Abstract
Previous studies have demonstrated that leptin and its receptors (LepRb) in the central nervous system play an important role in regulating depression- and anxiety-related behaviors. However, the physiological functions of LepRb in specific brain regions for mediating different emotional behaviors remain to be defined. In this study, we examined the behavioral effects of LepRb ablation in the adult hippocampus using a series of behavioral paradigms for assessing depression- and anxiety-related behaviors. Targeted deletion of LepRb was achieved using the Cre/loxP site-specific recombination system through bilateral stereotaxic delivery of an adeno-associated virus expressing Cre-recombinase (AAV-Cre) into the dentate gyrus of adult mice homozygous for a floxed leptin receptor allele. AAV-Cre-mediated deletion of the floxed region of LepRb was detected 2 weeks after injection. In accordance with this, leptin-stimulated phophorylation of Akt was attenuated in the hippocampus of AAV-Cre injected mice. Mice injected with AAV-Cre displayed normal locomotor activity and anxiety-like behavior, as determined in the elevated plus maze, light dark box and open field tests, but showed increased depression-like behaviors in the tail suspension, sucrose preference and learned helplessness tests. Taken together, this data suggests that deletion of LepRb in the adult hippocampus is sufficient to induce depression-like behaviors. Our results support the view that leptin signaling in the hippocampus may be essential for maintaining positive mood states and active coping to stress.
Keywords: leptin receptor LepRb, hippocampus, depression, learned helplessness, anhedonia
INTRODUCTION
Leptin is a hormone synthesized by adipose tissue. Once released into circulation, leptin can be transported across the blood-brain barrier and bind to receptors on neurons in various brain areas to exert its biological functions. Several lines of evidence have suggest that leptin plays a role in depression- and anxiety-related behaviors. Our group is the first to show that administration of leptin produces antidepressant-like effects rats and mice (Garza et al., 2011; Liu et al., 2010; Lu et al., 2006). The antidepressant-like properties of leptin have recently been confirmed independently by other investigators (Yamada et al., 2011). Also, we found that leptin stimulates adult hippocampal neurogenesis, a feature shared by antidepressants (Czeh and Lucassen, 2007; Jacobs, 2002; Malberg et al., 2000; Santarelli et al., 2003), under basal and chronic stress conditions, accompanied by mood improvement (Garza et al., 2008a; Garza et al., 2011). Moreover, we have demonstrated that acute leptin administration has anxiolytic effects (Liu et al., 2010). By contrast, leptin-deficient and leptin receptor deficient mice display anxiogenic behavior (Dinel et al., 2011; Finger et al., 2010).
Among six isoforms of the leptin receptor (LepRa–f) that have been identified, the LepRb isoform is principally responsible for signal transduction events stimulated by leptin, leading to biological actions (Chua et al., 1996; Lee et al., 1996). The physiological roles of endogenous leptin receptor signaling in depression- and anxiety-related behaviors have been investigated in previous studies using conventional Lepr knockout or conditional LepRb knockout mice (Guo et al., 2012; Liu et al., 2011; Sharma et al., 2010) (Dinel et al., 2011. While selective deletion of LepRb in midbrain dopaminergic neurons causes increased firing of dopamine neurons and an anxiogenic phenotype (Liu et al., 2011), loss of LepRb specifically in forebrain glutamatergic neurons in the dorsal cerebral cortex and hippocampus induces depressive-like behaviors (Guo et al., 2012). These studies support the view that LepRb signaling in specific neuronal populations plays essential but distinct roles in regulating depression- and anxiety-related behaviors.
The hippocampus has been implicated in the pathophysiology of depression and anxiety disorders. Within the hippocampus, LepRb mRNA is primarily expressed in the dentate gyrus, with a lower level in CA3 (Guo et al., 2012; Scott et al., 2009). Although it has been previously shown that intra-hippocampal microinjection of leptin produces antidepressant-like effects (Lu et al., 2006), the physiological roles that hippocampal LepRb signaling in depression- and anxiety-related behaviors remain to be determined. In this study, the Cre-loxP system and adeno-associated viral vectors (AAV) were used to achieve site-specific deletion of LepRb in the hippocampus. AAV expressing Cre-recombinase was delivered to the dentate gyrus of the hippocampus of adult LepRbflox/flox mice. The effects of AAV-Cre mediated deletion of LepRb in adult hippocampus on depression- and anxiety-like behaviors weight were examined using a battery of behavioral tests.
MATERIALS and METHODS
Animals
Adult male Leprflox/flox mice (McMinn et al., 2004) were housed in groups of four or five at 22°C on a 14 hr light/10 hr dark cycle (lights on at 7:00 h) with ad libitum access to food and water. Mice were genotyped using PCR-based genotyping with the following primers: 5’-ATGCTATCGACAAGCAGCAGAATGA-3’ and 5’-CAGGCTTGAGAACATGAACACAACAAC-3’. The presence of the LoxP sites was verified by digesting the PCR products with Hind III. All animal procedures were conducted in accordance with National Institutes of Health guidelines, and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Generation of viral vectors
The AAV-Cre-GFP vector was constructed by generating an expression cassette consisting of the Cre coding sequence with a 5’ nuclear localization signal (NLS) preceded by the cytomegalovirus (CMV) promoter. Downstream of the Cre coding sequence, an internal ribosomal entry sequence (IRES) was used to express green fluorescent protein (GFP) followed by a 3’ simian virus 40 polyadenylation signal [SV40 poly(A)]. The construct was inserted into an AAV2 vector. The control AAV-GFP vector was constructed similarly by cloning the GFP coding sequence with the SV40 poly(A) signal under the control of CMV promoter into the AAV2 vector. As previously described (Garza et al., 2008b), the viral vectors were generated by co-transfection of AAV vector plasmid and pDC2 helper plasmid (provided by Mark Kay, Stanford University) into HEK 293 cells by calcium phosphate transfection. The pDC2 helper plasmid was designed to include the AAV2 rep and cap genes as well as adenoviral helper functions necessary for AAV packaging. Viral vectors were purified by three cycles of cesium chloride density gradient centrifugation and concentrated using a nanosep centrifugation column (Pall Life Sciences, East Hills, NY). The AAV virion titer was determined by infecting HEK-293 cells with serial dilutions of the purified virus and counting the number of GFP-positive cells.
Stereotaxic surgery
Mice were anesthetized with an intramuscular injection of a cocktail containing ketamine 60 mg/ml, acepromazine 1 mg/ml and xylazine 8 mg/ml (0.1 ml/kg, i.m.) and mounted onto a stereotaxic frame. AAV-Cre-GFP vectors and control AAV-GFP vectors (with titers of 1×1012 infectious units/ml) were injected bilaterally into the dentate gyrus of hippocampus (coordinates relative to bregma: anterior-posterior = −2.1 mm; medial-lateral = ±1.5 mm; dorsal-ventral = −2.1 mm) according to the mouse brain atlas (Paxinos et al., 2001). A volume of 1.0 µl of AAV vectors was delivered into the hippocampus bilaterally with a slow injection rate (1.0 µl/10 min) through a 33-gauge stainless steel microinjector attached to a digital stereotaxic arm and connected to an infusion pump. After injection was completed, the injector was left in place for an additional 5 min to minimize backflow while withdrawing the injector. All of the behavioral experiments were conducted 14 days after AAV vector injection to allow sufficient time for viral vector transduction and Cre expression. For verification of injection sites, brains were sectioned at a thickness of 20 µm using a cryostat and the presence of GFP was inspected with a fluorescent microscope.
RT-PCR
Tissue was dissected from the hippocampus and hypothalamus of Leprflox/flox mice and total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA). SuperScript™ II reverse transcriptase (Invitrogen, Carlsbad, CA) was used to generate cDNA using the oligo(dT)25 as the template primer. The resulting cDNA was used for PCR amplification of LepR exon 17 or β-actin with Accuprime Pfx Supermix (Invitrogen, Carlsbad, CA). The PCR conditions consisted of an initial denaturing stage at 94°C for 5 min, followed by 35 cycles of 94°C for 1 min, 60°C for 1 min and 72°C for 1 min followed by a final incubation at 72°C for 10 minutes. The primer sequences used to amplify each product are as follows: LepR exon 17, forward: 5’-GGGACGATGTTCCAAACCCCA-3’ and reverse: 5’-AGGCTCCAGAAGAAGAGGACC-3’; β-actin, forward: 5’-AGCCATGTACGTAGCCATCC-3’ and reverse: 5’-TGTGGTGGTGAAGCTGTAGC-3’. The PCR products were analyzed on a 1% agarose gel stained with ethidium bromide.
Western blot analysis
Leptin-induced phosphorylation of Akt was determined by Western blot in the hippocampus of adult Leprflox/flox mice 14 days after intra-DG injection of AAV-Cre-GFP and AAV-GFP vectors. Animals were injected intracerebroventricularly (i.c.v.) with 2 µg of leptin in 1 µl of artificial cerebrospinal fluid (aCSF). The hippocampus was dissected out on ice 10 min after i.c.v. injection and immediately placed in liquid nitrogen and stored at −80°C until further processing. The brain tissue samples were homogenized in lysis buffer (50 mM Hepes, pH 7.6, 1% Triton X-100, 150 mM NaCl, 20 mM sodium pyrophosphate, 20 mM β-glycerophosphate, 10 mM NaF) containing a mixture of phosphatase inhibitors (leupeptin, aprotinin, sodium orthovanadate, phenylmethylsulfonyl fluoride, Ser/Thr phosphatase inhibitor mixture, Tyr phosphatase inhibitor mixture). Total protein was extracted and the concentration was determined using the Bradford assay. A total amount of 40 µg of protein was separated on an SDS-PAGE gel, transferred to a nitrocellulose membrane, blocked in a solution of 1% dry milk and 0.1% Tween 20 in 1× Tris-buffered saline, and subsequently incubated in primary antibodies diluted in a solution of 1% bovine serum albumin and 0.1% Tween 20 in 1× Tris-buffered saline (anti-Akt, 1:1000; anti-phosphorylated Akt-Thr308, 1:1000) overnight at 4 °C. Next, the membrane was washed and incubated in secondary antibody conjugated to horseradish peroxidase (1:10,000) in blocking solution for 1 hr. Western blot results were visualized using an electrogenerated chemiluminescence (ECL) reaction and exposed to X-ray film.
Food intake and body weight
Mice injected with AAV-Cre-GFP or AAV-GFP were weighed on the day of surgery, and at days 14, 21 and 46 after surgery. Food intake was measured for 5 consecutive days beginning 21 days after AAV injection.
Behavioral procedures
All behavioral tests were performed during the late light phase, except sucrose preference, which was measured every 24 h. On the test day, animals were individually housed in a new cage with some home cage bedding to avoid the stressful effect of sequential removal of the mice from the cage. Animals were transferred to the testing room and habituated to the room conditions for 3–4 h prior to the beginning of the experiments. After each individual test session, the apparatus was thoroughly cleaned with 20% alcohol to eliminate the odor and trace of the previously tested animal. We used three separate cohorts of male Leprflox/flox mice for behavioral tests. One cohort of mice was sequentially subjected to the tail suspension test, forced swim test, and learned helplessness test. A second cohort of mice was sequentially tested in the open field, elevated plus-maze, light/dark box, and learned helplessness tests. The behavioral tests were spaced by 5–7 days. The learned helplessness data were pooled from two cohorts of mice. A third cohort of mice was used only for saccharin preference. All behaviors were scored by experimenters who were blind to the treatment and genotype of the animals.
Locomotor activity
On the testing day, the home cage lid was removed and replaced with clear Plexiglas® over the top of the cage to allow the animal’s activity to be recorded. The activity of the mice was recorded for 30 minutes and the Noldus EthoVision 3.0 system was used to determine distance travelled for the 30-minute test in 2-minute bins.
Tail Suspension Test
The apparatus consisted of a box (30×30×30 cm). The front of the box was open, and a bar was placed horizontally 1 cm from the top with an attached vertical bar hanging down in the center. Mice were individually suspended by the tail to the vertical bar with adhesive tape affixed 2 cm from the tip of the tail. A camera positioned in front of the TST box was used to record the animals’ behavior for a 6-minute test session. Immobility in this test was defined as the absence of any limb or body movements, except those caused by respiration.
Forced Swim Test
Mice were placed in a clear Plexiglas® cylinder (25 cm high; 10 cm in diameter) filled to a depth of 15 cm with 24°C water. A camera positioned directly above the cylinder recorded the 6-minute swim session. For each test session, the first 2 min served as a habituation period. The immobility of the mice was measured during the last 4 min of the test. Immobility in this test was defined as the absence of any movement except for that required for keeping the animal’s head above water.
Saccharin preference
Mice were habituated to drinking from two bottles of water for one week prior to testing. To measure the preference for sweet solutions, the animals were singly housed and given access to one bottle of water and one bottle of 0.01% saccharin for 4 consecutive days. Water and saccharin intake was measured daily and the position of two bottles was switched every day to avoid any side preference. Saccharin preference was calculated as the volume of saccharin intake over the total volume of fluid intake.
Learned helplessness test
The learned helplessness test was performed in a shuttle cage divided equally into two chambers with an auto-controlled guillotine door between the two chambers. Learned helplessness was induced in mice by administering 200 scrambled, inescapable foot shocks (0.3 mA shock amplitude, 2-sec duration, 16-sec average interval) over a 1 h session. Control animals were placed in the apparatus for the same period of time but did not receive foot shocks. Escape performance was tested 24 h later in the same shuttle cage. Each mouse was given 30 shuttle escape trials with 25 sec maximum duration and 30-sec intervals. On the first 5 trials, a sound cue and the shock took place at the same time as the door to the safe compartment opened. For the remaining trials, the door opened 2 sec after the shock was delivered. Each trial was terminated when the mouse crossed into the non-shock compartment. The latency to escape and the number of escape failures were recorded automatically by the Graphic State software (Coulbourn Instruments INC, Allentown, PA).
Elevated plus-maze
The EPM was constructed with white acrylic, and has four arms (30-cm long and 5-cm wide) arranged in the shape of a "plus" sign and elevated to a height of 70 cm from the floor. Two arms have no side or end walls (open arms). The other two arms have side and end walls (12-cm high) but are open on top (closed arms). The open and closed arms intersect, having a central 5 × 5 cm square platform giving access to all arms. The mice were placed in the central square facing the corner between a closed arm and an open arm, and allowed to explore the elevated plus-maze for 5 min. Their activity on the elevated plus-maze was recorded. The time spent on the open and closed arms and the numbers of entries made into each arm were measured. Entry was defined as all four paws being positioned within one arm. The degree of anxiety was assessed by calculating the percentage of open arm entries (entries into the open arms/total entries into all arms) and percentage of open arm time (time spent in the open arms/total time spent in all arms).
Open-field test
The apparatus consisted of a 60 × 60 cm open arena with 40 cm high walls. The entire test arena was adjusted to even illumination. Mice were placed in the center of the arena, and their activity was recorded for 5 min. For analysis, a 3×3 grid was placed over the video and the center square was defined as the central zone, in which animal’s activity is usually regarded as a measure of anxiety. The distance mice traveled in the central zone over total distance traveled in the open arena was also quantified using the Noldus EthoVision 3.0 system. The total distance traveled was used as a measure of overall motor activity.
Light dark test
The apparatus consists of two equally sized compartments (17.8 × 17.8 × 30.5 cm) divided by a wall with an open door between the two compartments. One compartment (light compartment) was illuminated to a light intensity of 700 lx while the other compartment (dark compartment) was black-walled. For each test, the mouse was placed in the center of the light compartment facing away from the opening and the behavior was recorded for 5 min. The number of transitions between two compartments and time spent in the light compartment were measured.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). Statistical analyses were performed using one-way ANOVA with repeated measures on body weight gain and locomotor activity, two-way ANOVA on Western blot assays and number of failures to escape in the learned helplessness test. Bonferroni/Dunn or Tukey/Kramer (for unequal n) post hoc comparisons followed ANOVAs. Two-tailed Student t test was used for statistical analysis of the rest of the experimental results. P < 0.05 was considered statistically significant.
RESULTS
Targeted deletion of LepRb in the hippocampus
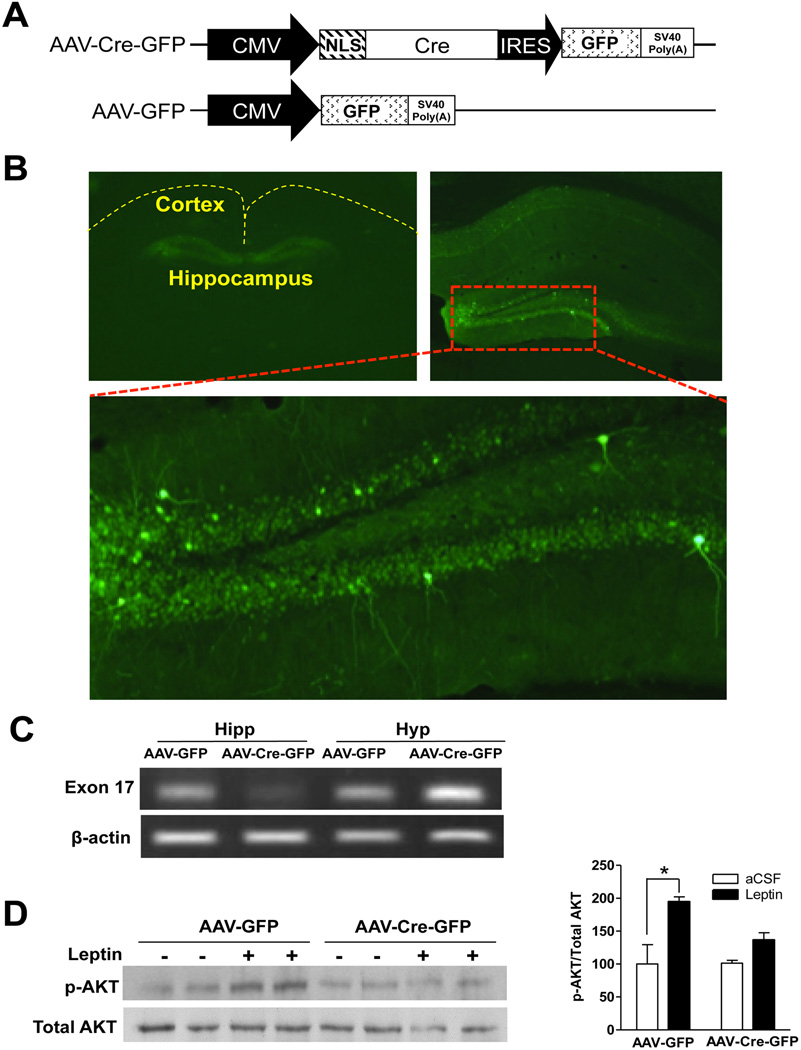
Targeted deletion of LepRb in a region-specific manner was achieved using AAV-mediated Cre recombinase expression in Lepr-floxed (Leprflox/flox) mice, in which exon 17, a critical exon involved in LepRb signaling, is floxed (McMinn et al., 2004). In the AAV-Cre-GFP vector, GFP was linked to the CMV promoter-driven Cre via an IRES (Figure 1A), allowing them to be expressed simultaneously and used for identification of injection sites (Figure 1B). Adult male Leprflox/flox mice were stereotaxically injected bilaterally with AAV-Cre-GFP into the dentate gyrus where LepRb is predominantly expressed (Guo et al., 2012; Scott et al., 2009). Leprflox/flox mice were bilaterally injected with AAV-GFP as a control. The injection sites were verified in each animal after completion of the behavioral experiments. The behavioral data from those mice with missed injection were removed from statistical analysis.
Figure 1.
Deletion of LepRb in the adult hippocampus. A. Schematic diagrams showing design of the AAV-Cre-GFP (upper) and AAV-GFP (lower) constructs. B. GFP expression in the dentate gyrus. Low magnification image (top, left) shows GFP distribution restricted in the dentate gyrus. Higher magnification (top, right and bottom). C. RT-PCR analysis of LepRb exon 17 mRNA in the hippocampus. Hipp, hippocampus; Hyp, hypothalamus. D. Western blot analysis. Hippocampal lysates from AAV-Cre-GFP and AAV-GFP injected mice were immunoblotted for total AKT and AKT phosphorylated on Thr308. n = 4. *P < 0.05, compared to aCSF treatment.
To examine the efficiency of AAV-Cre-mediated excision of the floxed exon 17, we used RT-PCR to measure exon 17 mRNA levels in hippocampus at 2 weeks after intra-dentate gyrus injection of AAV-Cre-GFP or AAV-GFP. Exon 17 mRNA in the hippocampus was drastically decreased in AAV-Cre-GFP injected mice in comparison with control AAV-GFP injected mice (Figure 1C). Akt phosphorylation, a downstream target of LepRb, in response to i.c.v. injection of leptin, was determined to confirm the functional loss of LepRb. Western blot demonstrated that leptin stimulated phosphorylation of Akt in the hippocampus of AAV-GFP injected mice, but this effect was greatly attenuated by AAV-Cre-GFP injection (Figure 1D). Taken together, these observations support the functional loss of LepRb induced by AAV-Cre-GFP.
Food intake and body weight
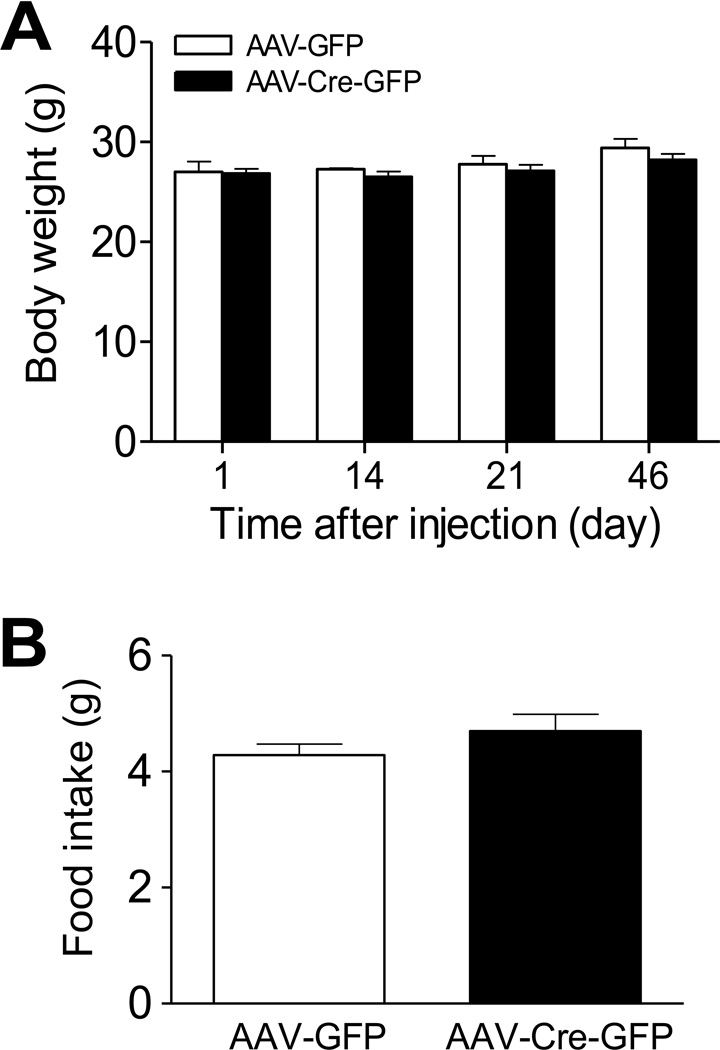
To determine whether deletion of LepRb in the adult hippocampus affects energy homeostasis, body weight was measured on days 1, 14, 21 and 46 after stereotaxic delivery of AAV-Cre-GFP or AAV-GFP into the dentate gyrus. ANOVA with repeated measures indicated that treatment had no significant effect on body weight (F(1,60) = 1.084, P > 0.1). There was no difference in body weight between AAV-Cre-GFP and AAV-GFP groups at any of the time points measured. Three weeks after intra-dentate gyrus AAV injection, food intake was measured for 5 consecutive days. No difference in food intake was found between AAV-Cre-GFP and AAV-GFP treated mice (Figure 2).
Figure 2.
Body weight gain and food intake in mice with deletion of LepRb in the dentate gyrus of adult male mice. A. Body weight was monitored at different time points after stereotaxic injection of AAV-Cre-GFP or AAV-GFP vectors into the dentate gyrus. B. Food intake was measured for 5 days at 3 weeks after intra-dentate gyrus injection of AAV-Cre-GFP and AAV-GFP vectors. Average food intake per day was calculated. n = 11–12 per group. Data are presented as mean ± SEM.
Locomotor activity
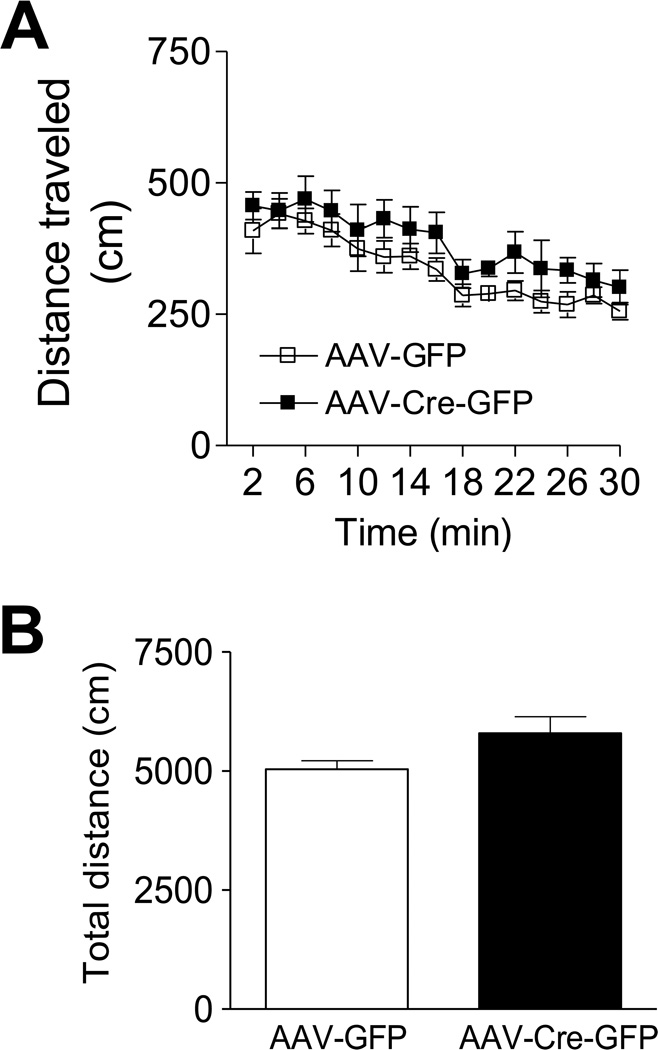
Locomotor activity was examined in AAV-Cre-GFP and AAV-GFP mice in an open field arena for 30 min and the distance traveled in 2-min bins was measured for the duration of the test. ANOVA with repeated measures revealed no significant effect of treatment on locomotor activity (F(1,196) = 3.96, P > 0.05 for the time course of the distance traveled every 2 min; t(15) =2.016, P > 0.05 for the total distance traveled within 30 min) (Figure 3).
Figure 3.
Locomotor activity after LepRb deletion. Male mice injected with either AAV-Cre-GFP or AAV-GFP in the dentate gyrus exhibited a similar level of locomotor activity during the testing period whether analyzed as 2 minute increments or total distance traveled over the entire 30 min period. n = 8–9 per group. Data are presented as mean ± SEM.
Anxiety-related behaviors
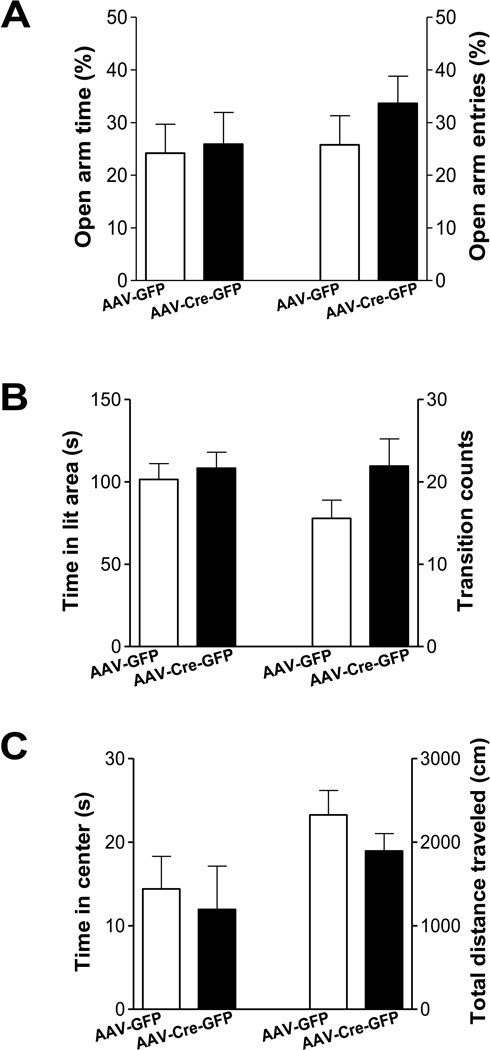
To assess whether deletion of LepRb in the dentate gyrus affects anxiety-related behavior, three behavioral tests were performed: i.e. the elevated plus maze, open field test, and light/dark choice. In the elevated plus maze, a reduction in time spent in the open arms and number of entries made into the open arms within the 5 min test are interpreted as indices of anxiety. AAV-Cre-GFP and AAV-GFP mice showed similar time spent in the open arms (t(17) =1.146 , P > 0.1) and number of entries into the open arms (t(17) = 0.209, P > 0.5) (Figure 4A). In the light/dark choice test, there was no difference in the amount of time spent in the light box (t(17) = 0.494, P > 0.5) or the total number of transitions between light and dark box (t(17) = 1.557, P > 0.1) (Figure 4B). In an open field arena, a decrease in time spent in the central area of the arena is considered as an index of anxiety. Mice that received an intra-dentate gyrus injection of AAV-Cre-GFP or AAV-GFP spent a similar amount of time in the central zone (t(18) = 0.011, P > 0.5) and traveled similar total distances within the arena (t(18) = 1.404, P > 0.1) (Figure 4). These data indicate that anxiety-like behaviors are not affected by loss of LepRb in the dentate gyrus.
Figure 4.
Anxiety levels in mice with deletion of LepRb in the dentate gyrus. A. The elevated plus maze. Left, the percentage of time spent in the open arms/total time spent in all arms. Right, the percentage of entries made into the open arms/total entries made into all arms. n = 9–10 per group. B. The light/dark choice. Left, the time spent in the light compartment. Right, the number of transitions between light and dark compartments. n = 9–10 per group. C. The open field test. Left, the time spent in the center area. Right, the total distance traveled in the entire open field. n = 10 per group. Data are presented as mean ± SEM.
Depression-related behaviors
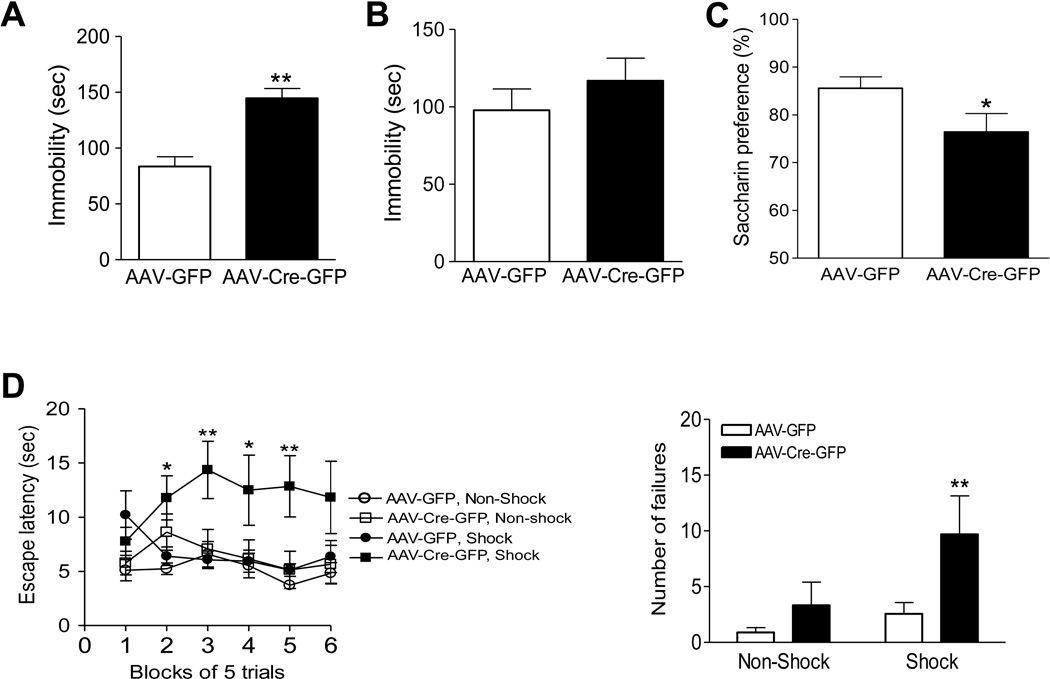
To determine whether loss of LepRb in the dentate gyrus affects depression-related behaviors, four behavioral tests were performed. The tail suspension test and forced swim test have been widely used to assess ‘despair behavior’ (Porsolt et al., 1977; Steru et al., 1985). Immobility in these two tests is referred to as ‘despair’, and a variety of antidepressants, as well as leptin, reduce this behavior (Cryan et al., 2005; Garza et al., 2011; Liu et al. 2010; Lu et al., 2006; Petit-Demouliere et al., 2005). Mice with an intra-dentate gyrus injection of AAV-Cre-GFP or AAV-GFP were first evaluated in the tail suspension test. The immobility time was significantly increased in AAV-Cre-GFP injected mice compared to those injected with control AAV-GFP (t(20) = 4.349 , P < 0.01) (Fig. 5A). In the forced swim test, the immobility time in AAV-Cre-GFP injected mice showed a tendency to increase but this did not reach statistical significance (t(18) = 0.941, P > 0.1 ) (Figure 5B).
Figure 5.
AAV-Cre-induced LepRb deletion in the adult dentate gyrus results in depression-like behavior. A. Tail suspension test. Immobility time was measured during the 6-min test period. n = 11 per group. **P < 0.01 compared to AAV-GFP treatment. B. Forced swim test. Immobility time was measured during the last 4 min of the 6-min test period. n = 8–10 per group. C. Saccharin preference was expressed as a ratio of the volume of saccharin solution intake to the volume of water intake for a 4-day test. n = 11 per group. *P < 0.05 compared to AAV-GFP treatment. D. Performance in the learned helplessness test. Left, mean escape latencies in six blocks of five trials. Right, number of failures to escape over the 30 trials. n = 9–12 per group. *P < 0.05, **P < 0.01, compared with the AAV-GFP, Shock control group. Data are presented as mean ± SEM.
Anhedonia, a core symptom of human depression, was assessed using the saccharin preference test. Mice were habituated to two drinking bottles in their home cages and then allowed access to a choice of water or a saccharin (0.1%) solution for 4 days. The preference for the saccharin solution was significantly lower in AAV-Cre-GFP injected mice compared with control AAV-GFP injected mice (t(20) = 2.706, P < 0.05) (Figure 5C).
Depression-like behavior was also evaluated using the learned helplessness paradigm, which resembles the passive, withdrawn behavior of human depression. In this test, AAV-Cre-GFP injected mice and AAV-GFP injected mice were exposed to inescapable foot shock and subsequently tested for a deficit in avoidance-escape performance. ANOVA revealed a significant treatment × shock interaction for escape latencies (F(1, 185) = 4.812, P < 0.05) and number of failures to escape (F(1, 37) = 6.593, P < 0.01). Mice injected with AAV-Cre-GFP showed increased escape latencies and number of failures to escape in comparison with AAV-GFP-treated control mice (Figure 5D).
DISCUSSION
In this study, we demonstrate that AAV-Cre mediated deletion of LepRb in the dentate gyrus results in depressive-like behaviors without significantly affecting locomotor activity and anxiety-related behaviors. These results suggest that disruption of LepRb in the dentate gyrus is sufficient to induce depressive-like symptoms.
The hippocampus has been implicated in both depression- and anxiety-related behaviors (Bannerman et al., 2004; Campbell and Macqueen, 2004; Engin and Treit, 2007; Soares and Mann, 1997). Our recent studies have shown that selective deletion of LepRb in forebrain glutamatergic neurons, specifically localized in the hippocampus and cerebral cortex, causes depression-like behaviors in a battery of behavioral tests (Guo et al., 2012). Infusion of exogenous leptin into the hippocampus targeting the dentate gyrus elicits antidepressant-like effects in the forced swim test (Lu et al., 2006). The results from the present study provide evidence that LepRb signaling in the dentate gyrus is physiologically relevant to mood regulation. Both the tail suspension test and forced swim test were used to assess behavioral ‘despair’ as measured by immobility time. Mice with deletion of LepRb in the dentate gyrus showed significantly increased immobility in the tail suspension test but not in the forced swim test. This could be due to different sensitivity of the mouse strain to these two tests (Liu and Gershenfeld, 2001; Lucki et al., 2001). One possible confounding factor in the ‘behavioral despair’ tests is altered locomotor activity. Mice received intra-dentate gyrus injection of AAV-Cre-GFP showed no change in locomotor activity, suggesting that increased ‘behavioral despair’ in these mice is unlikely to be a result of general hypolocomotion. The ‘behavioral despair’ tests are commonly used to evaluate antidepressant efficacy. We further examined depression-like behavior using sweet solution preference as a measure of anhedonia-like behavior (Liu et al., 2011; Snaith, 1993; Willner et al., 1992). AAV-Cre mediated deletion of LepRb in the adult dentate gyrus resulted in a significant reduction of saccharin preference. Furthermore, we tested mice in the learned helplessness test, a paradigm that has been used as an animal model of depression (Seligman and Beagley, 1975; Seligman and Maier, 1967). Mice were exposed to inescapable shock stress and subsequently tested in an avoidance-escape task. AAV-Cre mediated deletion of LepRb in the adult dentate gyrus caused increased learned helplessness as indicated by increased escape latency and increased number of failures to escapes. These behavioral deficits suggest that disruption of LepRb function in the adult hippocampus is sufficient to induce depressive-like behaviors under basal and stressed conditions. It was noticed that the depressive-like behaviors in conditional LepRb knockout mice induced by transgenic Cre targeting hippocampal and cortical neurons appear more severe than those observed in mice injected with AAV-Cre-GFP in the adult dentate gyrus (Guo et al., 2012). This could be explained by incomplete deletion of LepRb in the adult hippocampus and possible involvement of LepRb in the prefrontal cortex.
The hippocampus is also a brain structure involved in the modulation of anxiety-related behaviors (Gray, 1982). Anxiety-like behaviors were assessed in three tests, i.e. the elevated plus maze, light/dark box and open field; however, the performance on these behavioral tests was not significantly different between mice injected with AAV-Cre-GFP and control mice injected with AAV-GFP, suggesting that hippocampal LepRb may not participate in mediating anxiety behaviors. These results are in consistence with the findings in mice with selective ablation of LepRb in hippocampual and cortical neurons (Guo et al., 2012). A recent study reported that administration of leptin into the hippocampus reduces food intake and blocks the expression of a conditioned place preference for food (Kanoski et al., 2011). However, we found no significant change in daily food intake and body weight gain in mice received intra-dentate gyrus injection of AAV-Cre-GFP compared to mice received control AAV-GFP injection. Similar findings were made in conditional LepRb knockout mice lacking LepRb in the hippocampal and cortical neurons (Guo et al., 2012). The discrepancies between our studies using LepRb knockdown/knockout models and the studies by Kanoski et al. with intra-hippocampal infusion of leptin may reflect the differences between the effects of psychological versus pharmacological treatments. In addition, targeted deletion of LepRb in the hippocampus occurs in local neurons, whereas infusion of exogenous leptin into the hippocampus could activate LepRb located on both local neurons and presynaptic terminals originating from other brain regions.
Leptin stimulates multiple intracellular signaling pathways via LepRb, including the PI3-kinase -Akt, the signal transducer and activator of transcription-3 (STAT3) and the extracellular signal-regulated kinase (ERK) signaling pathways (Ahima and Osei, 2004; Bjorbaek et al., 2001; Morris and Rui, 2009; Munzberg and Myers, 2005; Niswender et al., 2001; Xu et al., 2005; Zhang et al., 2004). In this study, we demonstrated that leptin-stimulated phosphorylation of Akt was attenuated in the hippocampus of mice with AAV-Cre mediated deletion of LepRb. Akt is an important negative regulator of glycogen synthase kinase-3β (GSK-3β) (Cohen and Frame, 2001; Cross et al., 1995). Recent studies have reported alterations in Akt and its downstream target, GSK-3β, in depression and suicide (Dwivedi et al., 2010; Karege et al., 2007; Pandey et al., 2010; Yoon and Kim, 2010). A detailed analysis of the intracellular signaling pathways responsible for leptin action on depressive-like behaviors will be investigated in future studies.
In summary, we provide evidence that LepRb in adult hippocampus plays an important role in depression-related behaviors. Our results suggest that normal hippocampal LepRb activity may be required to maintain positive mood states and implicate that dysfunction of hippocampal LepRb signaling may contribute to the pathogenesis or pathophysiology of depression.
ACKNOWLEDGEMENT
This work was supported by NIH grants NIMH 076929 and NIMH 073844 (XYL).
Footnotes
INTEREST STATEMENT
The authors declare no conflict of interest.
References
- Ahima RS, Osei SY. Leptin signaling. Physiology & behavior. 2004;81(2):223–241. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, et al. Regional dissociations within the hippocampus--memory and anxiety. Neuroscience and biobehavioral reviews. 2004;28(3):273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Buchholz RM, Davis SM, Bates SH, et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. The Journal of biological chemistry. 2001;276(7):4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. Journal of psychiatry & neuroscience : JPN. 2004;29(6):417–426. [PMC free article] [PubMed] [Google Scholar]
- Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271(5251):994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- Cohen P, Frame S. The renaissance of GSK3. Nature reviews. Molecular cell biology. 2001;2(10):769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29(4–5):571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? European archives of psychiatry and clinical neuroscience. 2007;257(5):250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- Dinel AL, Andre C, Aubert A, Ferreira G, et al. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS One. 2011;6(9):e24325. doi: 10.1371/journal.pone.0024325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Zhang H, Roberts RC, et al. Modulation in activation and expression of phosphatase and tensin homolog on chromosome ten, Akt1, and 3-phosphoinositide-dependent kinase 1: further evidence demonstrating altered phosphoinositide 3-kinase signaling in postmortem brain of suicide subjects. Biological Psychiatry. 2010;67(11):1017–1025. doi: 10.1016/j.biopsych.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Treit D. The role of hippocampus in anxiety: intracerebral infusion studies. Behavioural pharmacology. 2007;18(5–6):365–374. doi: 10.1097/FBP.0b013e3282de7929. [DOI] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF. Leptin-deficient mice retain normal appetitive spatial learning yet exhibit marked increases in anxiety-related behaviours. Psychopharmacology (Berl) 2010;210(4):559–568. doi: 10.1007/s00213-010-1858-z. [DOI] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, Lu XY. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. The Journal of biological chemistry. 2008a;283(26):18238–18247. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, Lu XY. Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3beta/beta-catenin signaling. Molecular psychiatry. 2011 doi: 10.1038/mp.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza JC, Kim CS, Liu J, Zhang W, et al. Adeno-associated virus-mediated knockdown of melanocortin-4 receptor in the paraventricular nucleus of the hypothalamus promotes high-fat diet-induced hyperphagia and obesity. The Journal of endocrinology. 2008b;197(3):471–482. doi: 10.1677/JOE-08-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. The Neuropsychology of Anxiety. Oxford University Press; 1982. [Google Scholar]
- Guo M, Lu Y, Garza JC, Li Y, et al. Forebrain glutamatergic neurons mediate leptin action on depression-like beahviors and synaptic depression. Translational Psychiatry. 2012;2 doi: 10.1038/tp.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL. Adult brain neurogenesis and depression. Brain, behavior, and immunity. 2002;16(5):602–609. doi: 10.1016/s0889-1591(02)00015-6. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, et al. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology. 2011;36(9):1859–1870. doi: 10.1038/npp.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Perroud N, Burkhardt S, Schwald M, et al. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biological Psychiatry. 2007;61(2):240–245. doi: 10.1016/j.biopsych.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Liu J, Garza JC, Bronner J, Kim CS, et al. Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology (Berl) 2010;207(4):535–545. doi: 10.1007/s00213-009-1684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Perez SM, Zhang W, Lodge DJ, et al. Selective deletion of the leptin receptor in dopamine neurons produces anxiogenic-like behavior and increases dopaminergic activity in amygdala. Molecular psychiatry. 2011;16(10):1024–1038. doi: 10.1038/mp.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gershenfeld HK. Genetic differences in the tail-suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biological Psychiatry. 2001;49(7):575–581. doi: 10.1016/s0006-3223(00)01028-3. [DOI] [PubMed] [Google Scholar]
- Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5):1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155(3):315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn JE, Liu SM, Dragatsis I, Dietrich P, et al. An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mammalian genome : official journal of the International Mammalian Genome Society. 2004;15(9):677–685. doi: 10.1007/s00335-004-2340-1. [DOI] [PubMed] [Google Scholar]
- Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. American journal of physiology. Endocrinology and metabolism. 2009;297(6):E1247–E1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzberg H, Myers MG., Jr Molecular and anatomical determinants of central leptin resistance. Nature Neuroscience. 2005;8(5):566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, et al. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413(6858):794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Ren X, Rizavi HS, Dwivedi Y. Glycogen synthase kinase-3beta in the platelets of patients with mood disorders: effect of treatment. Journal of psychiatric research. 2010;44(3):143–148. doi: 10.1016/j.jpsychires.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177(3):245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–336. [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Scott MM, Lachey JL, Sternson SM, Lee CE, et al. Leptin targets in the mouse brain. The Journal of comparative neurology. 2009;514(5):518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman ME, Beagley G. Learned helplessness in the rat. Journal of comparative and physiological psychology. 1975;88(2):534–541. doi: 10.1037/h0076430. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Maier SF. Failure to escape traumatic shock. Journal of experimental psychology. 1967;74(1):1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- Sharma AN, Elased KM, Garrett TL, Lucot JB. Neurobehavioral deficits in db/db diabetic mice. Physiol Behav. 2010;101(3):381–388. doi: 10.1016/j.physbeh.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith P. Anhedonia: a neglected symptom of psychopathology. Psychological medicine. 1993;23(4):957–966. doi: 10.1017/s0033291700026428. [DOI] [PubMed] [Google Scholar]
- Soares JC, Mann JJ. The anatomy of mood disorders--review of structural neuroimaging studies. Biological Psychiatry. 1997;41(1):86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neuroscience and biobehavioral reviews. 1992;16(4):525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, et al. PI3K integrates the action of insulin and leptin on hypothalamic neurons. The Journal of clinical investigation. 2005;115(4):951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Katsuura G, Ochi Y, Ebihara K, et al. Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology. 2011;152(7):2634–2643. doi: 10.1210/en.2011-0004. [DOI] [PubMed] [Google Scholar]
- Yoon HK, Kim YK. Association between glycogen synthase kinase-3beta gene polymorphisms and major depression and suicidal behavior in a Korean population. Progress in neuro-psychopharmacology & biological psychiatry. 2010;34(2):331–334. doi: 10.1016/j.pnpbp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(45):16064–16069. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]