Abstract
Background
The antioxidant lycopene may be beneficial for patients with heart failure (HF). Processed tomato products are a major source of lycopene, although they are also high in sodium. Increased sodium intake may counter the positive antioxidant effect of lycopene.
Methods
This was a prospective study of 212 patients with HF. Dietary intake of lycopene and sodium was obtained from weighted 4-day food diaries. Patients were grouped by the median split of lycopene of 2471 μg/day and stratified by daily sodium levels above and below 3 g/day. Patients were followed for 1 year to collect survival and hospitalization data. Cox proportional hazards modeling was used to compare cardiac event-free survival between lycopene groups within each stratum of sodium intake.
Results
Higher lycopene intake was associated with longer cardiac event-free survival compared with lower lycopene intake (p = 0.003). The worst cardiac event-free survival was observed in the low lycopene intake group regardless of sodium intake (> 3 g/day HR = 3.01; p = 0.027 and ≤ 3 g/day HR= 3.34; p = 0.023).
Conclusion
These findings suggest that increased lycopene intake has the potential to improve cardiac event-free survival in patients with HF independent of sodium intake.
Keywords: Lycopene, antioxidants, heart failure, sodium
Introduction
Heart failure (HF) remains a major cause of early death and is associated with high morbidity resulting in significant personal, societal and economic burden.1 Hospitalizations account for the majority of expenses incurred in the care of patients with HF. Rates of HF hospitalizations have increased by approximately 200% over the past decade.2 Additional strategies for managing HF are needed to reduce the personal and economic costs of HF.
Recent evidence suggests that nutrition may play a role in improving outcomes in patients with HF.3–6 Nutritional interventions may be more justified in individuals with HF who have body wasting and cachexia.1 Findings point to specific macro- and micronutrients that may slow the trajectory of HF by decreasing the inflammatory process associated with HF, and reducing the amount of oxidative stress.3,7–10 Antioxidants such as lycopene, selenium and vitamin D may be targets for nutritional therapy. Lycopene in particular may be beneficial in HF due to its antioxidant effect in reducing inflammatory status.11 Numerous investigations in humans and animal models have demonstrated an inverse relationship between serum lycopene levels and risk of cardiovascular disease (CVD).12–15 Specific investigations between serum lycopene and HF are not as abundant in the literature.
The major source of dietary lycopene in the USA is fresh and processed fruits and vegetables, particularly tomatoes. Heat during processing alters the molecular structure of lycopene, making it more bioavailable to human tissue.13,16,17 Unfortunately, processed foods containing high levels of lycopene also contain high levels of sodium. Research investigating the potential benefits of lycopene must also consider the sodium content of such foods.18,19 Therefore the purpose of this study was to determine the association between lycopene intake and cardiac event-free survival in patients with HF stratified by sodium intake levels.
Methods
Study design
This was a longitudinal prospective study in which levels of dietary lycopene and sodium intake were measured at baseline. Patients were followed for an average of 1 year, and rehospitalizations and cardiac mortality data were obtained to examine the relationship between dietary lycopene intake and cardiac event-free survival after stratifying patients by sodium intake levels.
Sample and setting
Patients were recruited from outpatient HF clinics associated with academic health centers in Kentucky, Indiana and Georgia. Patients were included if they had a diagnosis of chronic HF confirmed by a cardiologist and had been on a stable medication regimen for at least 3 months. Patients were excluded if they had a history of acute myocardial infarction or cerebrovascular attack within the previous 6 months, if they had a comorbid terminal illness or an inflammatory condition that suppressed appetite and if they were cognitively impaired. A total of 246 patients were invited to participate. Six patients declined, 7 patients withdrew, 5 patients were lost to follow-up and 16 provided incomplete food diaries, resulting in a final sample size of 212 patients. The investigation conforms to the principles outlined in the Declaration of Helsinki.2
Measurement of variables
Dietary lycopene and sodium intake
Dietary intake was measured by weighted 4-day food diaries that included three weekdays and one weekend day. Food diaries were analyzed using the Nutrition Data System for Research (NDSR; Nutrition Coordinating Center, Minneapolis, MN).20 The analysis provided 4-day averages of each patient's intake, with data on 156 individual nutrients including lycopene and sodium.
Event-free survival
The primary outcome of this study was event-free survival. This is defined as the composite end point of time to first HF hospitalization (emergency department or inpatient admission with a primary diagnosis of HF) or a cardiac-related death during the follow-up period. Data on hospitalization events or death were collected from patients/family member interviews, medical record review, hospital administrative records, and death certificates. A clinical expert on the research team reviewed all the data on reasons for hospitalization to ensure accurate categorization. Because of the possibility that a patient was admitted to various hospitals, patients/families were interviewed to obtain self-reports of admissions, which were then used to validate or augment electronic medical record data.
Covariates
Covariates included New York Heart Association (NHYA) functional classification, age, gender, body mass index (BMI), left ventricular ejection fraction (LVEF), and prescribed medications (i.e. ACE inhibitors, beta-blockers, angiotensin II receptor blockers, digoxin, and diuretics). Total comorbidity score was obtained using the Charlson Comorbidity Index.21,22
Procedure
The study received approval from the Institutional Review Board at each enrollment site. Patients with HF were referred to the study by cardiologists and nurse practitioners. All patients gave written informed consent prior to participating. Demographic and clinical characteristics were collected at baseline. Patients were visited in their homes by a trained research nurse who provided detailed oral and written instructions for recording all food and beverages consumed during the 4-day food diary collection. Digital scales were provided to measure the weight of each food. Food models were also provided for patients to estimate serving sizes when they found that weighing food was impractical (e.g. when eating at a restaurant). Patients were asked to provide a return demonstration of food diary recording and food measurement as a review of the procedure. Patients were also telephoned on the first day of the food diary recording to answer any questions they had. The morning after completion of the food diary, the patient met with the dietitian who reviewed the completed diary. This process was to verify the serving sizes, obtain any missing information, and clarify food preparation techniques. These review sessions typically lasted 30–45 minutes. Lycopene and sodium levels were then determined using the NDSR software program for nutrient analysis. Lycopene and sodium daily consumption levels were totaled and then averaged over the 4 days of record keeping.
Statistical analysis
Data were analyzed using SPSS for Windows 17.0. Patients were dichotomized based on the median value for lycopene intake and the two groups were further stratified by the cutoff point of 3 g/day of sodium intake. For the purpose of data analysis, patients with a lycopene intake less than the median were defined as the low lycopene group and those with an intake of greater than the median split were defined as the high lycopene group. Independent t-tests or chi-square tests were used to compare the differences in sample characteristics between lycopene groups. Cox proportional hazard regression was used to compare the differences in cardiac event-free survival of patients in the lycopene groups stratified by sodium intake while controlling for age, gender, HF etiology, BMI, NYHA classification, LVEF and total comorbidity burden. The proportional hazard assumption was confirmed through visual inspection of the log (−log) survival curves.
Results
Patient characteristics
Patient characteristics of the total sample and of the high and low lycopene intake groups are shown in Table 1. The average age of patients enrolled in this study was 60 ± 12 years (range 23–97 years). Almost one-third of patients had preserved systolic function with LVEF > 40%. The common comorbidities were hypertension and diabetes mellitus. The mean 4-day average intake of lycopene was 4091 ± 4940 μg/day (range 0–31,529) and the median was 2471 (25th percentile = 915, 75th percentile = 5076) μg/day. These levels of lycopene intake are comparable to other investigations of dietary lycopene intake measured by food diaries.23,24 Fifty-one per cent of patients had daily sodium intake greater than 3 g/day. The average sodium intake of the high lycopene group was greater than that of the low lycopene group (Table 1). The majority of patients in both lycopene groups were classified as NYHA functional Class II or III, and were on appropriate HF medication regimens of ACE inhibitors, diuretics and beta-blockers.
Table 1.
Patient characteristics N = 212.
| Characteristics |
N (%) or mean ± SD |
||
|---|---|---|---|
| Total (N = 212) | Lycopene ≤ 2471 μg/day | Lycopene > 2471 μg/day | |
| Age (years) | 60 ± 12 | 59 ± 12 | 61 ± 13 |
| Gender | |||
| Male | 143 (67.5) | 66 (62.3) | 77 (72.6) |
| Female | 69 (32.5) | 40 (37.7) | 29 (27.4) |
| Body mass index (kg/m2) | 30.5 ± 7.6 | 30.5 ± 7.4 | 30.4 ± 7.8 |
| Normal weight (< 25.0) | 49 (23.1) | 24 (22.6) | 25 (23.6) |
| Overweight (25.0 to 29.9) | 60 (28.3) | 26 (24.5) | 34 (32.1) |
| Obese (≥ 30.0) | 103 (48.6) | 56 (52.8) | 47 (44.3) |
| NYHA functional class | |||
| I | 15 (7.1) | 4 (3.8) | 11 (10.4) |
| II | 81 (38.2) | 40 (37.7) | 41 (38.7) |
| III | 87 (41.0) | 48 (45.3) | 39 (36.8) |
| IV | 29 (13.7) | 14 (13.2) | 15 (14.2) |
| Heart failure etiology | |||
| Non-ischemic heart disease | 122 (57.5) | 64 (60.4) | 58 (54.7) |
| Ischemic heart disease | 90 (42.5) | 42 (39.6) | 48 (45.3) |
| Medication | |||
| ACE inhibitors | 143 (67.5) | 71 (67.0) | 72 (67.9) |
| ARB II* | 39 (18.4) | 14 (13.5) | 25 (23.8) |
| Digoxin | 62 (29.2) | 30 (28.8) | 32 (30.2) |
| Beta-blocker | 185 (87.3) | 95 (89.6) | 90 (84.9) |
| Diuretics | 163 (76.9) | 78 (74.3) | 85 (81.0) |
| Aldosterone antagonist | 45 (21.2) | 25 (23.8) | 20 (18.9) |
| Left ventricular ejection fraction (%) | 33.9 ± 14.0 | 34.0 ± 14.9 | 33.8 ± 13.2 |
| < 40% | 136 (64.2) | 66 (62.3) | 70 (66.0) |
| Total comorbidity score | 3.0 ± 2.0 | 3.0 ± 1.9 | 3.0 ± 2.1 |
| Hypertension | 147 (69.3) | 77 (73.3) | 70 (67.3) |
| Diabetes mellitus | 80 (37.7) | 41 (38.7) | 39 (36.8) |
| Sodium (mg/day)* | 3256 ± 1193 | 3029 ± 1087 | 3483 ± 1254 |
| Less than 3000 mg/day | 109 (51.4) | 60 (56.6) | 49 (46.2) |
| Greater than 3000 mg/day | 103 (48.6) | 46 (43.4) | 57 (53.8) |
ACE: angiotensin converting enzyme;
ARB II: angiotension II receptor blocker;
NYHA: New York Heart Association.
p < 0.05 in the independent t-test.
Cardiac events
During the follow-up period there were a total of two patients who died (1.0%) and 41 patients (19.3%) who were hospitalized with a primary diagnosis of HF or experienced a cardiac-related death (Table 2).
Table 2.
Event rates and median time to first event in patients classified as four groups by 3 g of dietary sodium intake and median split of lycopene (N = 212).
| Total (N = 212) | < 3 g of dietary sodium intake (n = 109) |
≥ 3 g of dietary sodium intake (n = 103) |
|||
|---|---|---|---|---|---|
| Lycopene ≤ 2471 μg/day (n = 46) | Lycopene > 2471 μg/day (n = 57) | Lycopene ≤ 2471 μg/day (n = 60) | Lycopene > 2471 μg/day (n = 49) | ||
| Cardiac events | 43 (20.3) | 13 (28.2) | 8 (14.0) | 17 (28.3) | 5 (10.2) |
| Median time to first event (days) | 201 (111, 393) | 209 (123, 332) | 624 (407, 769) | 83 (58, 207) | 193 (107, 306) |
Values are n (%) or median (25th, 75th quartile).
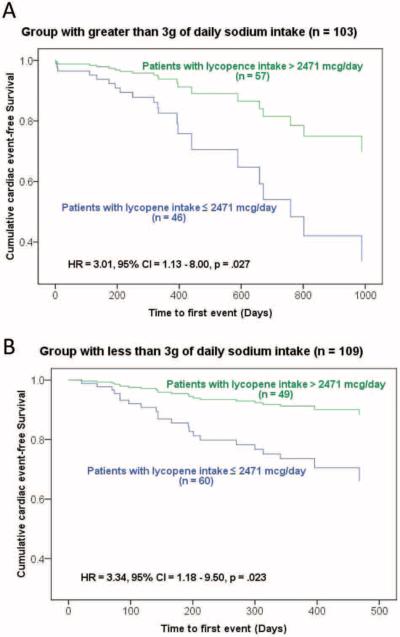
Using Cox hazard regression modeling, patients with a dietary lycopene intake above the median had longer event-free survival than those with a dietary lycopene intake below the median after multivariate adjustment. In the stratum of patients with sodium intake levels above 3 g/day, the low lycopene group demonstrated three times greater risk of having a cardiac event compared to patients in the high lycopene group (Table 3). Adjusted survival curves for high versus low lycopene groups with each stratum of sodium intake are depicted in Figures 1A and 1B. Higher intake of lycopene was associated with longer event-free survival in both strata of sodium intake. In the stratum of patients with sodium intake less than 3 g/day, low lycopene intake presented a 3.3 times greater risk of cardiac events when compared to the high lycopene group. The median time to first event for the total sample was 201 days (111, 393). In the sample with < 3 g/day dietary sodium intake, the low lycopene group had a median time of 209 days (123, 332) to first event, whereas the high lycopene group had a median time of 624 days. In the sample with ≥ 3 g/day of dietary sodium intake, the low lycopene group had a median time of 83 days (58, 207) until first event, whereas the high lycopene group had a median time of 193 days until first event (Table 2).
Table 3.
Independent predictors of event-free survival in patients with heart failure, N = 212.
| Characteristic | Adjusted hazard ratio | 95% CI | P |
|---|---|---|---|
| Age (years) | 1.00 | 0.97–1.03 | 0.881 |
| Gender | |||
| Male | |||
| Female | 0.50 | 0.09–1.13 | 0.093 |
| Body mass index (kg/m2) | 0.97 | 0.92 1.01 | 0.148 |
| NYHA functional class | |||
| I/II | |||
| III | 1.50 | 0.75–3.00 | 0.254 |
| IV | 1.41 | 0.54–3.66 | 0.485 |
| Heart failure etiology | |||
| Non-ischemic heart disease | |||
| Ischemic heart disease | 1.33 | 0.68–2.59 | 0.410 |
| Left ventricular ejection fraction (%) | 0.98–1.03 | 0.596 | |
| Total comorbidity score | 1.12 | 0.94–1.33 | 0.198 |
| Lycopene intake | |||
| > 2471 μg/day | |||
| ≤ 2471 μg/day | 2.79 | 1.40–5.55 | 0.003 |
NYHA: New York Heart Association.
Figure 1A and 1B.
Difference in time to event between two groups with higher and lower lycopene intake stratified by sodium intake. Survival curves were adjusted for age, gender, HF etiology, BMI, LVEF, NYHA class, and total comorbidity score after stratifying by sodium intake.
HR: hazard ratio, CI: confidence interval
Discussion
This is the first study to demonstrate that higher intake of lycopene independently predicted longer cardiac event-free survival regardless of sodium intake in patients with HF. These findings are important because they suggest a fruitful avenue for future research that some have been reluctant to follow due to the high sodium content of many tomato-based foods. Our findings suggest that the antioxidant properties of dietary lycopene may be more important to the health of patients with HF than reducing sodium intake alone.
Our findings about lycopene are consistent with those of prior researchers who have reported inverse relationships between lycopene and the risk of CVD.25 Previous evidence in support of the role of lycopene in the prevention of CVD comes primarily from observational studies on normal and at-risk populations.12,13,15,26–30 There have been only a few reports on lycopene interventions in samples of patients with HF.31–33
For example, in the Rotterdam Study, using case-control design, investigators assessed the presence of calcified plaques in the abdominal aorta as a clinical indication of atherosclerosis.34 The study sample consisted of 108 individuals with moderate to severe atherosclerosis compared to 108 age- and gender-matched controls without atherosclerosis. The investigators reported an inverse relationship between serum levels of lycopene and the presence of atherosclerosis when using a logistic regression model with multivariate adjustment.34 The odds ratio for the highest compared to the lowest quartile of serum lycopene was 0.55 (95% CI, 0.25–1.22).34
The strongest population-based evidence for lycopene comes from a multicenter case-control study (EURAMIC) that evaluated the relationship between lycopene levels and acute myocardial infarction (AMI).35 Plasma lycopene levels were found to be protective against AMI, independent of other cardiovascular risk factors, with an odds ratio of 0.52 for the comparison of the 10th and 90th percentiles (95% CI, 0.33–0.82; p = 0.005).35 A dose-response was observed between each quintile of lycopene levels and the risk of myocardial infarction. The protective potential of lycopene was at the highest among individuals with the highest polyunsaturated fat stores.35
Evidence about the effectiveness of lycopene in heart disease has recently been extended to patients with HF. In a series of observational studies, Polidori et al. evaluated plasma levels of malondialdehyde (MDA) and F2 isoprostane as biomarkers of oxidative stress and antioxidant levels (vitamin A, vitamin E, lutein, zeaxanthin and lycopene) in 30 HF patients with NYHA class II and III compared to 30 age- and gender-matched controls (18 male, 12 female, 80.0 ± 17.4 years).31,32 Class II NYHA patients with HF had significantly lower levels of markers of oxidative stress (MDA and F2 isoprostane levels) and significantly higher levels of antioxidant intake (vitamin A, vitamin E, lutein and lycopene) than NYHA class III patients. Ejection fraction was inversely correlated with MDA levels in HF patients when compared to the controls. A positive correlation was found between plasma levels of lycopene, lutein and vitamin A and ejection fraction, thus suggesting increased hemodynamic function of the heart in the presence of dietary antioxidants. These findings further suggest that antioxidants are depleted in patients with worse HF, which may be linked to increased oxidative stress or perhaps as a result of lifestyle, disease or age-related reasons that can facilitate oxidative stress.31,32
In a retrospective analysis of subjects enrolled in the Third National Health and Nutrition Examination Survey, Wood and Johnson reported that a relationship was found among tomato consumption, serum lycopene levels and HF risk in individuals with periodontitis.33 Individuals with periodontitis had a dose-response relationship between tomato consumption and self-reported CHF risk, moderate tomato consumption (RR 3.15; 95% CI, 1.03–9.67), low tomato consumption (RR 3.31; 95% CI, 1.33–8.24) (p < 0.05). This dose-response relationship existed after adjusting for demographic, medical and lifestyle factors. When data were further adjusted for serum lycopene levels, the relationship between tomato consumption and HF risk in periodontally involved individuals remained high (p ≤ 0.05).33
Approximately 80% of the sodium in the average American diet comes from processed foods, and for the past two decades the average daily sodium intake among adults was greater than 3.2 g.36 Dietary sodium indiscretion is considered to be a precipitant in > 20% of patients hospitalized for decompensated HF and high sodium intake is an independent risk factor for HF exacerbation.37–39 Because the richest sources of lycopene (processed tomato-based products such as ketchup, tomato juice, and pizza and spaghetti sauces) are also some of the highest in sodium, clinicians commonly have patients with HF avoid these foods. There is evidence that patients may respond differently to sodium restriction depending on symptom severity.3 In a previous study we demonstrated that a higher sodium intake diet has detrimental effects on event-free survival in patients with HF, but only in those with NYHA class III and IV classification.40 Our data suggest that foods containing high levels of lycopene may be beneficial without regard to the amount of sodium content. Patients often experience symptoms at varying levels of intensity and will often seek advice from healthcare providers about changes in diet and lifestyle that impacts their daily life and quality of life, thus promoting self-care management is a vital component in the care of patients with HF.4
A potential limitation of this study was the use of self-recorded food diaries. We recognize the greater number of days food intake is recorded, the more reliable are estimates of actual food intake.5 We used a 4-day recording period, which optimizes estimates while minimizing risk of unreliable data due to recorder fatigue. Participants must be motivated, so we also used a combination of strategies to increase the accuracy of these diaries.6 As food intake varies by day of week and season, we asked our participants to record intake on one weekend day and three weekdays. All instructions for the diary were given by an experienced HF research nurse. In addition to the verbal instruction, written and graphic materials were provided to each participant. Patients were also given digital food scales to help them measure food portions. Food models were used to help estimate serving sizes. Food diaries were reviewed with the patient by a dietician. Further motivation to accurately complete the food diary was enhanced by providing a nutritional analysis report to the patient. The nutritional analysis report was also provided as an encouraging feedback mechanism for participants to prevent the possibility of potentially changing their normal dietary intake during the reporting phase.
A second limitation to this study is that we did not obtain a direct measurement of oxidative stress or inflammation and therefore we cannot conclude the potential mechanism responsible for our findings. Our findings do support the potential cardioprotective benefit of higher lycopene intake in the presence of increased sodium levels in food products consumed by patients with HF as evidenced by longer event-free survival rates. Increased sodium intake in patients with HF is associated with an increase in fluid retention that is often responsible for the exacerbation of HF symptoms. However, the potentially unfavorable effect of increased sodium content in processed food products may not overshadow the benefit of lycopene in HF patients. This finding may be a result of overall diet quality or a dietary pattern; however, further studies are needed to clearly delineate the interaction of lycopene and sodium in patients with HF, and the results from our study should not be interpreted as suggesting that sodium restriction is unnecessary in patients with HF, even in the presence of high lycopene intake.
Conclusion
Future research should include the use of randomized control trials of various levels of dietary lycopene intake in HF patients stratified by their usual sodium intake. A diet with a high lycopene intake has the potential to improve cardiac event-free survival outcomes in HF patients. The study of dietary factors that led to a diet rich in antioxidants is essential for optimization of interventions aimed at reducing the burden of HF. Additional research should be conducted to examine the potential impact of lycopene from a variety of food products on the inflammatory process in patients with HF.
“What's new?”
A diet rich in foods that are high in lycopene have the potential to improve cardiac event-free survival outcomes in HF patients.
A novel new intervention to increase dietary antioxidants may reduce the symptom burden of heart failure.
Acknowledgments
Funding (1) NIH/National Institute Nursing Research 3R01 NR 009280 (Lennie, T.A., PI); (2) NIH/National Institute Nursing Research 1P20 NR 010679 (Moser, D.K., PI).
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics – 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 3.Lennie TA. Nutritional recommendations for patients with heart failure. J Cardiovasc Nurs. 2006;21:261–268. doi: 10.1097/00005082-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Lennie TA, Chung ML, Habash DL, et al. Dietary fat intake and proinflammatory cytokine levels in patients with heart failure. J Card Fail. 2005;11:613–618. doi: 10.1016/j.cardfail.2005.06.434. [DOI] [PubMed] [Google Scholar]
- 5.Serdar A, Yesilbursa D, Serdar Z, et al. Relation of functional capacity with the oxidative stress and antioxidants in chronic heart failure. Congest Heart Fail. 2001;7:309–311. doi: 10.1111/j.1527-5299.2001.00261.x. [DOI] [PubMed] [Google Scholar]
- 6.Ershow AG, Costello RB. Dietary guidance in heart failure: a perspective on needs for prevention and management. Heart Fail Rev. 2006;11:7–12. doi: 10.1007/s10741-006-9187-3. [DOI] [PubMed] [Google Scholar]
- 7.Keith M, Geranmayegan A, Sole MJ, et al. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 8.Keith ME, Jeejeebhoy KN, Langer A, et al. A controlled clinical trial of vitamin E supplementation in patients with congestive heart failure. Am J Clin Nutr. 2001;73:219–224. doi: 10.1093/ajcn/73.2.219. [DOI] [PubMed] [Google Scholar]
- 9.Singal P, Khaper N, Farahmand F, et al. Oxidative stress in congestive heart failure. Curr Cardiol Rep. 2000;2:206–211. doi: 10.1007/s11886-000-0070-x. [DOI] [PubMed] [Google Scholar]
- 10.Rogowski O, Shnizer S, Wolff R, et al. Increased serum levels of oxidative stress are associated with hospital read-missions due to acute heart failure. Cardiology. 2011;118:33–37. doi: 10.1159/000324192. [DOI] [PubMed] [Google Scholar]
- 11.Blum A, Monir M, Wirsansky I, et al. The beneficial effects of tomatoes. Eur J Intern Med. 2005;16:402–404. doi: 10.1016/j.ejim.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Arab L, Steck S. Lycopene and cardiovascular disease. Am J Clin Nutr. 2000;71:1691S–1695S. doi: 10.1093/ajcn/71.6.1691S. discussion 1696S-1697S. [DOI] [PubMed] [Google Scholar]
- 13.Bose KS, Agrawal BK. Effect of lycopene from cooked tomatoes on serum antioxidant enzymes, lipid peroxidation rate and lipid profile in coronary heart disease. Singapore Med J. 2007;48:415–420. [PubMed] [Google Scholar]
- 14.Das S, Otani H, Maulik N, et al. Lycopene, tomatoes, and coronary heart disease. Free Radic Res. 2005;39:449–455. doi: 10.1080/10715760500053685. [DOI] [PubMed] [Google Scholar]
- 15.Rao AV. Lycopene, tomatoes, and the prevention of coronary heart disease. Exp Biol Med (Maywood) 2002;227:908–913. doi: 10.1177/153537020222701011. [DOI] [PubMed] [Google Scholar]
- 16.Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev. 1998;56:35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 17.Basu A, Imrhan V. Tomatoes versus lycopene in oxidative stress and carcinogenesis: conclusions from clinical trials. Eur J Clin Nutr. 2007;61:295–303. doi: 10.1038/sj.ejcn.1602510. [DOI] [PubMed] [Google Scholar]
- 18.Bentley B, Moser DK. Dietary sodium in heart failure: what to tell your patients. Prog Cardiovasc Nurs. 2007;22:41–42. doi: 10.1111/j.0889-7204.2007.06501.x. [DOI] [PubMed] [Google Scholar]
- 19.Paterna S, Gaspare P, Fasullo S, et al. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clin Sci (Lond) 2008;114:221–230. doi: 10.1042/CS20070193. [DOI] [PubMed] [Google Scholar]
- 20.Schakel SF. Maintaining a nutrient database in a changing marketplace: Keeping pace with changing food products – a research perspective. J Food Comp Anal. 2001;14:315–322. [Google Scholar]
- 21.Kieszak SM, Flanders WD, Kosinski AS, et al. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol. 1999;52:137–142. doi: 10.1016/s0895-4356(98)00154-1. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Charlson RE, Peterson JC, et al. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61:1234–1240. doi: 10.1016/j.jclinepi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Mayne ST, Cartmel B, Silva F, et al. Plasma lycopene concentrations in humans are determined by lycopene intake, plasma cholesterol concentrations and selected demographic factors. J Nutr. 1999;129:849–854. doi: 10.1093/jn/129.4.849. [DOI] [PubMed] [Google Scholar]
- 24.Jacob K, Periago MJ, Bohm V, et al. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Br J Nutr. 2008;99:137–146. doi: 10.1017/S0007114507791894. [DOI] [PubMed] [Google Scholar]
- 25.Kristenson M, Zieden B, Kucinskiene Z, et al. Antioxidant state and mortality from coronary heart disease in Lithuanian and Swedish men: concomitant cross sectional study of men aged 50. BMJ. 1997;314:629–633. doi: 10.1136/bmj.314.7081.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao AV, Agarwal S. Role of antioxidant lycopene in cancer and heart disease. J Am Coll Nutr. 2000;19:563–569. doi: 10.1080/07315724.2000.10718953. [DOI] [PubMed] [Google Scholar]
- 27.Upritchard JE, Sutherland WH, Mann JI. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care. 2000;23:733–738. doi: 10.2337/diacare.23.6.733. [DOI] [PubMed] [Google Scholar]
- 28.Riccioni G, Mancini B, Di Ilio E, et al. Protective effect of lycopene in cardiovascular disease. Eur Rev Med Pharmacol Sci. 2008;12:183–190. [PubMed] [Google Scholar]
- 29.Ahuja KDK, Pittaway JK, Ball MJ. Effects of olive oil and tomato lycopene combination on serum lycopene, lipid profile, and lipid oxidation. Nutrition. 2006;22:259–265. doi: 10.1016/j.nut.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Ahuja KD, Kunde D, Ball MJ. Effects of olive oil and tomato lycopene combination on heart disease risk factors. Asia Pac J Clin Nutr. 2003;12(Suppl):S21. [Google Scholar]
- 31.Polidori MC, Pratico D, Savino K, et al. Increased F2 isoprostane plasma levels in patients with congestive heart failure are correlated with antioxidant status and disease severity. J Card Fail. 2004;10:334–338. doi: 10.1016/j.cardfail.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Polidori MC, Savino K, Alunni G, et al. Plasma lipophilic antioxidants and malondialdehyde in congestive heart failure patients: relationship to disease severity. Free Radic Biol Med. 2002;32:148–152. doi: 10.1016/s0891-5849(01)00782-1. [DOI] [PubMed] [Google Scholar]
- 33.Wood N, Johnson RB. The relationship between tomato intake and congestive heart failure risk in periodontitis subjects. J Clin Periodontol. 2004;31:574–580. doi: 10.1111/j.1600-051X.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 34.Klipstein-Grobusch K, Launer LJ, Geleijnse JM, et al. Serum carotenoids and atherosclerosis. The Rotterdam Study. Atherosclerosis. 2000;148:49–56. doi: 10.1016/s0021-9150(99)00221-x. [DOI] [PubMed] [Google Scholar]
- 35.Kohlmeier L, Kark JD, Gomez-Gracia E, et al. Lycopene and myocardial infarction risk in the EURAMIC Study. Am J Epidemiol. 1997;146:618–626. doi: 10.1093/oxfordjournals.aje.a009327. [DOI] [PubMed] [Google Scholar]
- 36.Jacobson MF. Sodium content of processed foods: 1983–2004. Am J Clin Nutr. 2005;81:941–942. doi: 10.1093/ajcn/81.4.941. [DOI] [PubMed] [Google Scholar]
- 37.He J, Ogden LG, Bazzano LA, et al. Dietary sodium intake and incidence of congestive heart failure in overweight US men and women: first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med. 2002;162:1619–1624. doi: 10.1001/archinte.162.14.1619. [DOI] [PubMed] [Google Scholar]
- 38.He J, Ogden LG, Vupputuri S, et al. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA. 1999;282:2027–2034. doi: 10.1001/jama.282.21.2027. [DOI] [PubMed] [Google Scholar]
- 39.Bentley B, De Jong MJ, Moser DK, et al. Factors related to nonadherence to low sodium diet recommendations in heart failure patients. Eur J Cardiovasc Nurs. 2005;4:331–336. doi: 10.1016/j.ejcnurse.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Lennie TA, Song EK, Wu JR, et al. Three gram sodium intake is associated with longer event-free survival only in patients with advanced heart failure. J Card Fail. 2011;17:325–330. doi: 10.1016/j.cardfail.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]