Abstract
Purpose
Among individuals with peripheral artery disease (PAD), we compared annual change in six-minute walk performance between participants who neither underwent lower extremity revascularization nor walked for exercise (Group 1, reference), those who walked regularly for exercise (Group 2), and those who underwent lower extremity revascularization (Group 3).
Methods
Participants were recruited from Chicago-area vascular laboratories and followed annually. Change in six-minute walk was calculated beginning at the study visit preceding lower extremity revascularization or exercise behavior and continuing for one additional year after the therapy was reported. Results adjust for age, sex, race, comorbidities, and other confounders.
Results
Of 348 PAD participants, 43 underwent revascularization during a median follow-up of 84 months. Adjusted annual declines in six-minute walk were Group 1: −96.6 feet/year, Group 2: −49.9 feet/year, and Group 3: −32.6 feet/year(p<.001). Forty-one percent of revascularizations were not associated with ankle brachial index (ABI) improvement. When Group 3 was limited to participants with ABI improvement of ≥ 0.15 after revascularization, annual adjusted changes in six-minute walk were Group 1:−97.7 feet/year; Group 2:−46.5 feet/year, and Group 3:+68.1 feet/year (P value<.001). When Group 3 was limited to participants without ABI improvement ≥ 0.15 after revascularization, annual adjusted changes in six-minute walk were Group 1:−99.2 feet/year, Group 2:−48.0 feet/year; and Group 3:−61.7 feet/year. (P value<.001).
Conclusion
A large proportion of PAD participants did not have an ABI improvement of at least 0.15 at their follow-up study visit after revascularization. The benefits of lower extremity revascularization in patients with PAD appear closely tied to improvements in the ABI after revascularization.
Keywords: Peripheral artery disease, intermittent claudication, physical functioning, exercise
Men and women with lower extremity peripheral arterial disease (PAD) have greater functional impairment and faster rates of functional decline compared to people without PAD (1–3). Rates of lower extremity revascularization and related medical care costs are increasing (4). The Institute of Medicine has identified PAD as among the high priority medical conditions in need of comparative effectiveness research (5).
The Walking and Leg Circulation Study (WALCS) cohort is a longitudinal natural history study of men and women with PAD followed for up to eight years (1–3). The primary aim of the current study was to compare average annual decline in six-minute walk among PAD participants in the WALCS cohort according to whether they a) reported neither lower extremity revascularization nor engaged in walking exercise during follow-up (Group 1, reference group); b) reported that they engaged in regular walking exercise (Group 2); or c)) reported lower extremity revascularization during follow-up (Group 3);. We hypothesized that PAD participants who participated in regular walking exercise and those who underwent lower extremity revascularization, respectively, would experience less annual decline in six-minute walk performance compared to participants who neither underwent lower extremity revascularization nor engaged in walking exercise. Average annual decline in four-meter walking velocity was a secondary outcome. In secondary aims, we repeated our analyses, limiting the revascularized group first to participants with an ABI increase ≥ 0.15 in the revascularized leg and then to participants without an ABI increase ≥ 0.15 in the revascularized leg. Our results provide real-world information on the relative benefits of self-directed walking exercise and lower extremity revascularization among men and women with PAD.
METHODS
Methods for the WALCS longitudinal observational study of men and women with PAD have been described (1–3). The protocol was Institutional Review Board-approved by Northwestern University and participating medical centers.
Participant Identification
Participants were age 55 and older at baseline and were identified from among consecutive patients diagnosed with PAD in three Chicago-area non-invasive vascular laboratories. In order to be eligible for WALCS, PAD participants were required to have been diagnosed with PAD in one of the non-invasive vascular laboratories and have an ABI < 0.90 at their baseline study visit. A small number of PAD participants were identified from among consecutive patients in a large general medicine practice who were screened for PAD with the ankle brachial index (ABI). Baseline visits occurred between October 1998 and January 2000. Follow-up visits occurred annually for up to eight years. Data collection at baseline and at annual follow-up visits included a detailed medical history, confirmed with medical record review, six-minute walk and four-meter walking velocity measures, and the ankle brachial index. PAD was defined as ABI < .90 at the baseline study visit (1–3).
Exclusion Criteria
Exclusion criteria have been reported (3). Patients with dementia, recent major surgery, or foot or leg amputations were excluded. Nursing home residents and wheelchair-bound patients were excluded. Non-English-speaking patients were excluded because investigators were not fluent in non-English languages.
Ankle Brachial Index Measurement
A hand-held Doppler probe (Nicolet Vascular Pocket Dop II, Golden, CO) was used to obtain systolic pressures in the right and left brachial, dorsalis pedis, and posterior tibial arteries at baseline and at each follow-up visits (1–3). Each pressure was measured twice. The ABI was calculated in each leg by dividing the average of the dorsalis pedis and posterior tibial pressures in each leg by the average of the left and right brachial pressures (6). Average brachial pressures in the arm with highest pressure were used in the denominator when one brachial pressure was higher than the opposite brachial pressure in both measurement sets, and the two brachial pressures differed by 10 or more mm Hg in at least one measurement set, since in such cases subclavian stenosis was possible (7). The lowest leg ABI was used in analyses. An ABI <0.90 was required at the baseline visit for inclusion.
Comorbidities
Algorithms developed for the Women’s Health and Aging Study were used to document angina, diabetes, myocardial infarction, stroke, heart failure, pulmonary disease, spinal stenosis, disk disease, and hip fracture (8). American College of Rheumatology criteria were used to diagnose knee and hip osteoarthritis (9,10).
Functional measures
Six-Minute Walk
Following a standardized protocol (1–3), participants walk up and down a 100- foot hallway for six minutes after instructions to cover as much distance as possible.
Four-meter walking velocity
Walking velocity was measured with a four-meter walk performed at “usual” and “fastest” pace. For the “usual” paced walk, participants were instructed to walk at their usual pace, “as if going down the street to the store.” Each walk was performed twice. The faster walk in each pair was used in analyses (1–3).
Self-reported Exercise
At baseline and each annual follow-up visit, participants were asked, “During the past two weeks, have you gone walking for exercise?” Participants who answered “yes” were queried about the number of walking exercise sessions they engaged in during the past two weeks and the duration of walking exercise for each session. We classified participants as engaging in regular walking exercise (Group 2) if they described walking exercise activity at least three times per week for a a total of at least 90 minutes per week (11).
Ascertainment of Lower Extremity Revascularization
At each annual follow-up visit, participants were asked, “Since your last WALCS visit on<date>did you have a procedure to improve the blood flow to your legs?” Medical records were obtained and abstracted by a physician to identify lower extremity revascularizations after the baseline visit. A primary care physician questionnaire was also mailed after each study visit to identify and verify lower extremity revascularization since the previous study visit. The presence of lower extremity revascularization required documentation in the medical record or confirmation by the primary care physician.
An ABI increase of at least 0.15 in the revascularized leg at the study visit immediately following revascularization was considered a clinically meaningful ABI improvement. An ABI increase of less than 0.15 in the revascularized leg at the study visit immediately following revascularization were considered not to have had a clinically meaningful improvement in ABI after revascularization.
Leg Symptoms
Leg symptoms were classified into one of five groups using the San Diego Claudication Questionnaire, based on previous study (1,2,12): 1) intermittent claudication (IC) (exertional calf pain that does not begin at rest and resolves within 10 minutes after walking ceases); 2) Leg pain on exertion and rest (exertional leg pain that sometimes begins at rest); 3) Atypical exertional leg pain/carry on (exertional leg symptoms that do not begin at rest and do not stop the individual while walking); 4) Atypical exertional leg pain/stop (exertional leg symptoms that do not meet criteria for the other three symptom groups); 5) Asymptomatic (no pain in either leg or buttock on walking).
Other Measures
Height and weight were measured at each visit. Body mass index (BMI) was calculated as weight (kilograms)/height (meters)2. Cigarette smoking (pack-years) was assessed annually with self- report. Physical activity was measured using a validated measure of patient-reported blocks walked in the past week (13).
Follow-up
Individuals for whom data collection forms indicated that the participant was unable to complete functional measures at follow-up due to wheelchair-confinement, exhaustion, or other significant symptoms were classified as too disabled to complete functional measures (2). A previously developed algorithm incorporating participant-reported physical activity, and performance on standing balance tests and time for five repeated chair rises was used to classify participants who refused functional measures at follow-up into “disabled” or “non-disabled” (2). Those classified as “disabled” were assigned the minimum score of all participants who completed the six-minute walk or four-meter walking velocities at the study visit for which they were classified as disabled (2).
Statistical Analyses
At each follow-up visit, participants were categorized into Group 1 (no revascularization and no regular walking exercise since the last visit, reference), Group 2 (self-reported regular walking exercise since the last visit), and Group 3 (report of lower extremity revascularization since the last visit). Participants who reported both revascularization and walking exercise at a follow-up visit were classified in Group 3. Baseline characteristics across these three groups were compared using general linear models for continuous variables and chi-square tests for categorical variables.
In comparing change in six minute walk distance across the groups, a longitudinal or repeated measures analysis of covariance (ANCOVA) was carried out using the mixed-effects linear regression analysis, adjusting for age, sex, race, BMI, ABI, comorbidities, smoking, leg symptoms, and functional performance at the immediately preceding study visit. Group status was entered into the model as a time-dependent variable. For example, when revascularization was reported at the first annual follow-up visit (FV-1), change in six-minute walk associated with revascularization was evaluated by the average annual change in six-minute walk between the second annual follow-up visit (FV-2) and the visit occurring one year prior to FV-1, when revascularization was reported. When regular walking exercise was reported at FV-2, change in six-minute walk associated with walking exercise was evaluated by the average annual change in six-minute walk between the third-annual follow-up visit (FV-3) and the visit occurring one year prior to FV-2 (i.e. FV-1). Analyses were performed using person-years of follow-up. Thus, individuals contributed multiple person-years of follow-up for example if they were categorized into Group 1 (reference) at their FV-1 visit, but reported lower extremity revascularization at their FV-3 visit.
Longitudinal analyses were repeated a) after restricting Group 3 to participants whose ABI in the revascularized leg increased by at least 0.15 at the study visit immediately following revascularization, compared to the ABI in the same leg at the study visit immediately prior to revascularization and b) after restricting Group 3 to participants whose ABI in the revascularized leg did not increase by at least 0.15 at the study visit immediately following revascularization, compared to the ABI in the same leg at the study visit immediately prior to revascularization. If a participant underwent bilateral revascularization, the leg with lowest ABI prior to revascularization was considered in analyses, since prior study shows that the ABI in the leg with lowest ABI is more closely associated with the degree of functional impairment than the leg with higher ABI (6). Of the 25 participants with bilateral lower extremity revascularization, only three participants had an ABI increase ≥ 0.15 in the leg with highest ABI without a concomitant increase > 0.15 in the leg with lowest ABI prior to the revascularization. For all longitudinal analyses, Pairwise comparisons were also performed, comparing Group 2 and Group 3, respectively, to Group 1. Analyses were performed using SAS statistical software (version 9.1, SAS Institute Inc, Cary, NC).
RESULTS
Of 460 PAD participants in the WALCS cohort, 35 were missing information about exercise behavior and 76 were missing data on functional performance during follow-up, leaving 349 participants. Of these, one was missing data for BMI, leaving 348 eligible participants for analyses. Compared to potential participants who were missing data on functional performance during follow-up, those included in analyses had a higher BMI (27.6 vs. 26.1 kg/M2, P=.005) and lower prevalences of heart failure (24.4% vs. 38.4%, P=.004), pulmonary disease (30.0% vs. 42.0%, P=.018), and cancer (12.9% vs. 25.9%, P=.001).
Of the 348 PAD participants, the average age was 71.45 ± 8.47, 60.3% were male, 15.8% were African American, 21.3% were current smokers, and 30.5% had diabetes mellitus. Eighty-two percent had any exertional leg symptoms and 32.5% had classic claudication symptoms. The mean ABI among the 348 participants was 0.65 ± 0.15. Forty-three of the 348 PAD participants underwent 61 lower extremity revascularization procedures during follow-up (Group 3), 160 did not undergo revascularization but reported self-directed walking exercise during at least one follow-up visit (Group 2), and 145 reported neither therapy at any follow-up visits (Group 1, reference). PAD participants who underwent revascularization (Group 3) were younger and had lower baseline BMI compared to participants in the other two groups. PAD participants who engaged in regular self-directed walking exercise (Group 2) included lower proportions of current smokers and African-American participants compared to the other groups (Table 1).
Table 1.
Baseline Characteristics of Peripheral Arterial Disease Participants According to Treatment During Follow-up
| Exercise ≥ 3 times per week during follow-up a |
Lower Extremity Revascularization during follow-upb |
Neither revascularization nor walking exercise during follow-up c. |
P value | |
|---|---|---|---|---|
| N | 160 | 43 | 145 | |
| Age (years) | 72.60 (8.19) | 68.91 (8.29) | 70.92 (8.66) | 0.024 |
| ABI | 0.67 (0.13) | 0.61 (0.14) | 0.65 (0.16) | 0.069 |
| BMI (Kg/M2) | 27.67 (4.19) | 25.83 (4.07) | 27.95 (5.31) | 0.032 |
| Male Gender (%) | 61.3 | 60.5 | 59.3 | 0.942 |
| African American (%) | 10.0 | 18.6 | 21.4 | 0.021 |
| Current smoker (%) | 15.0 | 23.3 | 27.6 | 0.026 |
| Diabetes (%) | 28.8 | 27.9 | 33.1 | 0.660 |
| Angina (%) | 31.3 | 41.9 | 37.9 | 0.302 |
| Myocardial infarction (%) | 26.9 | 16.3 | 28.3 | 0.279 |
| Stroke (%) | 9.4 | 16.3 | 12.4 | 0.408 |
| Heart Failure (%) | 25.6 | 11.6 | 26.9 | 0.110 |
| Cilostazol (%) | 1.3 | 4.7 | 0.7 | 0.195 |
| Pentoxifylline (%) | 6.9 | 4.7 | 8.3 | 0.706 |
| Classic Intermittent | 33.1 | 44.2 | 28.3 | 0.143 |
| Claudication (%) |
Participants included in this group reported engaging in walking exercise at least once during follow-up and did not report lower extremity revascularization during follow-up.
Participants in this group reported lower extremity revascularization at least once during follow-up.
Participants in this group reported neither regular self-directed walking exercise nor lower extremity revascularization at any annual visit during follow-up.
ABI data were collected both before and after 54 of the 61 revascularization procedures. Of these, 22 (41%) revascularizations were associated with an ABI increase less than 0.15 at the study visit immediately following revascularization. Overall, the mean ABI change in legs undergoing revascularization was +0.061 ± 0.24. The mean change in the lowest leg ABI among participants who engaged in regular exercise was −0.01 ± 0.13 and the mean change in the lowest leg ABI among participants who neither engaged in regular exercise nor underwent lower extremity revascularization was + 0.01 ±0.16.
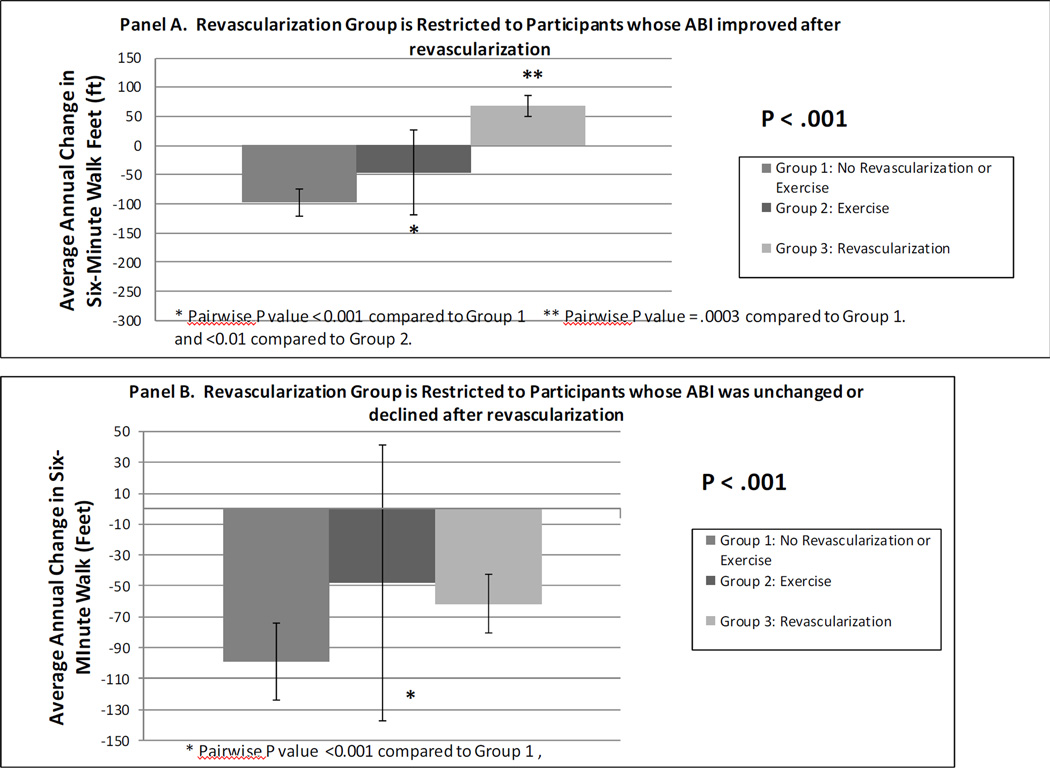
Overall, average annual declines in six-minute walk were −96.6 feet in Group 1 (no revascularization or exercise), −49.9 feet in Group 2 (self-directed walking exercise), and −32.6 feet in Group 3 (participant reported revascularization) (p<.001), adjusting for age, sex, race, prior year performance, baseline ABI, BMI, smoking, comorbidities, and leg symptoms. Among participants in Group 3, the correlation between change in ABI and change in six-minute walk after revascularization was 0.528 (P<0.001). In contrast, there was no association of change in ABI and change in six-minute walk among Group 2 (correlation coefficient=−0.017, P=0.716) or among Group 1 (correlation coefficient=−0.009, P=0.792). When analyses were repeated after restricting Group 3 to participants whose ABI improved by at least 0.15 after revascularization, Group 3 had greater improvement in the six-minute walk, compared to Group 1 (P=0.003) and Group 2 (P<0.001), respectively (Figure 1, Panel A). Participants engaging in regular walking exercise (Group 2) had less six-minute walk decline than participants in Group 1 (P=.0002) (Figure 1, Panel A).
Figure 1. Associations of walking exercise, lower extremity revascularization, and neither therapy with average annual change in six-minute walk performance.
Panel A. Revascularization group is restricted to participants whose ankle brachial index improved after revascularization.
Panel B. Revascularization group is restricted to participants whose ankle brachial index was unchanged or declined after revascularization.
When adjusted analyses were repeated after restricting Group 3 to PAD participants whose ABI did not improve by at least 0.15 following revascularization, adjusted annual decline in six-minute walk performance was greatest in Group 3 and least in Group 2 (P<.001) (Figure 1, Panel B). In pair-wise analyses, PAD participants who engaged in self-directed walking exercise (Group 2) had less six-minute walk decline than participants in Group 1 (P<.001) (Figure 1, Panel B).
Adjusting for age, sex, race, prior year performance, ABI, BMI, smoking, comorbidities, and leg symptoms, there were no significant differences in average annual change in the usual-paced or fastest paced four-meter walking velocity between Groups 1, 2, and 3 (Table 2). When analyses were repeated after restricting Group 3 to participants with ABI improvement of at least 0.15 after revascularization and to participants without an ABI improvement of at least 0.15 after revascularization, results were similar to those for the entire group (Table 2).
Table 2.
Adjusted Associations of Regular Self-Directed Walking Exercise and Lower Extremity Revascularization with Average Change in Walking velocity among Participants with Peripheral Arterial Disease a
| Group 1 Regular Self-Directed Walking Exercise |
Group 2 Lower Extremity Revascularization |
Group 3 No Revascularization or Exercise |
P Value | |
|---|---|---|---|---|
| Usual Pace Four Meter Walking Velocity (Meters/second) | ||||
| All Participants | −0.061 (−0.094 to −0.028) | −0.047 (−0.063 to −0.032) | −0.062 (−0.074 to −0.049) | 0.2164 |
| Group 2 restricted to participants with ABI improvement after revascularization. |
−0.059 (−0.124 to 0.006) | −0.049 (−0.065 to −0.032) | −0.063 (−0.077 to −0.050) | 0.2262 |
| Group 2 restricted to participants with no ABI improvement after revascularization. |
−0.050 (−0.090 to −0.009) | −0.049 (−0.064 to −0.033) | −0.063 (−0.076 to −0.051) | 0.1991 |
| Fastest Pace Four Meter Walking Velocity (Meters/second) | ||||
| All Participants | −0.071 (−0.117 to −0.025) | −0.072 (−0.094 to −0.051) | −0.094 (−0.112 to −0.076) | 0.1393 |
| Group 2 restricted to participants with ABI improvement after revascularization. |
−0.079 (−0.166 to 0.009) | −0.075 (−0.098 to 0.053) | −0.096 (−0.114 to −0.078) | 0.1968 |
| Group 2 restricted to participants with no ABI improvement after revascularization. |
−0.064 (−0.120 to −0.008) | −0.0741 (−0.096 to −0.052) | −0.097 (−0.115 to −0.079) | 0.0996 |
Analyses adjust for age, sex, race, prior year performance, ABI, BMI, smoking, comorbidities, and leg symptoms.
The difference between groups 2 and 3 is statistically significant (P=0.0375).
Of 61 revascularization procedures, 21 were classified as surgical and 27 were classified as endovascular procedures. Four procedures were a hybrid of endovascular and surgical revascularization. Medical record data were insufficient to classify procedure type for nine revascularizations (see Appendix). When analyses were repeated, limiting Group 3 to surgical revascularization procedures, average annual declines in six-minute walk were −98.6 feet in Group 3, −46.9 feet in Group 2 and −34.0 feet in Group 3. -, adjusting for age, sex, race, prior year performance, ABI, BMI, smoking, comorbidities, and leg symptoms (P<.001). When Group 3 was limited to endovascular procedures, overall average annual declines in six-minute walk were −98.7 feet in Group 1, −46.4 feet in Group 2, and −45.5 feet in Group 3 (P<.001).
Results for our primary analyses did not substantially differ when analyses were repeated using propensity scoring to adjust for differences in the three groups at baseline (data not shown). Our results did not substantially differ when analyses were repeated, imputing missing data for participants who did not complete follow-up testing (data not shown).
DISCUSSION
Few therapies are available for improving lower extremity functional impairment in men and women with PAD. Only two medications are FDA approved for treating PAD-related walking impairment, and one is no better than placebo (14,15). Although supervised treadmill walking exercise improves functional performance in PAD (16,17), medical insurance typically does not pay for supervised treadmill exercise for patients with PAD. Thus, most PAD patients do not participate (18). Clinical trials comparing lower extremity revascularization head-to-head with walking exercise are relatively few in number, are limited to those meeting eligibility criteria, and have yielded inconsistent results (19–22). Real-life data on actual changes in lower extremity functioning after lower extremity revascularization among PAD participants not enrolled in randomized controlled clinical trials are lacking.
Results reported here demonstrate that 41% of revascularization procedures were not associated with at least a 0.15 unit improvement in ABI in the ipsilateral leg at the study visit immediately following revascularization. PAD participants with revascularization that was associated with ABI improvement of at least 0.15 had significantly greater improvement in six-minute walk performance compared to PAD participants who engaged in regular self-directed walking exercise (Group 2) and as compared to PAD participants who engaged in neither therapy (Group 1). However, PAD participants with revascularization that was not associated with ABI improvement of at least 0.15 had no significant difference in average annual change in six-minute walk, compared to Group 1. PAD participants who engaged in regular self-directed walking exercise (Group 2) had less decline in the six-minute walk compared to PAD participants who engaged in neither therapy (Group 1). Together, these findings were consistent with our a priori hypotheses. The high proportion of PAD participants whose ABI did not improve by at least 0.15 following revascularization was unexpected. It is conceivable that these participants may have had rapid ABI declines that was not captured by the annual study visits but led to immediate revascularization. It is also possible that initial improvement in the ABI following revascularization was followed by an ABI decline prior to the next annual study visit.
Our study has limitations. First, the observational study design prevents definitive conclusions, since selection bias is likely to influence treatment decisions in PAD. Second, deterioration in functional performance or the ABI that occurred after an annual WALCS visit but immediately before revascularization were not measured. Third, we do not have data on indications for lower extremity revascularization. Fourth, our data, collected between 1998 and 2004, may be less applicable to lower extremity revascularization procedures performed currently. Fifth, we did not collect data on the technical success of the operation, measured by change in the ABI immediately after revascularization. Sixth, our results regarding self-directed walking exercise may be less favorable than what might be achieved in supervised treadmill walking exercise (23).
In conclusion, our data suggest that the benefits of lower extremity revascularization in patients with PAD may be closely tied to improvements in the ABI after revascularization. Our results support the benefits of self-directed exercise.
ACKNOWLEDGEMENTS
Kiang Liu, PhD of Northwestern University Feinberg School of Medicine and Luigi Ferrucci MD, PhD made important contributions to this work and to the manuscript.
Funding: Supported by #R01-HL58099, R01-HL64739, R01-HL071223, R01-HL076298, and R01-HL083064 from the NHLBI and by grant #RR-00048 from the National Center for Research Resources, NIH. Supported in part by the Intramural Research Program, NIA, NIH.
Role of Authors: All authors had access to the data and a role in writing the manuscript.
Appendix. Types of Lower Extremity Revascularlization Procedures
|
Procedure |
Number of Procedures |
|---|---|
| Endovascular Procedures | |
| Angioplasty-not otherwise specified. | 5 |
| Angioplasty + stent-not otherwise specified | 8 |
| Femoral angioplasty | 1 |
| Femoral angioplasty and stent | 4 |
| Iliac angioplasty | 3 |
| Iliac angioplasty with stent. | 7 |
| Aortic stent | 2 |
| Angioplasty with endarterectomy | 1 |
| Surgical Revascularization Procedures | |
| Bypass surgery-not otherwise specified. | 5 |
| Anterior-tibial bypass surgery. | 1 |
| Aorto-femoral bypass surgery | 2 |
| Axillary-femoral bypass surgery | 2 |
| Femoro-popliteal bypass surgery | 8 |
| Femoral bypass surgery, not otherwise specified. | 1 |
| Ilial-femoral bypass surgery | 1 |
| Popliteal to posterior tibial bypass surgery. | 1 |
| Bypass to anterior tibial artery. | 1 |
| Femoro-tibial bypass | 1 |
| Femoro-femoro bypass surgery with endarterterectomy. |
1 |
| Femoro-femoral bypass graft | 1 |
| Other revascularizations | 2 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None of the authors has any conflicts of interest.
REFERENCES
- 1.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286(13):1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292(4):453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136(12):873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 4.Egorova NN, Guillerme S, Gelijns A, Morrissey N, Dayal R, McKinsey JF, et al. An analysis of the outcomes of a decade of experience with lower extremity revascularization including limb salvage, lengths of stay, and safety. J Vasc Surg. 2010;51(4):878–885. doi: 10.1016/j.jvs.2009.10.102. [DOI] [PubMed] [Google Scholar]
- 5. [Accessed 5/20/12]; http://www.iom.edu/~/media/Files/Report%20Files/2009/ComparativeEffectivenessResearchPri orities/CER%20report%20brief%2008-13-09.pdf.
- 6.McDermott MM, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, et al. The lower ankle brachial index calculated by averaging the dorsalis pedis and posterior tibial arterial pressures is most closely associated with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 7.Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Gamst AC, et al. Subclavian artery stenosis: Prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. The Women’s Health and Aging Study: Health and social characteristics of older women with disability. Bethesda, MD: National Institute on Aging; 1995. NIH publication No. 95-4009, Appendix E. [Google Scholar]
- 9.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 10.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. for the American College of Rheumatology. Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–1049. [Google Scholar]
- 11.McDermott MM, Liu K, Ferrucci L, Criqui MH, Greenland P, Guralnik JM, et al. Ann Intern Med. 2006;144:10–20. doi: 10.7326/0003-4819-144-1-200601030-00005. [DOI] [PubMed] [Google Scholar]
- 12.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 13.Garg PK, Liu K, Tian L, Ferruci L, Criqui MH, Tan J, et al. Physical activity during daily life and functional decline in peripheral arterial disease. Circulation. 2009;119:251–260. doi: 10.1161/CIRCULATIONAHA.108.791491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson DL, Cutler BS, Meissner MH, Strandness DEJ. Cilostazol has beneficial effects in treatment of intermittent claudication: Results from a multi-center, randomized, prospective, double-blind trial. Circulation. 1998;98:678–686. doi: 10.1161/01.cir.98.7.678. [DOI] [PubMed] [Google Scholar]
- 15.Girolami B, Bernardi E, Prins MH, Ten Cate JW, Hettiarachchi R, Prandoni P, et al. Treatment of intermittent claudication with physical training, smoking cessation, pentoxifylline, or nafronyl: A meta-analysis. Arch Intern Med. 1999;159:337–345. doi: 10.1001/archinte.159.4.337. [DOI] [PubMed] [Google Scholar]
- 16.McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301(2):165–174. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2008;4:CD000990. doi: 10.1002/14651858.CD000990.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Falcone RA, Hirsch AT, Regensteiner JG, Treat-Jacobson D, Williams MA, Hiatt WR, et al. Peripheral arterial disease rehabilitation: A review. Journal of Cardiopulmonary Rehabilitation. 2003;23:170–175. doi: 10.1097/00008483-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Spronk S, Bosch JL, Den Hoed PT, Veen HF, Pattynama PMT, Hunink MGM, et al. Intermittent claudication: clinical effectiveness of endovascular revascularization versus supervised hospitalbased exercise training--randomized controlled trial. Radiology. 2009;250:586–595. doi: 10.1148/radiol.2501080607. [DOI] [PubMed] [Google Scholar]
- 20.Mazari FAK, Gulati S, Rahman MN, Lee HL, Mehta TA, McCollum PT, et al. Early outcomes from a randomized, controlled trial of supervised exercise, angioplasty, and combined therapy in intermittent claudication. Ann Vasc Surg. 2010;24(1):69–79. doi: 10.1016/j.avsg.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Fowkes FG, Gillespie IN. Angioplasty (versus non surgical management) for intermittent claudication. Cochrane Database Syst Rev. 2000;2:CD000017. doi: 10.1002/14651858.CD000017. [DOI] [PubMed] [Google Scholar]
- 22.Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: Six-month outcome from the Claudicaton Exercise Versus Endoluminal Revascularization (CLEVER) Study. Circulation. 2012;125 doi: 10.1161/CIRCULATIONAHA.111.075770. 00-00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frans FA, Bipat S, Reekers JA, Legemate DA, Koelemay MJ. Systematic review of exercise training or percutaneous transluminal angioplasty for intermittent claudication. Br J Surg. 2012;99:16–28. doi: 10.1002/bjs.7656. [DOI] [PubMed] [Google Scholar]
- 24.Bendermacher BL, Willigendael EM, Teijink JA, Prins MH. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev. 2006;2:CD005263. doi: 10.1002/14651858.CD005263.pub2. [DOI] [PubMed] [Google Scholar]