.Dunn RW, Reed TA, Copeland PD, Frye CA. The nitric oxide synthase inhibitor 7-nitroindazole displays enhanced anxiolytic efficacy without tolerance in rats following subchronic administration. Neuropharmacology. 1998;37:899–904. doi: 10.1016/s0028-3908(98)00076-8. [DOI] [PubMed] [Google Scholar]
- 49.Baum M. Extinction of an avoidance response motivated by intense fear: social facilitation of the action of response prevention (flooding) in rats. Behav Res Ther. 1969;7:57–62. doi: 10.1016/0005-7967(69)90049-7. [DOI] [PubMed] [Google Scholar]
- 50.File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- 51.Erskine MS. Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomizedadrenalectomized hormone-primed rats. Behav Neurosci. 1985;99:151–161. doi: 10.1037//0735-7044.99.1.151. [DOI] [PubMed] [Google Scholar]
- 52.Frye CA, Erskine MS. Influence of time of mating and paced copulation on induction of pseudopregnancy in cyclic female rats. J Reprod Fertil. 1990;90:375–385. doi: 10.1530/jrf.0.0900375. [DOI] [PubMed] [Google Scholar]
- 53.Hardy DF, DeBold JF. Effects of coital stimulation upon behavior of the female rat. J Comp Physiol Psychol. 1972;78:400–408. doi: 10.1037/h0032536. [DOI] [PubMed] [Google Scholar]
- 54.Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3α,5α-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- 55.Rodbard D, Hutt DM. Statistical analysis of radioimmunoassay and immunoradiometric assays: a generalized, weighted iterative, least squares method for logistic curve fitting. International Atomic Energy Agency; Symposium on Radioimmunoassay and Related Procedures in Medicine; New York, Uniput. 1974. pp. 209–223. [Google Scholar]
- 56.Zuluaga MJ, Agrati D, Pereira M, Uriarte N, Fernandez-Guasti A, Ferreira A. Experimental anxiety in the black and white model in cycling, pregnant and lactating rats. Physiol Behav. 2005;84:279–286. doi: 10.1016/j.physbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Diaz-Veliz G, Butron S, Benavides MS, Dussaubat N, Mora S. Gender, estrous cycle, ovariectomy, and ovarian hormones influence the effects of diazepam on avoidance conditioning in rats. Pharmacol Biochem Behav. 2000;66:887–892. doi: 10.1016/s0091-3057(00)00283-5. [DOI] [PubMed] [Google Scholar]
- 58.Komisaruk BR, Diakow C. Lordosis reflex intensity in rats in relation to the estrous cycle, ovariectomy, estrogen administration and mating behavior. Endocrinology. 1973;93:548–557. doi: 10.1210/endo-93-3-548. [DOI] [PubMed] [Google Scholar]
- 59.Frye CA, Rhodes ME. Infusions of 3α,5α-THP to the ventral tegmental area, but not substantia nigra, enhance exploratory, anti-anxiety, social, and sexual behaviours and concomitantly increase 3α,5α-THP concentrations in the hippocampus, diencephalon, and cortex of ovariectomized estrogenprimed rats. J Neuroendocrinol. 2006 doi: 10.1111/j.1365-2826.2006.01494.x. (in revision) [DOI] [PubMed] [Google Scholar]
- 60.Frye CA, Rhodes ME. Infusions of 3α,5α-THP to the VTA enhance exploratory, anti-anxiety, social, and sexual behavior and increase levels of 3α,5α-THP in midbrain, hippocampus, diencephalon, and cortex of female rats. Horm Behav. 2006 doi: 10.1016/j.bbr.2007.08.031. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frye CA, McCormick CM, Coopersmith C, Erskine MS. Effects of paced and non-paced mating stimulation on plasma progesterone, 3α-diol and corticosterone. Psychoneuroendocrinology. 1996;21:431–439. doi: 10.1016/0306-4530(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 62.Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci USA. 1999;96:13512–13517. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 64.Lopez NL, Vazquez DM, Olson SL. An integrative approach to the neurophysiological substrates of social withdrawal and aggression. Dev Psychopathol. 2004;16:69–93. doi: 10.1017/s0954579404044414. [DOI] [PubMed] [Google Scholar]
- 65.McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 66.Vassout A, Veenstra S, Hauser K, Ofner S, Brugger F, Schilling W, Gentsch C. NKP608: A selective NK-1 receptor antagonist with anxiolytic-like effects in the social interaction and social exploration test in rats. Regul Pept. 2000;96:7–16. doi: 10.1016/s0167-0115(00)00194-4. [DOI] [PubMed] [Google Scholar]
- 67.Gans SE, Stamper JL, Butler T, McClintock MK. Endocrine basis for two types of individual differences in lordosis reflex intensity. Horm Behav. 1995;29:367–391. doi: 10.1006/hbeh.1995.1026. [DOI] [PubMed] [Google Scholar]
- 68.Li X, Bertics PJ, Karavolas HJ. Regional distribution of cytosolic and particulate 5α-dihydroprogesterone 3α-hydroxysteroid oxidoreductases in female rat brain. J Steroid Biochem Mol Biol. 1997;60:311–318. doi: 10.1016/s0960-0760(96)00195-1. [DOI] [PubMed] [Google Scholar]
- 69.Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78:29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- 70.Cheng YJ, Karavolas HJ. Conversion of progesterone to 5α-pregnane-3,20-dione and 3α-hydroxy-5 α-pregnan-20-one by rat medical basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93:1157–1162. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- 71.Vongher JM, Frye CA. Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacol Biochem Behav. 1999;64:777–785. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]