Abstract
A number of serious clinical cognitive syndromes occur following surgery and anesthesia. Postoperative delirium is a behavioral syndrome that occurs in the perioperative period. It is diagnosed through observation and characterized by a fluctuating loss of orientation and confusion. A distinct syndrome that requires formalized neurocognitive testing is frequently referred to as postoperative cognitive dysfunction (POCD). There are serious concerns as to whether either postoperative delirium or postoperative cognitive dysfunction lead to dementia. These concerns are reviewed in this article.
Keywords: Postoperative Delirium, Postoperative Cognitive Dysfunction, Long term cognitive impairment, Dementia
Introduction
Since the 1950’s, it has been clear that patients undergoing surgery and anesthesia suffer from postoperative cognitive changes. Two distinct syndromes have been described. Postoperative delirium is identified in the postoperative period and described as a fluctuating alteration of consciousness. Postoperative delirium is associated with postoperative cognitive dysfunction (POCD), which refers to measureable decreases in neuropsychiatric function, most commonly alterations in memory and executive function. Cognitive domains found affected in POCD overlap greatly with the deficits encountered in patients with formal dementia. In this article, we explore the relationship between delirium and dementia.
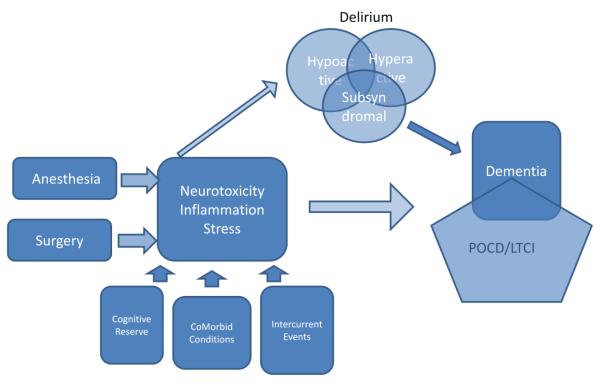
Apart from direct toxicity, anesthesia may have secondary effects that result in delirium or POCD. Surgical intervention and the response to surgery may be involved in the generation of these states. Either delirium, POCD, or both of these states may be an early manifestation of a continuum of pathology leading to dementia. Figure 1 maps some of these potential relationships. The questions posed by this figure include:
Does anesthesia and/or surgery cause delirium? Short Answer: Surgery/major illness – yes; anesthesia, maybe
Does delirium cause POCD? Answer: mixed picture with highly divergent methods and small sample sizes makes conclusions difficult, but there appears to be more of an association than not.
Does delirium have a direct impact on dementia? There is a definite association, but how to determine if a patient with dementia is more likely to get delirium versus a patient who gets delirium is more likely to get dementia.
Does delirium have an indirect impact of dementia by way of POCD? A version of this question is whether POCD leads to or is a prodromal form of dementia? So far, POCD appears to be primarily transient and not related to the trajectory of AD.
Figure 1.
Potential relationships between anesthesia, surgery and the ultimate development of long term cognitive impairment. This conception indicates that neurotoxicity, inflammation and stress appear to be the mediators of both delirium and LTCI. Delirium may represent an intermediate step on the road to LTCI for some patients. Other conditions such as pre-existing impairment (lack of cognitive reserve), co-morbid illness and intercurrent events (e.g. postoperative complications) may also play a role in the development of these problems.
In exploring these questions, we will first explore how these entities are defined, as some of the methodological problems evolve from the overlap of definition and symptomatology. We will briefly describe similarities in potential pathogenesis that would be consistent with an association between delirium and dementia. We will review the evidence to date suggesting that there is an important relationship between delirium and POCD, and discuss whether this extends to dementia. Finally, we will make suggestions as to the important research activities needed to make progress in this area.
What is delirium?
Delirium is a neuropsychological syndrome characterized by disorganized thinking, lack of orientation and a fluctuating course. Delirium has become a focal point for much recent study and the formation of specialty societies in both Europe and the United States.1 The basis for the concerns about delirium include the additional difficulty and cost associated with the care of individuals who develop delirium, the association of delirium with other complications, increasing morbidity and mortality, and the concern about its relationship to POCD and dementia.2,3
The definition of delirium has evolved over recent years. The Diagnostic and Statistical Manual of the American Psychiatric Association represents the consensus definition for most psychiatric conditions. The current proposal for the DSM V is listed in table 1. As this represents a minor change from the DSM IV-TR, table 1 also notes the alterations from the previous definition. It is important to appreciate that delirium is a behavioral syndrome that is diagnosed by observing a patient. The primary instruments for diagnosis of delirium, the Confusion Assessment Method (CAM)4 and the Delirium Rating Scale (DRS) are not interchangeable5. There is considerable variation in how the instruments are utilized. Some groups use multiple instruments to inform the CAM and others use short interviews. There are alternative versions and foreign language versions of both.
Table 1.
DSM V delirium (with notes regarding alteration of definitions from the DSM IV)
| A. Disturbance in level of awareness and reduced ability to direct, focus, sustain, and shift attention. (This is represents a minor change from : Disturbance of consciousness (i.e., reduced clarity of awareness of the environment) with reduced ability to focus, sustain or shift attention.) |
| B. A change in cognition, (such as deficits in orientation, executive ability, language, visuoperception, learning and memory) |
| -Cannot be assessed in face of severely reduced level of awareness |
| -Should not be better accounted for by a preexisting neurocognitive disorder |
| (A minor change from A change in cognition or the development of a perceptual disturbance that is not betteraccounted for by a preexisting, established or evolving dementia. |
| C. There is evidence from the history, physical examination, or laboratory findings that the disturbance is caused by the direct physiologic consequences of a general medical condition. (no change from DSM IV) |
| D. The disturbance develops over a short period of time (usually hours to a few days) and tends to fluctuate in severity during the course of a day. (no change from DSM IV) |
Postoperative delirium is a specific subset of the panorama of deliria. Two different syndromes are discussed but only one is consistent with the classic definition of delirium.6 Upon the cessation of general anesthesia, is it somewhat common for patients to be confused and uncooperative. This phenomenon has been referred to as emergence delirium or emergence excitation. Characteristically, the patient is reacting to the continued presence of the endotracheal tube, i.e. their gag reflex has returned, but they are not yet responding to verbal commands. Patients in this state can be very difficult to manage and frequently require additional sedation. Interestingly, they typically emerge from this additional sedation in a calmer and more manageable manner. This behavior does not fluctuate. The most standard diagnostic instruments are not usable in this situation, which is generally extremely short lived.7 Emergence excitation is probably not a real subset of delirium. It occurs across all age groups and with rare exceptions is extremely limited in time.
Postoperative delirium (PD) is predominantly a diagnosis of older patients in that the prevalence becomes significant after age 60-65. It has been referred to as interval delirium in that its presentation is typically 24-72 hours after the completion of a surgical procedure. Unlike emergence excitation, postoperative delirium is diagnosed using the same criteria and instruments used for medical patients. For general surgery, the incidence of PD is reported from 5-15%, ranging up to 65% for patients with hip fractures.3 The reported incidence in patients undergoing surgery for hip fracture is higher, ranging from 16-62%, with an average rate of 35% across 12 studies of 1,823 patients.8 Delirium occurs in up to 80% patients admitted to intensive care. In this scenario, the age specificity is less marked. The diagnosis of ICU delirium has required the development of instruments that adjust to patients who are intubated for mechanical ventilation, but the general criteria are similar.9 Prior to the extensive description of ICU delirium by Ely and colleagues,10 this phenomenon was frequently termed ICU psychosis and was a diagnosis of exclusion.
Delirium is frequently characterized as hyperactive, in which agitation and restlessness are predominant and hypoactive, in which lack of responsiveness, slow or absent movement and slowed speech are predominant.11 Subsequently, a mixed subtype has been used to describe patients who vacillate between those two poles. In addition, the presence of some but not all symptoms has resulted in the discussion of subsyndromal delirium.12 The most recent assessment of these motor types found that 35% manifested hypoactive delirium, 26% had a mixed picture and 15% showed hyperactive symptoms. In this study, 24% had no subtype, most of which were cases referred to as subsyndromal delirium.11
The pathophysiology underlying delirium, as described below, is unknown. There are no biomarkers or external diagnostic tests that assist in the diagnosis of delirium and no anatomic or histologic finding that confirms the diagnosis. A wide range of precipitating factors including acute illness, surgery, drugs and trauma are associated with the development of delirium.13
The primary feature that makes postoperative delirium particularly interesting is the ability to evaluate patients prior to surgery and serially observe them for development of delirium. This is somewhat less true in critically ill patients, but certainly does apply to surgical intensive care patients, such as cardiac surgical patients. It is not clear if there is a meaningful distinction between the delirium noted in the postoperative period and that noted in patients with medical illness.14 The setting of postoperative delirium has provided an important window into the study of this phenomenon.
What is dementia
Dementia is a general term for series of specific diseases whose common feature is a decline in mental ability that interferes with daily life. Dementia is not a specific disease. Alzheimer’s disease is the most common form, accounting for 60-80% of cases. (http://www.alz.org/alzheimers_disease_what_is_dementia.asp?gclid=CKzUkcLl3K4CF YNo4Aod-jDJaA) The second most common is vascular dementia which is generally associated with neural damage from strokes. Dementia associated with certain conditions, such a vitamin deficiencies or thyroid disorders may be reversible. In addition, conditions such as depression can have overlapping symptoms. The definitive diagnosis of Alzheimer’s disease requires examination of the brain so it is almost always made postmortem. While the pre-mortem diagnosis may not be 100% accurate, it is nonetheless vitally important to make the diagnosis to assure that the symptoms are not related to a reversible disease. The diagnosis is usually made by a combination of history, physical examination, blood tests to eliminate other causes, imaging of the brain with either CT or MRI and neuropsychological tests. Scales to assess dementia, such as the clinical dementia rating (CDR) scale include multiple domains of function in making a determination of dementia.
What is (are) Post Operative Cognitive Dysfunction (POCD) and Long Term Cognitive Impairment (LTCI)?
For decades, it has been apparent that some patients have notable cognitive problems following surgery and anesthesia that were distinct and distant from delirium. Following a series of studies in the 1990’s this became known generically as postoperative cognitive dysfunction or POCD. The major studies evolving out of the International Studies of Postoperative Cognitive Dysfunction found that approximately 10% of patients had significant deterioration at 3 months following surgery.15,16,17 Twenty five percent were impaired at 1 week. Other researcher working, for example in the intensive care units have found even more profound effects on memory and executive function.18 These investigators frequently refer to their finds as Long Term Cognitive Impairment (LTCI) or Decline (LTCD).10,19 For the purposes of this article POCD and LTCI are used essentially synonymously
In contrast to delirium, assessing patients for cognitive change requires a pre and postoperative test. Determination and quantization of any change requires a baseline measure. There is no consensus on the magnitude of cognitive change that should be considered clinically relevant.7 Test batteries usually include assessment of immediate and short term memory and various assessments of executive function.17 There are multiple analytical approaches to making a present or absent determination of POCD. In a review of studies following cardiac surgery, Mahanna et al made the important point that the analytical approach taken has a significant impact on the determined incidence of POCD.20 In that review of a single prospective data set, the incidence of decline ranged from 66% to 15.3% before discharge, 34% to 1.1% at 6 weeks, and 19.4% to 3.4% at 6 months depending upon which of four approaches were applied. The timing of cognitive testing also varies. Most tests of POCD occur in the 3 – 6 month range. The incidence appears to drop over time with most POCD appearing to be transient over the course of a year.21 POCD and LTCI remain research findings. Neither is a recognized disease or syndrome. As noted by many articles, standardization of an approach to the determination of POCD and/or LTCI would greatly expand our ability to compare studies.
Studies of POCD have failed to capture a distinct set of patients that seem to have had a catastrophic event associated with surgery and anesthesia. Most investigators who work in this area have heard one or more stories in which a patient who was relatively intact and functional undergoes an elective procedure becomes confused, develops a few complications and end up barely functional in a nursing facility, the most noticeable aspect being that they seem to have rapidly progressive dementia. An example of this perception is noted in the description provided by a daughter on an AD association blog. (http://alzheimers.infopop.cc/eve/forums/a/tpc/f/3621033452/m/1721067562?r=466300483 #466300483) “My dad had NO DEMENTIA prior to surgery, and was an Estimator for a Paving Company, after surgery...he couldn’t add 2+2!” We, as well as other authors, have both noted that these cases exist and that we have almost no information about them.22 For such cases it is hard to argue that surgery and anesthesia did not play a role. However, as noted in another articles in this issue and reviewed briefly below, the data suggesting a general trend toward anesthesia and surgery causing a major change in the trajectory of dementia are still lacking.
Relationship of Symptomatology/Overlap in Diagnosis
The overlap in symptoms provides some clues as to the interrelationship between dementia and delirium but it also points out one of the true problems in sorting out a clear understanding. There are substantial concerns about the accuracy of diagnosis for both entities in standard clinical practice. http://www.acnp.org/g4/gn401000133/ch130.html )23 Delirium is thought to be majorly under diagnosed24,11 and there is concern about the relative value of the testing instruments in use.25,26
In table 2, we provide a tabular overview of symptomatology noted in evolving definitions of delirium and dementia, which we have modified from the work of Holtta.23 Two points are illustrated by this table. First, the definition of delirium is evolving. While this is appropriate, a reader must distinguish which criteria are in use and appreciate that the diagnosis has changed over time. The second important point is the are the areas that are common between various definitions of delirium and a test for dementia called the neuropsychiatric inventory (NPI)27. In evaluating 425 consecutive patients, Hollta and colleagues sought to study the frequency of overlapping delirium and neuropychiatric symptoms (NPS) among 255 patients with dementia. Of these patients, 66 developed delirium. Eight-five percent of these patients also had multiple neuropsychiatric symptoms. They also noted that only the patients with a confirmed diagnosis of delirium had a poor prognosis. Thus, is may be important to distinguish whether specific neuropsychiatric symptoms derive directly from dementia or whether the result from an acute episode of delirium, because only the diagnosis of delirium was associated with poor outcomes. It also points out how difficult this can be. Meagher and colleagues explored the overlapping features that complicate the differential diagnosis of delirium and dementia in a study of patients with delirium, dementia, comorbid delirium-dementia and cognitively intact controls in a palliative care setting.28 Acknowledging the primary distinguishing features appear to be reversibility and temporal course, they sought to determine first which features best differentiate controls from dementia and or delirium and second to compare the neurocognitive profiles of these three group. DRS-R98 and CTD total scores were comparable in the delirium and comorbid delirium-dementia groups and higher than in dementia or control groups. Inattention and disorientation were more severe in delirium groups compared with dementia-alone. They identified one test, the spatial span forward, (but not the spatial span backward) that appeared to disproportionately diminish in delirium. This overlap of symptoms makes the idea that delirium leads to dementia both intuitively appealing and hard to prove. It suggests to clinicians trying to assess geriatric patients in that distinguishing delirium from neuropsychiatric symptoms without delirium can be challenging, but seems to be important. Finally, it challenges researchers to seek clear and mutually agreeable definitions of these neuropsychiatric syndromes to make studies comparable.
| ICD-10 | DSM-II | DSMII-R | DSM IV | DSM V | NPI | |
|---|---|---|---|---|---|---|
| Clouding of conciousness | x | x | x | x | ||
| Impariment of Attention | x | x | x | x | x | |
| Preceptual Disturbance | x | x | x | x | x | x |
| Disorganized thinking/speech |
x | x | x | x | ||
| Distrubance of Sleep | x | x | x | x | ||
| Motor Activity change | x | x | x | x | ||
| Disorientation | x | x | x | x | x | |
| Memory Impairment | x | x | x | x | x | |
| Impairment of Abstract thinking or comprehension |
x | |||||
| Emotional Disturbance | x | x | ||||
| Rapid Onset and fluctuating symptoms |
x | x | x | x | x | |
| Causative Factor | x | x | x | x | x |
Relationship of Pathophysiology
In 1996, Inouye et. al described what they called predisposing and precipitating factors for delirium.29 In essence they observed that a patient’s status preoperatively was the baseline upon which various events eventually tipped the patient from coherence into fluctuating incoherence. The predisposing factors, which are perhaps erroneously referred to as risk factors, have been determined through a number of studies. Risk factors for which high level evidence exists include: pre-existing cognitive impairment such as dementia,- depression, abnormal serum sodium, age 70 years or more; or increasing age.29-33 Other factors for which there is relatively strong evidence for an associated with increased risk of post-operative delirium include: exposure to meperidine, exposure to benzodiazepine, particularly longer acting preparations, a previous history of delirium, alcohol abuse, pre-operative use of narcotic analgesics, and both pain and use of narcotics. 34,35,30,36,37 Many of these factors are thought to be fixed in the sense that not much can be done to alter them. In our search for means of preventing complications in the elderly, a more aggressive view of changing risk factors may be in order. An evolving concept of frailty38 is an area which may be both associated with delirium39 and potentially amendable to preoperative habilitation.40,41
The precipitating factors have generated more research, perhaps because they are easier to study and portend a greater chance of subsequent intervention. Within the realm of precipitating factor, complications associated with surgery are important. The development of infections, in particular, is thought to predispose a patient to delirium.42 At it is routinely the intention of all medical teams to prevent complications and the prevention of many postoperative complications is itself an extensive literature, we have focused our discussion on areas that are concomitant to standard care.
We propose that three major areas have been explored. Progress in all areas is hampered by an absence of an animal model of delirium. There are excellent animal models of cognitive function that should not be confused with or thought a substitute for a functional model of delirium. The literature on animal cognition in the postoperative period is interesting literature and beyond the reach of this review.43 Progress in distinguishing surgical insult from anesthetic toxicity is limited by the rarity of administering anesthetic drugs to human subjects in the absence of a procedure. Thus, direct effects of medications are hard to isolate from the stress associated with surgery.
The first area is the idea that direct and indirect neurotoxicity or neuromodulatory effects from drugs primarily administered for anesthesia may cause delirium. For years some sort of misbalance of the cholinergic system has been implicated. Severe cholinergic toxicity causes a syndrome consistent with delirium.44 Measurement of cholinergic activity in blood and epidemiological evidence regarding cholinergic drugs administered to have produced mixed results in terms of association with delirium.45,46 Perhaps the primary efforts in this area have focused on the difference between regional and general anesthesia. Regional anesthesia, usually either epidural or subarachnoid, produces a complete lack of sensation by blocking neural transmission at the level of the spinal cord. In theory, patients having operations amenable to regional anesthesia could be wide awake during surgery, as opposed to the effect of general anesthesia which produces a profound but reversible coma. This has led many a clinician to recommend regional anesthesia, particularly for hip fracture. To date, a reasonably extensive literature of sufficient substance to allow meta-analytic review for both postoperative delirium47,48 and postoperative cognitive dysfunction40,49 have failed to any advantage. An important clinical issue confounds this literature in that few patients undergoing regional anesthesia fail to receive significant sedation during their procedure. A recent small trial that is currently being followed up with a larger clinical trial suggests that the depth of sedation can be an important factor in determining the incidence of postoperative delirium.50
As described more in depth by other articles in this issue, there are many clues suggesting a biochemical relationship between anesthetic agents and Alzheimer’s Disease (AD) pathology.51 For example, Eckenhoff has demonstrated that inhaled anesthetic agents enhance the cytotoxicity and oligomerization of Alzheimer disease associated peptides.52 Using nuclear magnetic resonance (NMR) techniques, Mandal demonstrated the induction of amyloid oligomerization with clinically relevant concentration of isoflurane and desflurane,53 while Perucho demonstrated an increase in amyloid pathology in mice using isoflurane.54 NMR experiments have also noted that small sized anesthetics "inhaled" do initiate the amyloid beta oligomerization but at clinically relevant concentration bigger sized anesthetics (intravenous) do not oligomerize amyloid beta peptide.55 The clinical relevance of these findings is evolving. One small human study demonstrated cognitive decline due to isoflurane but not desflurane on a clinical study evaluating patients only 7 days following surgery.56 A recent study by Liu et al investigated the role of sevoflurane in the potential development of dementia.57 Evaluating patients with MCI, they demonstrated that sevoflurane, but not propofol or lidocaine epidural anesthesia accelerated the progression of amnestic mild cognitive impairment. This is an extremely important study that requires additional follow-up. There is some animal evidence that cognitive processes are altered after exposure to anesthesia, but in the absence of an animal model of delirium, there is no information that anesthetic agents lead to delirium in animals. There is a parallel literature on neurotoxicity in the developing brain.58,59 In the neonate, there seems to be more direct toxicity during somewhat defined intervals of neuronal susceptibility. Another line of evidence in this area suggests that inflammation, rather than the directe effect of anesthetics, is a more important factor for any cognitive decline.60 The reader is referred to other articles accompanying this paper as well as recent reviews for details.61
The clinical implication of this discussion rests on the possibility that preventing delirium could prevent POCD and/or dementia. The relationship between specific drugs and the incidence of postoperative delirium is difficult to assess as the current practice of anesthesia routinely involves the use of multiple medications. Propofol was found to be associated with a higher incidence (67.5 vs 49.4%; p=0.018) of delirium than desflurane in patients have coronary artery bypass.62 However, as reported by Sieber, depth of anesthesia rather than the drug employed may be the primary variable.50 Benzodiazepines have generated mixed reports. One trial comparing midazolam to dexmedetomidine suggested that delirium was more common with midazolam.63and a Cochrane review recommends that benzodiazepines not be used to treat delirium that is not related to alcohol withdrawal.64 Although it is common for experts to recommend that benzodiazepines be avoided in the elderly to prevent paradoxical agitation, there is relatively little data suggesting a direct link to postoperative delirium.65 The relationship of opioid analgesics to delirium is similarly complicated by the need to distinguish the role of pain in the generation of delirium.66 Meperidine, which has been thought to have a relationship to delirium, is not a good medication to use in the elderly for many reasons, including myocardial depression.67 Thus, while it would be convenient to attribute postoperative delirium to a single drug, the literature to date does not support this idea.
There is sufficient data to suggest that at least the incidence of delirium can be diminished primarily by attention to details associated with care of the elderly.35 Approaches including an alteration of the anesthetic plan50 or postoperative pain management68 could be important as well, however definitive evidence awaits the results of larger ongoing clinical trials
The second major area regarding the pathophysiology of delirium is that of ischemia or relative ischemia. As in other areas, there is a mixed set of results, with hypoxemia found related to69,70 and not related to71 the development of delirium. This limited and confusing evidence was nonetheless sufficient for a recent NIH clinical guideline to include assessment for hypoxia and optimization of oxygenation as one of 13 recommendations for the prevention of delirium. 72
The third general area is that of inflammation and the neurophysiology of stress, with delirium seen as to the neuropsychiatric effects of various cytokines. 73-75 The current state of the art is that stress and inflammation seem to be temporally related to delirium, but no serum or spinal fluid marker has developed anything approaching diagnostic or prognostic significance.76 Recent hypothesis generating studies have suggested that white matter disruption at discharge and at 3months is associated with the duration of delirium in patients undergoing intensive care.77 There is currently no idea regarding the potential pathophysiology of this finding, but it provides an intriguing neuroanatomic clue as to the underlying changes associated with delirium.
A number of hypothetical approaches have recently been put forward that attempt to synthesize current knowledge into a working model. Sanders has recently proposed a model in which network connectivity is viewed as the primary organizing principle and that 1) baseline connectivity varies due to primarily non-modifiable risk factors and 2) inhibitory tone, acting on this baseline level of connectivity, increases, enhancing the risk of delirium.78 In a sense, this is a neural network level description of Inouye’s predisposing and precipitating factors.29 In this model, the effects of both stress and specific drugs are related to alterations in inhibitory tone, and in this sense propose as strong role for anesthetic drugs in pathophysiology of delirium. At least in theory, drugs which provided sedation with less impact on the GABAA mediated inhibitory circuits would decrease the incidence of delirium. Another approach was put forward by Cerejeira based on a prospective study of cholinesterase activity and inflammation. 79 In this study delirium was associated with an increased production of proinflammatory cytokines. The magnitude of the increase was predicted by preoperative cholinergic tone, suggesting the balance between immune response and cholinergic activity may play a role in the development of delirium.
Pathophysiology of POCD
Neither POCD nor LTCI are the primary focus of this review but rather a state that is frequently encountered and sought after in patients developing delirium. For this review, the primary question is whether there is a link between delirium and POCD that leads to early dementia, that is reviewed bellow. Therefore, we will limit out comments on the pathophysiology of POCD to indicating that essentially of all of the issues addressed in, as well as the organization of those issues, described above for delirium apply to POCD and that results have not been conclusive in any manner.6,80
Does delirium lead to POCD/LTCI
For the purpose of discussion, we will refer to LTCI in describing the following data although most of the data is postoperative. Jackson and colleagues assessed the literature in 2004 to determine what was known about delirium and LTCI.81 This review and others sight a number of difficulties in comparing studies. The methodology for assessing delirium and the means and assessment of cognitive impairment vary widely between reports. Delirium assessment are made using the confusion assessment method (CAM), the CAM-ICU, the Delirium Rating Scale (DRS), as well as clinician determination using various DSM criteria. For cognitive assessment, approaches range from complete neurosychological batteries to forms of the MMSE. In 2009, Maclullich and colleagues added nine additional reports and further analyzed the relationship of delirium to LTCI first evaluated by Jackson.81,82 Three of the nine studies evaluated patients with hip fractures. These are patients who sustain a painful trauma and almost always have an anesthetic and surgical procedure to repair the fracture within a few days. Estimates for the incidence of delirium run very high (30-65%) in hip fracture patients. Elective orthopedic surgery (hip and knee) and two studies of elective abdominal and cardiac surgery were also included. In 2008, Bickel and colleague reported on a prospective cohort study of 200 patients 60 years of age and over undergoing hip surgery and found delirium in 40 patients. 83 “Logistic regression analysis adjusted for age, sex, medical comorbidity and preoperative cognitive performance revealed highly significant associations between delirium and cognitive impairment (OR = 41.2; 95% CI = 4.3-396.2), subjective memory decline (OR = 6.2; 95% CI = 1.5-25.8) and incident need for long-term care (OR = 5.6; 95% CI = 1.6-19.7). “ So, while two of the articles reviewed by MacLullich concluded that there was no relationship between delirium and LTCI,84,85 the authors concluded that the results broadly confirm a link between delirium and LTCI. The most recent addition to this literature evaluated 225 patients over the age of 65 following cardiac surgery.86 This cohort had a 46% incidence of delirium. These patients had lower preoperative determinations on the mini mental state examination (MMSE) and lower MMSE scores at 1 month and 1 year. However, with adjustment for baseline differences, difference in mean MMSE scores were different at 30 days but not 6 or 12 months. Because there is such a variety of methodologies there has not been any formal meta-analyses. There are a number of prospective current trials that should shed a clearer light on this issue. (see for example: NCT00561678 on www.clinicaltrials.gov or SAGES at www.hebrewseniorlife.org/sages). To date there is no clinical data suggesting that the prevention of delirium avoids or diminishes the risk for dementia. This question will require large prospective studies.
Does surgery and anesthesia impact the incidence or trajectory to AD?
In an attempt to understand this problem, a number of investigators have used various registries of dementia patients to determine if there was any demonstrable effect of anesthesia and surgery. Avidan, used a group of patients enrolled in the Alzheimer’s Disease Research Center at Washington University to determine if a history of major surgery could be found to have an impact on the trajectory of cognitive decline.87 This group had raised a series of important concerns regarding POCD and its relationship to and relevance to dementia. They were concerned that essentially all studies of POCD start from a single preoperative baseline test, so if a patient were in fact deteriorating progressively leading up to the surgery, most studies would not capture that clinical information and therefore unfairly implicate anesthesia and surgery. Their analysis addressed the question as to whether there is anything special regarding the perioperative period in that patients with medical illnesses also frequently have delirium, cognitive change and an impact on progression of dementing illness. Their review of 575 patients divided into those with no medical event, a surgical event or a medical event in the year preceding their follow-up test in the ADRC showed no difference in the trajectory to advancing dementia. There has been extensive discussion regarding the limitations of this approach, however it seems fair to say that the preponderance of studies using this epidemiologic approach have failed to find a significant signal suggesting that the perioperative experience has a significant impact on the progression of a population followed in an AD registry.
If surgery and anesthesia are associated with the deterioration of patients with dementia, one would expect patients with early forms of dementia to be at particular risk. The literature on early forms of dementia can be confusing, with multiple different states described including various forms of mild cognitive impairment (MCI), Age Associated Memory Impairment, Age Associated Cognitive Decline, and Cognitive Impairment No Dementia. The current recommendation is based on the idea of MCI.88,89 In a retrospective review, Bekker demonstrated that patients with MCI and surgery had a greater deterioration on the digit forward span test.90 They interpreted their findings as the first demonstration that MCI leads to POCD. This finding awaits the results of currently ongoing prospective trials for confirmation.
On balance, it appears that a small cadre of undefined size suffers from profound delirium from which dementia appears to evolve. It is hard to know if these rare but catastrophic events have anything to do with the types of neurotoxicity described in this issue. A variable number of patients depending on surgical type and risk factors develop delirium. There may be an association between delirium and POCD/LTCI. To date there is insufficient evidence to link POCD/LTCI to ling term dementia.
Does postoperative delirium lead to dementia?
In a population of patients with Alzheimer’s Disease, an episode of delirium is associated with a significant acceleration of the slope of cognitive decline.91 The literature reviewed herein has not, for the most part, included patients with significant cognitive impairment before surgery. Patients with mild cognitive impairment may be at risk for POCD.90 At the moment, there is little convincing evidence that an incidence of postoperative delirium in an otherwise intact patient is commonly associated with rapidly progressive dementia.
Does dementia cause delirium?
Patients with dementia are prone to episodes of delirium as well as mixed cognitive pictures that include delirium like symptoms.23 Larger programs, in the sense that they are not focused on perioperative care, such as the hospital elder life program seek to address some of the needs of these patients.92 Patients with pre-exisiting cognitive impairment are at high risk for delirium.
Conclusions
As there is some data to suggest that delirium could be prevented, there is great interest in understanding the relationship between delirium and dementia. We have seen how overlap in the definitions of the various entities makes it both logical that there is a relationship and difficult to make clear determinations, particularly in complex patients. We have noted that surgery and anesthesia are potent stimuli to the development of delirium. We have reviewed the literature that seems, on balance, to suggest that patients who develop delirium are at high risk for POCD/LTCI. We have noted that a small cadre of patients who are poorly categorized appear to have catastrophic course, but that these patients are rarely in studies. There is relatively little data at the moment to suggest that POCD is either permanent or that surgery and anesthesia accelerate the progression of dementia.
There is much work that needs to be done in this area. Unfortunately, there remains controversy regarding the determination of delirium. With the introduction of the DSM V, there is hope that the definition of delirium will remain stable for a period of time. Consensus regarding instruments for the determination of delirium would be valuable. For POCD/LTCI there is so much variability in the methods that limit the ability to make a coherent assessment. One difficulty is that studies tended to include either high quality delirium or cognitive assessment, but not both. There are currently a number of NIA funded prospective trials that will use both state of the art delirium assessments and cognitive batteries are being employed, so we should have additional prospective datasets to review in a few years.
Highlights.
Delirium is a common problem that has been studied primarily in postoperative patients
Delirium may cause long term cognitive impairment
Long term cognitive impairment may be associated with development of dementia.
Abbreviations
- Postoperative Cognitive Dysfunction
(POCD)
- Long Term Cognitive Impairment
(LTCI)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MacLullich AM, Hall RJ. Who understands delirium? Age Ageing. 2011;40:412–4. doi: 10.1093/ageing/afr062. [DOI] [PubMed] [Google Scholar]
- 2.Leslie DL, Inouye SK. The importance of delirium: economic and societal costs. J Am Geriatr Soc. 2011;59(Suppl 2):S241–3. doi: 10.1111/j.1532-5415.2011.03671.x. doi:S241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg. 2011;112:1202–11. doi: 10.1213/ANE.0b013e3182147f6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823–30. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–42. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 6.Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009;103(Suppl 1):i41–46. doi: 10.1093/bja/aep291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverstein JH, Timberger M, Reich DL, Uysal S. Central nervous system dysfunction after noncardiac surgery and anesthesia in the elderly. Anesthesiology. 2007;106:622–8. doi: 10.1097/00000542-200703000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Bitsch M, Foss N, Kristensen B, Kehlet H. Pathogenesis of and management strategies for postoperative delirium after hip fracture: a review. Acta Orthop Scand. 2004;75:378–89. doi: 10.1080/00016470410001123. [DOI] [PubMed] [Google Scholar]
- 9.Vasilevskis EE, Girard TD, Ely EW. The bedside diagnosis of ICU delirium: specificity is high, let’s optimize sensitivity. Am J Respir Crit Care Med. 2012;185:107, 8. doi: 10.1164/ajrccm.185.1.107. author reply 108. [DOI] [PubMed] [Google Scholar]
- 10.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meagher DJ, Leonard M, Donnelly S, Conroy M, Adamis D, Trzepacz PT. A longitudinal study of motor subtypes in delirium: Frequency and stability during episodes. J Psychosom Res. 2012;72:236–41. doi: 10.1016/j.jpsychores.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Meagher D, Adamis D, Trzepacz P, Leonard M. Features of subsyndromal and persistent delirium. Br J Psychiatry. 2012;200:37–44. doi: 10.1192/bjp.bp.111.095273. [DOI] [PubMed] [Google Scholar]
- 13.Steiner LA. Postoperative delirium. Part 1: pathophysiology and risk factors. Eur J Anaesthesiol. 2011;28:628–36. doi: 10.1097/EJA.0b013e328349b7f5. [DOI] [PubMed] [Google Scholar]
- 14.Brauer C, Morrison RS, Silberzweig SB, Siu AL. The cause of delirium in patients with hip fracture. Arch Intern Med. 2000;160:1856–60. doi: 10.1001/archinte.160.12.1856. [DOI] [PubMed] [Google Scholar]
- 15.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 16.Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 17.Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology. 2008;108:8–17. doi: 10.1097/01.anes.0000296072.02527.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson JE, Tandon N, Mercado AF, Camhi SL, Ely EW, Morrison RS. Brain dysfunction: another burden for the chronically critically ill. Arch Intern Med. 2006;166:1993–9. doi: 10.1001/archinte.166.18.1993. [DOI] [PubMed] [Google Scholar]
- 19.Girard TD, Ely EW. Delirium in the critically ill patient. Handb Clin Neurol. 2008;90:39–56. doi: 10.1016/S0072-9752(07)01703-4. [DOI] [PubMed] [Google Scholar]
- 20.Mahanna EP, Blumenthal JA, White WD, et al. Defining neuropsychological dysfunction after coronary artery bypass grafting. Ann Thorac Surg. 1996;61:1342–7. doi: 10.1016/0003-4975(95)01095-5. [DOI] [PubMed] [Google Scholar]
- 21.Caza N, Taha R, Qi Y, Blaise G. The effects of surgery and anesthesia on memory and cognition. Prog Brain Res. 2008;169:409–22. doi: 10.1016/S0079-6123(07)00026-X. [DOI] [PubMed] [Google Scholar]
- 22.Sauer AM, Kalkman C, van Dijk D. Postoperative cognitive decline. J Anesth. 2009;23:256–9. doi: 10.1007/s00540-009-0744-5. [DOI] [PubMed] [Google Scholar]
- 23.Holtta E, Laakkonen ML, Laurila JV, et al. The overlap of delirium with neuropsychiatric symptoms among patients with dementia. Am J Geriatr Psychiatry. 2011;19:1034–41. doi: 10.1097/JGP.0b013e31820dcbb6. [DOI] [PubMed] [Google Scholar]
- 24.Brown LJ, Ferner HS, Robertson J, et al. Differential effects of delirium on fluid and crystallized cognitive abilities. Arch Gerontol Geriatr. 2011;52:153–8. doi: 10.1016/j.archger.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 25.van Eijk MM, van den Boogaard M, van Marum RJ, et al. Routine use of the confusion assessment method for the intensive care unit: a multicenter study. Am J Respir Crit Care Med. 2011;184:340–4. doi: 10.1164/rccm.201101-0065OC. [DOI] [PubMed] [Google Scholar]
- 26.van Eijk MM, van Marum RJ, Klijn IA, de Wit N, Kesecioglu J, Slooter AJ. Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med. 2009;37:1881–5. doi: 10.1097/CCM.0b013e3181a00118. [DOI] [PubMed] [Google Scholar]
- 27.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10–6. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 28.Meagher DJ, Leonard M, Donnelly S, Conroy M, Saunders J, Trzepacz PT. A comparison of neuropsychiatric and cognitive profiles in delirium, dementia, comorbid delirium-dementia and cognitively intact controls. J Neurol Neurosurg Psychiatry. 2010;81:876–81. doi: 10.1136/jnnp.2009.200956. [DOI] [PubMed] [Google Scholar]
- 29.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852–7. [PubMed] [Google Scholar]
- 30.Rizzo JA, Bogardus ST, Jr, Leo-Summers L, Williams CS, Acampora D, Inouye SK. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value? Med Care. 2001;39:740–52. doi: 10.1097/00005650-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5:65–79. doi: 10.1007/BF02602312. [DOI] [PubMed] [Google Scholar]
- 32.Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49:523–32. doi: 10.1046/j.1532-5415.2001.49109.x. [DOI] [PubMed] [Google Scholar]
- 33.Leung JM. Postoperative delirium: are there modifiable risk factors? Eur J Anaesthesiol. 2010;27:403–5. doi: 10.1097/EJA.0b013e3283340a99. [DOI] [PubMed] [Google Scholar]
- 34.Jitapunkul S, Pillay I, Ebrahim S. Delirium in newly admitted elderly patients: a prospective study. Q J Med. 1992;83:307–14. [PubMed] [Google Scholar]
- 35.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–22. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 36.DeCrane SK, Sands L, Ashland M, et al. Factors associated with recovery from early postoperative delirium. J Perianesth Nurs. 2011;26:231–41. doi: 10.1016/j.jopan.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fong HK, Sands LP, Leung JM. The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review. Anesth Analg. 2006;102:1255–66. doi: 10.1213/01.ane.0000198602.29716.53. [DOI] [PubMed] [Google Scholar]
- 38.Deiner S, Silverstein JH. Long-term outcomes in elderly surgical patients. Mt Sinai J Med. 2012;79:95–106. doi: 10.1002/msj.21288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leung JM, Tsai TL, Sands LP. Brief report: preoperative frailty in older surgical patients is associated with early postoperative delirium. Anesth Analg. 2011;112:1199–201. doi: 10.1213/ANE.0b013e31820c7c06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chou CH, Hwang CL, Wu YT. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: a meta-analysis. Arch Phys Med Rehabil. 2012;93:237–44. doi: 10.1016/j.apmr.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 41.Inoue J, Ono R, Makiura D, et al. Prevention of postoperative pulmonary complications through intensive preoperative respiratory rehabilitation in patients with esophageal cancer. Dis Esophagus. 2012 doi: 10.1111/j.1442-2050.2012.01336.x. [DOI] [PubMed] [Google Scholar]
- 42.Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dement Geriatr Cogn Disord. 1999;10:393–400. doi: 10.1159/000017177. [DOI] [PubMed] [Google Scholar]
- 43.Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–43. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Hammon K, DeMartino BK. Postoperative delirium secondary to atropine premedication. Anesth Prog. 1985;32:107–8. [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas C, Hestermann U, Kopitz J, et al. Serum anticholinergic activity and cerebral cholinergic dysfunction: an EEG study in frail elderly with and without delirium. BMC Neurosci. 2008;9:86. doi: 10.1186/1471-2202-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tune LE. Serum anticholinergic activity levels and delirium in the elderly. Semin Clin Neuropsychiatry. 2000;5:149–53. doi: 10.153/SCNP00500149. [DOI] [PubMed] [Google Scholar]
- 47.Bryson GL, Wyand A. Evidence-based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction. Can J Anaesth. 2006;53:669–77. doi: 10.1007/BF03021625. [DOI] [PubMed] [Google Scholar]
- 48.Slor CJ, de Jonghe JF, Vreeswijk R, et al. Anesthesia and postoperative delirium in older adults undergoing hip surgery. J Am Geriatr Soc. 2011;59:1313–9. doi: 10.1111/j.1532-5415.2011.03452.x. [DOI] [PubMed] [Google Scholar]
- 49.Wu CL, Hsu W, Richman JM, Raja SN. Postoperative cognitive function as an outcome of regional anesthesia and analgesia. Reg Anesth Pain Med. 2004;29:257–68. doi: 10.1016/j.rapm.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Sieber FE, Zakriya KJ, Gottschalk A, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bittner EA, Yue Y, Xie Z. Brief review: anesthetic neurotoxicity in the elderly, cognitive dysfunction and Alzheimer’s disease. Can J Anaesth. 2011;58:216–23. doi: 10.1007/s12630-010-9418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eckenhoff RG, Johansson JS, Wei H, et al. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–9. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 53.Mandal PK, Fodale V. Isoflurane and desflurane at clinically relevant concentrations induce amyloid beta-peptide oligomerization: an NMR study. Biochem Biophys Res Commun. 2009;379:716–20. doi: 10.1016/j.bbrc.2008.12.092. [DOI] [PubMed] [Google Scholar]
- 54.Perucho J, Rubio I, Casarejos MJ, et al. Anesthesia with isoflurane increases amyloid pathology in mice models of Alzheimer’s disease. J Alzheimers Dis. 2010;19:1245–57. doi: 10.3233/JAD-2010-1318. [DOI] [PubMed] [Google Scholar]
- 55.Mandal PK, Bhavesh NS, Chauhan VS, Fodale V. NMR investigations of amyloid-beta peptide interactions with propofol at clinically relevant concentrations with and without aqueous halothane solution. J Alzheimers Dis. 2010;21:1303–9. doi: 10.3233/jad-2010-100396. [DOI] [PubMed] [Google Scholar]
- 56.Zhang B, Tian M, Zhen Y, et al. The effects of isoflurane and desflurane on cognitive function in humans. Anesth Analg. 2012;114:410–5. doi: 10.1213/ANE.0b013e31823b2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Pan N, Ma Y, et al. Inhaled Sevoflurane May Promote Progression of Amnestic Mild Cognitive Impairment: A Prospective, Randomized Parallel-Group Study. Am J Med Sci. 2012 doi: 10.1097/MAJ.0b013e31825a674d. [DOI] [PubMed] [Google Scholar]
- 58.Stratmann G. Review article: Neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg. 2011;113:1170–9. doi: 10.1213/ANE.0b013e318232066c. [DOI] [PubMed] [Google Scholar]
- 59.Jevtovic-Todorovic V. Anesthesia and the developing brain: are we getting closer to understanding the truth? Curr Opin Anaesthesiol. 2011;24:395–9. doi: 10.1097/ACO.0b013e3283487247. [DOI] [PubMed] [Google Scholar]
- 60.Eckenhoff RG, Laudansky KF. Anesthesia, surgery, illness and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2012 doi: 10.1016/j.pnpbp.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hudson AE, Hemmings HC., Jr Are anaesthetics toxic to the brain? Br J Anaesth. 2011;107:30–7. doi: 10.1093/bja/aer122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Royse CF, Andrews DT, Newman SN, et al. The influence of propofol or desflurane on postoperative cognitive dysfunction in patients undergoing coronary artery bypass surgery. Anaesthesia. 2011;66:455–64. doi: 10.1111/j.1365-2044.2011.06704.x. [DOI] [PubMed] [Google Scholar]
- 63.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–99. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 64.Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. doi: 10.1002/14651858.CD006379.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barnett SR. Polypharmacy and perioperative medications in the elderly. Anesthesiol Clin. 2009;27:377, 89. doi: 10.1016/j.anclin.2009.07.004. table of contents. [DOI] [PubMed] [Google Scholar]
- 66.Fong HK, Sands LP, Leung JM. The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review. Anesth Analg. 2006;102:1255–66. doi: 10.1213/01.ane.0000198602.29716.53. [DOI] [PubMed] [Google Scholar]
- 67.Shafer SL, Flood P. The Pharmacology of Opioids. In: Silverestein JH, Rooke GA, Reves JG, McLeskey CH, editors. Geriatric Anesthesiology. 2nd ed Springer; New York: 2008. p. 209. [Google Scholar]
- 68.Wang Y, Sands LP, Vaurio L, Mullen EA, Leung JM. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15:50–9. doi: 10.1097/01.JGP.0000229792.31009.da. [DOI] [PubMed] [Google Scholar]
- 69.Kazmierski J, Kowman M, Banach M, et al. Incidence and predictors of delirium after cardiac surgery: Results from The IPDACS Study. J Psychosom Res. 2010;69:179–85. doi: 10.1016/j.jpsychores.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 70.Janz DR, Abel TW, Jackson JC, Gunther ML, Heckers S, Ely EW. Brain autopsy findings in intensive care unit patients previously suffering from delirium: a pilot study. J Crit Care. 2010;25 doi: 10.1016/j.jcrc.2010.05.004. 538.e7,538.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guillamondegui OD, Richards JE, Ely EW, et al. Does hypoxia affect intensive care unit delirium or long-term cognitive impairment after multiple trauma without intracranial hemorrhage? J Trauma. 2011;70:910–5. doi: 10.1097/TA.0b013e3182114f18. [DOI] [PubMed] [Google Scholar]
- 72.O’Mahony R, Murthy L, Akunne A, Young J, Guideline Development Group Synopsis of the National Institute for Health and Clinical Excellence guideline for prevention of delirium. Ann Intern Med. 2011;154:746–51. doi: 10.7326/0003-4819-154-11-201106070-00006. [DOI] [PubMed] [Google Scholar]
- 73.Hall RJ, Shenkin SD, Maclullich AM. A systematic literature review of cerebrospinal fluid biomarkers in delirium. Dement Geriatr Cogn Disord. 2011;32:79–93. doi: 10.1159/000330757. [DOI] [PubMed] [Google Scholar]
- 74.McGrane S, Girard TD, Thompson JL, et al. Procalcitonin and C-reactive protein levels at admission as predictors of duration of acute brain dysfunction in critically ill patients. Crit Care. 2011;15:R78. doi: 10.1186/cc10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marcantonio ER, Rudolph JL, Culley D, Crosby G, Alsop D, Inouye SK. Serum biomarkers for delirium. J Gerontol A Biol Sci Med Sci. 2006;61:1281–6. doi: 10.1093/gerona/61.12.1281. [DOI] [PubMed] [Google Scholar]
- 76.Marcantonio ER, Rudolph JL, Culley D, Crosby G, Alsop D, Inouye SK. Serum biomarkers for delirium. J Gerontol A Biol Sci Med Sci. 2006;61:1281–6. doi: 10.1093/gerona/61.12.1281. [DOI] [PubMed] [Google Scholar]
- 77.Morandi A, Rogers BP, Gunther ML, et al. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: the VISIONS prospective cohort magnetic resonance imaging study*. Crit Care Med. 2012;40:2182–9. doi: 10.1097/CCM.0b013e318250acdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanders RD. Hypothesis for the pathophysiology of delirium: role of baseline brain network connectivity and changes in inhibitory tone. Med Hypotheses. 2011;77:140–3. doi: 10.1016/j.mehy.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 79.Cerejeira J, Nogueira V, Luis P, Vaz-Serra A, Mukaetova-Ladinska EB. The Cholinergic System and Inflammation: Common Pathways in Delirium Pathophysiology. J Am Geriatr Soc. 2012 doi: 10.1111/j.1532-5415.2011.03883.x. [DOI] [PubMed] [Google Scholar]
- 80.Monk TG, Price CC. Postoperative cognitive disorders. Curr Opin Crit Care. 2011;17:376–81. doi: 10.1097/MCC.0b013e328348bece. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 82.MacLullich AM, Beaglehole A, Hall RJ, Meagher DJ. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21:30–42. doi: 10.1080/09540260802675031. [DOI] [PubMed] [Google Scholar]
- 83.Bickel H, Gradinger R, Kochs E, Forstl H. High risk of cognitive and functional decline after postoperative delirium. A three-year prospective study. Dement Geriatr Cogn Disord. 2008;26:26–31. doi: 10.1159/000140804. [DOI] [PubMed] [Google Scholar]
- 84.Rothenhausler HB, Grieser B, Nollert G, Reichart B, Schelling G, Kapfhammer HP. Psychiatric and psychosocial outcome of cardiac surgery with cardiopulmonary bypass: a prospective 12-month follow-up study. Gen Hosp Psychiatry. 2005;27:18–28. doi: 10.1016/j.genhosppsych.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 85.Furlaneto ME, Garcez-Leme LE. Impact of delirium on mortality and cognitive and functional performance among elderly people with femoral fractures. Clinics (Sao Paulo) 2007;62:545–52. doi: 10.1590/s1807-59322007000500003. [DOI] [PubMed] [Google Scholar]
- 86.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–9. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Breshears JD, Roland JL, Sharma M, et al. Stable and dynamic cortical electrophysiology of induction and emergence with propofol anesthesia. Proc Natl Acad Sci U S A. 2010;107:21170–5. doi: 10.1073/pnas.1011949107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reiman EM, McKhann GM, Albert MS, Sperling RA, Petersen RC, Blacker D. Clinical impact of updated diagnostic and research criteria for Alzheimer’s disease. J Clin Psychiatry. 2011;72:e37. doi: 10.4088/JCP.10087tx2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bekker A, Lee C, de Santi S, et al. Does mild cognitive impairment increase the risk of developing postoperative cognitive dysfunction? Am J Surg. 2010;199:782–8. doi: 10.1016/j.amjsurg.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–5. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen CC, Lin MT, Tien YW, Yen CJ, Huang GH, Inouye SK. Modified hospital elder life program: effects on abdominal surgery patients. J Am Coll Surg. 2011;213:245–52. doi: 10.1016/j.jamcollsurg.2011.05.004. [DOI] [PubMed] [Google Scholar]