Abstract
Objective
To identify differences in the post-exercise phosphocreatine (PCr) recovery, an index of mitochondrial function, in diabetic patients with and without lower extremity complications.
Research Methods and Design
We enrolled healthy control subjects and three groups of T2DM patients: patients without complications, with peripheral neuropathy and both peripheral neuropathy and peripheral arterial disease. We employed Magnetic Resonance Spectroscopic (MRS) measurements to perform continuous measurements of phosphorous metabolites (PCr and Pi) during a 3-minute graded exercise at the level of the posterior calf muscles (gastrocnemius and soleus muscles). Micro- and macrovascular reactivity measurements were also performed.
Results
The resting Pi/PCr ratio and PCr at baseline and the maximum reached during exercise was similar in all groups. The post-exercise time required for recovery of Pi/PCr ratio and PCr levels to resting levels, an assessment of mitochondrial oxidative phosphorylation, was significantly higher in the diabetic patients with neuropathy and those with both neuropathy and PAD (p <0.01 for both measurements). These two groups had also higher levels of TNFα, (p<0.01) and G-CSF (p <0.05). Multiple regression analysis showed that only G-CSF, OPG and TNFα were significant contributing factors in the variation of the Pi/PCr ratio recovery time. No associations were observed between micro- and macrovascular reactivity measurements and Pi/Pcr ration or Pcr recovery time.
Conclusions
Mitochondrial oxidative phosphorylation is impaired only in T2DM patients with neuropathy whether PAD is present or not and is associated with the increased proinflammatory state that was observed in these groups.
Keywords: Magnetic Resonance Spectroscopy, Mitochondrial Function, Inflammation
INTRODUCTION
Mitochondria are the site of oxidative substrate utilization that results in production of adenosine triphosphate (ATP). In the majority of cells of the human body, mitochondria represent the energy-generating organelle. Mitochondrial function depends on oxygen supply and conditions that cause either acute or chronic tissue hypoxia, such as peripheral arterial disease (PAD), also lead to impaired mitochondrial function 1–3. However, accumulating evidence suggests that the impaired mitochondrial function that is present in PAD is not exclusively related to oxygen supply but is also caused by intrinsic mitochondrial defects in the claudicating muscle that are similar to the ones seen in mitochondrial myopathies 4–6. In addition, mitochondrial dysfunction has been associated to the presence of diabetes, although causality is not clear 7–10. Finally, animal studies have suggested that mitochondrial dysfunction contributed to the development of diabetic neuropathy 11–13.
Phosphorus-31 Magnetic Resonance Spectroscopy (31P MRS) has been used for more than three decades to document the muscle energy reserves, metabolism and function. This technique is unique in its ability to study continuously and noninvasively the biochemical pathways for the supply and utilization of energy 14. Moreover, by measuring the post exercise rate of phosphocreatine (PCr) resynthesis, an almost pure oxidative process, 31P MRS is capable of detecting defects in mitochondrial oxidative phosphorylation, one of the most important functions of the mitochondria 15.
Currently there is little information available regarding changes in the muscle energy reserves and the mitochondrial oxidative phosphorylation in diabetic patients with or without lower extremity complications. The main aim of this study was to identify the contribution of diabetic peripheral neuropathy (DPN), mild PAD and inflammation in these parameters. Our primary hypothesis was that peripheral neuropathy would affect mitochondrial oxidative phosphorylation and the combination of both DPN and PAD would result in further compromise.
RESEARCH DESIGN AND METHODS
Subjects
All research subjects were recruited from ambulatory patients who attended the Joslin- Beth Israel Deaconess Foot Center, located at the Beth Israel–Deaconess Medical Center. We studied five groups of subjects age 40–80 years: the first group included healthy control subjects (C), the second Type 2 diabetic (T2DM) patients without PAD or DPN (DM), the third T2DM patients with DPN but no PAD (DM-DPN), the fourth T2DM patients with both DPN and PAD (DM-DPN-PAD) and the fifth Type 1 diabetic patients (T1DM) with lower extremity complications (DPN with or without PAD). PAD was diagnosed as: ABI 0.9–0.41 and in case of non-compressible vessels, Rutherford 0 and 1 PAD based on toe pressures and PVR and/or Doppler measurements performed at the vascular lab. Exclusion criteria were: 1.) Presence of a foot ulcer. 2.) Cardiovascular disease as demonstrated in only these instances: a. congestive Heart Failure with severe peripheral edema, b. TIA within the past 6 months, c. Stroke with residual neurological damage, 3.) Uncontrolled hypertension: SBP> 180 mmHg or DBP > 105 mmHg, 4.) Presence of any serious disease including end stage renal failure requiring dialysis or renal transplantation, active malignant disease requiring treatment, hepatic, hematologic, neurologic, or immune disease. 5.) Subject with known alcohol or drug abuse problems, 6.) Treatment with oral or parenteral corticosteroids, immunosuppressive or cytotoxic agents, 7.) Presence of known infectious disease (including HIV, Hepatitis B and C), 8.) Subject has any condition(s) which seriously compromises the patient’s ability to complete this study including frailty, 9.) Known history of myopathy and/or a CK level during the screening visit 3 times higher than the upper normal limit; 10.) Smoking/tobacco use. The study was approved by the IRB of the Beth Israel Deaconess Medical Center and the McLean Hospital.
Methods
All participants were recruited in consecutive order at the Joslin-Beth Israel Deaconess Foot Center over a period of 12 months. All participants first attended the Joslin-Beth Israel Deaconess Foot Center and underwent the same set of tests that included a full medical history, a complete physical examination, characterization of neuropathy, ABIs, vascular reactivity, and blood drawing for the evaluation of cytokines and insulin resistance measurement. They subsequently attended the Radiology Department of the McLean Hospital where the muscle energy reserve measurements were performed.
Characterization of the neuropathy
The symptoms of Diabetic Peripheral Neuropathy (DPN) were evaluated by using a Neuropathy Symptom Score (NSS), and the clinical signs by using a Neuropathy Disability Score (NDS). Quantitative Sensory Testing (QTS) included the assessment of Vibration Perception Threshold using a Biothesiometer and Cutaneous Perception Threshold using Semmes-Weinstein monofilaments. Details about the above tests have been described elsewhere 16. Diabetic neuropathic patients were required to have severe neuropathy (NDS >5) and to have clinical neuropathy up to the mild-calf level. This ensured that peripheral neuropathy was present at the level of muscle measurements.
Evaluation of the peripheral arterial disease
Peripheral Arterial Disease (PAD) was diagnosed according to the ACC/AHA Guidelines 17. Diabetic patients with mild peripheral vascular disease, based on the ankle brachial index (ABI) and/or the clinical features were recruited.
Assessment of vascular reactivity
Vascular reactivity at the microcirculation of the dorsum of the foot employing the iontophoresis of acetylcholine (ACh) and sodium nitroprusside (SNP) was evaluated using a Laser Doppler Iontophoresis (MIC1 iontophoresis system, Monitor Moor Instruments Ltd, Millwey, Devon, England) and a Laser Doppler Perfusion Imager (Lisca PIM 2.0, Lisca Development AB, Linkoping, Sweden) as previously described 18. Skin oxygenation was measured using the Medical Hyperspectral Imaging (MHSI) technique (HyperMed OxyView MHSI System, HyperMed, Inc., Watertown, MA). MHSI images were obtained from same site where vascular reactivity was measured. Data was analyzed offline using decomposition, image processing, and image registration techniques as previously described 18. Endothelium dependent (Flow Mediated Dilation, FMD) and independent (Nitroglycerin Induced Dilation, NID) vasodilation of the macrocirculation was assessed at the brachial artery with a 10.0 MHz linear array transducer (Aloka Prosound a7, Hitachi Aloka Medical Ltd, Tokyo, Japan), according to established guidelines 19.
MRS Measurements of Muscle Energy Reserves [Phosphorus (31P) Metabolites]
The participants attended the MR Imaging Center of the McLean Hospital for the phosphorus (31P) spectroscopic measurements. All subjects were asked to be fasting and advised to avoid strenuous exercise for 24 hours before the testing. The subjects rested in a warm room for 30 minutes prior to the testing. Phosphorus-31 MRS data was acquired on a Siemens 3T whole body MR scanner (TIM Trio, Siemens AG, Germany). Subjects were placed supine on a MR compatible exercise bed. One leg, the one identified to have PAD according to the inclusion criteria, was restrained at the ankle and placed on a foot pedal mounted to an isotonic ergometer 1. A 1H/31P double tuned surface radio frequency (RF) coil designed for this study was fastened to the underbelly of the calf muscle. Prior to the 31P data acquisition, shimming was performed with proton signal to achieve a better homogeneity of the static field and proton (1H) spin-echo images were acquire for detailed images of the anatomy. The muscle exercise was performed using the isotonic ergometer placed in the MRI scanner, as previously described 1. The 31P metabolite spectra were acquired kinetically with 4 signal averages per spectrum within 6 seconds. Initially, twenty baseline spectra were collected with the subject resting. Subjects were then asked to push against the pedal to isometrically exercise the posterior compartment muscles for three minutes and then relaxed for approximately fifteen minutes. The amount of the force applied on the pedal was recorded and averaged over the three minute exercise period and was used to evaluate the working capacity of the calf muscles. Phosphorus-31 MRS data was acquired at a temporal resolution of 6 sections during resting and exercise. Pi and PCr information was extracted from the data as previously published by our unit 18, 20.
Cytokine measurements
The cytokines and growth factors were measured using a Luminex 200 apparatus (Luminex, Austin, TX) and Millipore multiplex immunoassay panels (Millipore, Chicago, IL). Serum specimens were obtained during resting and were stored in a −80 °C until they were all analyzed together during the first thaw at the end of the study. In order to test the reliability of the multiplex assay measurements, we measured insulin levels using our standard hospital lab and the multiplex assay. We found a very satisfactory correlation (r = 0.9). Using a 39-plex platform, we performed two repeated measurements on the same samples and we noticed very satisfactory correlations. The intra- and interassay coefficient variation of the performed cytokine measurements, as provided by the manufacturer (Millipore) is similar to measurements performed using standard ELISA techniques in our unit 21.
Insulin Resistance Measurements
Insulin resistance was assessed by using the homeostasis model assessment (HOMA), which is based on a mathematical correlation of fasting plasma glucose and insulin levels 22. Patients who were treated with insulin were excluded and as a result, measurements were available in the healthy control subjects, diabetic group with no complications and the diabetic group with neuropathy.
Data analysis
The analysis was undertaken by univariate techniques and modeling the data through multiple linear regression using the Minitab statistical package (State College, PA). For normally distributed data, the analysis of variance procedure was used, followed by the Fisher’s post hoc multiple comparison tests to identify differences among the various groups. For nonparametrically distributed data, the Kruskal - Wallis test was used. Multiple regression analysis was used to assess significant contributing factors in the variation of the Pi/PCr ratio and PCr recovery time between the four study groups. The Pearson correlation coefficient r was used for the correlation of different parameters in the same group.
RESULTS
The comparisons of the demographics and of the vascular reactivity results are summarized in Table 1. Flow Mediated Dilation (FMD) was lower in all three diabetic groups when compared to the healthy controls and further reduced in the groups with neuropathy and PAD when compared to the diabetic group without complications (p <0.05). As expected, a higher number of diabetic patients were treated with medications that can affect endothelial function such as antihypertensives (ACE inhibitors and ARBs), and statins when compared to the healthy controls. ABI was measurable in six patients with peripheral neuropathy and PAD (0.86±0.1, mean ± sd). The remaining four subjects had already diagnosed at the vascular lab before their recruitment in the study. All of them had tibial occlusive disease while on subject also had sub-critical iliac stenosis. Eight subjects had Rutherford 0 and two Rutherford 1 PAD.
Table 1.
Clinical Characteristics and Cytokines Results.
| Controls (C) | T2DM Patients, With No Complications (DM) | T2DM Patients, With Neuropathy (DM-PDN) | T2DM Patients, With Neuropathy-PAD (DM-PDN-PAD) | |
|---|---|---|---|---|
| No | 14 | 11 | 10 | 7 |
| Age (years) | 55 ± 11 | 63 ± 9 | 63 ± 10 | 66 ± 7 |
| Males (%) | 8 (57) | 6 (55) | 4 (40) | 5 (71) |
| DM type 2 (%) | - | 11 (100) | 10 (100) | 7 (100) |
| DM duration (years) | - | 12 ± 8 | 20 ± 14 | 23 ± 10 |
| BMI (kg.m−2) | 26.9 ± 6.4 | 30.7 ± 4.5 | 32.7 ± 8.7 | 32.2 ± 4.6 |
| Glucose a | 89 ± 12 | 119 ± 23 | 118 ± 48 | 158 ± 61 |
| HbA1c b | 5.8 ± 0.5 | 7.1 ± 0.7 | 6.7 ± 0.9 | 8.2 ± 1.2 |
| Creatinine (mg/dl) c | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.2 ± 0.5 | 1.3 ± 0.4 |
| Neuropathy Symptom Score (NSS) d | 0 ± 0 | 3 ± 3 | 5 ± 5 | 5 ± 5 |
| Neuropathy Disability Score (NDS) e | 0 ± 0 | 1 ± 1 | 11 ± 5 | 16 ± 5 |
| Vibration Perception Threshold (VPT, volts) f | 8 ± 4 | 19 ± 9 | 39 ± 15 | 39 ± 16 |
| Semmes-Weinstein Monofilaments g | 3.88 ± 0.14 | 4.29 ± 0.52 | 6.11 ± 1.45 | 6.47 ± 0.80 |
| Flow Mediated Dilation (FMD) (%) h | 6.9 ± 1.3 | 5.1 ± 1.4 | 4.6 ± 2.1 | 3.2 ± 1.3 |
| Nitroglycerin Induced Dilation (NID) (%) | 13.1 ± 5.2 | 11.5 ± 3.7 | 11.4 ±1.7 | 9.3 ± 6.7 |
| Resting Foot Skin blood Flow (arbitrary units) | 0.87 ± 0.15 | 0.88 ± 0.21 | 0.83 ± 0.17 | 0.75 ± 0.16 |
| Foot Oxyhemoglobin (arbitrary units) | 37 ± 15 | 38 ± 21 | 39 ± 21 | 30 ± 17 |
| Foot Deoxyhemoglobin (arbitrary units) | 45 ± 13 | 54 ± 12 | 50 ± 16 | 47 ± 10 |
| Statin Treatment % | 2 (14) | 9 (82) | 7 (70) | 6 (86) |
| Antihypertensives ACE or ARB % | 1 (7) | 6 (55) | 7 (70) | 2 (29) |
| Oral Hypoglycemic Agent Treatment (%) | 0 (0) | 7 (64) | 6 (60) | 2 (29) |
| Insulin Treatment % | 0 (0) | 2 (18) | 4 (40) | 6 (86) |
| TGFa (pg/ml) | 6.62 (2.77:9.47) | 6.24 (4.06:8.34) | 4.69 (2.37:10.79) | 11.0 (5.06:13.50) |
| VEGF (pg/ml) | 157 (64:268) | 136 (75:209) | 136 (33:262) | 232 (174:267) |
| INSULIN (pg/ml) i | 255 (112:622) | 343 (216:737) | 970 (255:1521) | 2358 (710:6006) |
| OPG (pg/ml) j | 617 (390: 1442) | 573 (440:713) | 726 (494:2550) | 1820 (800:3334) |
| OPN(ng/ml) | 26.1 (18.4:39.3) | 19.3 (11.2:23.2) | 31.2 (13.6:68.2) | 38.2 (20.6:70.3) |
| G-CSF (pg/ml) k | 18.9 (13.2:31.5) | 23.1 (17.4:32.0) | 35.8 (25.5:40.4) | 58.4 (40.8:119.0) |
| MCP-1 (pg/ml)l | 343 (208:437) | 575 (450:706) | 414 (284:558) | 516 (298:760) |
| TNFa (pg/ml) m | 4.41 (3.69:8.10) | 6.02 (4.97:7.79) | 9.11 (7.05:13.68) | 9.95 (8.99:28.10) |
| CRP (μg/ml) | 5.53 (1.35: 11.97) | 2.13 (1.08:4.04) | 14.30 (3.59:40.10) | 10.91 (6.29:24.94) |
Data are Mean ± SD or Median, (first : third quartiles)
C vs DM-PDN-PAD p <0.05
C vs DM, DM- PDN, DM-PDN-PAD; DM, DM- PDN vs DM-PDN-PAD: p<0.0001
C, DM vs DM-PDN-PAD p <0.05
C vs DM- PDN, DM-PDN-PAD p <0.05
C, DM vs DM- PDN, DM-PDN-PAD; DM- PDN vs DM-PDN-PAD, p <0.0001
C vs DM, DM- PDN, DM-PDN-PAD; DM, vs DM- PDN, DM-PDN-PAD: p <0.0001
C, DM vs DM, DM- PDN, DM-PDN-PAD: p <0.0001
C vs DM, DM- PDN, DM-PDN-PAD; DM vs DM-PDN-PAD: p <0.0001
C, DM vs DM-PDN-PAD: p <0.005
C, DM vs DM-PDN-PAD: p <0.05
C vs DM- PDN, DM-PDN-PAD; DM, DM- PDN vs DM-PDN-PAD: p<0.01
C vs DM, DM-PDN-PAD; DM vs DM-PDN: p<0.01
C, DM vs DM-PDN, DM-PDN-PAD p <0.01
DM vs DM-PDN, DM-PDN-PAD: p <0.05
The results from the measurements of the various growth factors, cytokines and cellular adhesion molecules are shown in Table 1. Tumor Necrosis factor (TNFα) was higher in both diabetic groups with complications when compared to the healthy controls and to the diabetic group without complications (p <0.01). The Granulocyte-Colony Stimulating Factor (G-CSF) was higher in the two diabetic groups with complications when compared to the healthy controls, whereas the diabetic group with neuropathy and PAD had higher levels when compared to the other two diabetic groups (p <0.05). Osteoprotegerin (OPG) was higher in the diabetic group with neuropathy and PAD when compared to the healthy controls and to the diabetic group without complications (p<0.01). Monocyte Chemoattractant Protein-1 (MCP-1) was higher in the diabetic patients without complications and the group with neuropathy and PAD when compared to healthy controls, and in the diabetic group without complications when compared to the diabetic group with neuropathy (p <0.01). CRP was also higher in the two groups with complications when compared to the diabetic group without complication (p<0.05). Insulin resistance, assessed by the HOMA model, was higher in the group with neuropathy [6.68 (1.90 – 14.72) (first, third quartiles), 6 patients] when compared to the healthy controls [1.28 (0.52 – 3.61), p <0.05]. However, no differences existed between the diabetic group without complications [4.21 (1.67 – 6.43), 9 patients] and the control subjects or the diabetic patients with neuropathy.
The muscle metabolism measurements, Pi/PCr ratio and PCr, are summarized in Table 2. There was no difference within groups for Pi/PCr levels at baseline, maximum level reached during exercise and post exercise levels. The time to Pi/PCr post exercise recovery was higher in the two diabetic groups with complications when compared to both the healthy controls and the diabetic group without complications (p<0.01) (Figure 1). Similar results were also observed when PCr levels were evaluated. Thus, no differences existed among all four groups at the PCr levels at baseline, minimum reached level during exercise and post exercise levels. However, the time to PCr post exercise recovery was higher in the diabetic groups with complications when compared to the other two groups (p <0.01).
Table 2.
Pi/PCr Ratio and PCr MRS Measurements
| Controls (C) | T2DM Patients, With No Complications (DM) | T2DM Patients, With Neuropathy (DM-PDN) | T2DM Patients, With Neuropathy-PAD (DM-PDN-PAD) | |
|---|---|---|---|---|
| Average Applied Force During Exercise | 15.8 ± 4.4 | 14.4 ± 5.9 | 13.4 ± 4.6 | 12.7 ± 4.0 |
| Resting Pi/PCr Ratio Before Exercise | 0.14 ± 0.04 | 0.14 ± 0.03 | 0.13 ± 0.04 | 0.12 ± 0.05 |
| Maximal Pi/PCr Ratio During Exercise | 0.50 ± 0.27 | 0.49 ± 0.25 | 0.57 ± 0.27 | 0.55 ± 0.24 |
| Resting Post-Exercise Pi/PCr Ratio | 0.13 ± 0.05 | 0.13 ± 0.03 | 0.12 ± 0.03 | 0.11 ± 0.06 |
| Pi/PCr Ratio Area Under the Curve (AUC) | 7.18 ± 6.30 | 7.19 ± 5.8 | 9.4 ± 5.6 | 9.4 ± 5.1 |
| Post-exercise Recovery Time for Pi/PCr Ratio (sec) a | 58 ± 25 | 61 ± 15 | 85 ± 27 | 90 ± 24 |
| Resting PCr Level Before Exercise | 41488 ± 14907 | 48863 ± 25069 | 44214 ± 16518 | 50608 ± 25193 |
| Minimal PCr Level During Exercise | 22313 ± 7687 | 30473 ± 17707 | 28211 ± 13940 | 27268 ± 18091 |
| Resting Post-Exercise PCr Level | 41704 ± 14895 | 48508 ± 24591 | 46218 ± 17520 | 50632 ± 25646 |
| PCr Area Under the Curve | 531 ± 378 | 511 ± 547 | 501 ± 312 | 723 ±355 |
| Post-exercise Recovery Time for PCr Level (sec) b | 87 ± 42 | 76 ± 32 | 121 ± 33 | 121 ± 15 |
Mean ± SD
C, DM vs DM-PDN, DM-PDN-PAD: p <0.01
Figure 1.
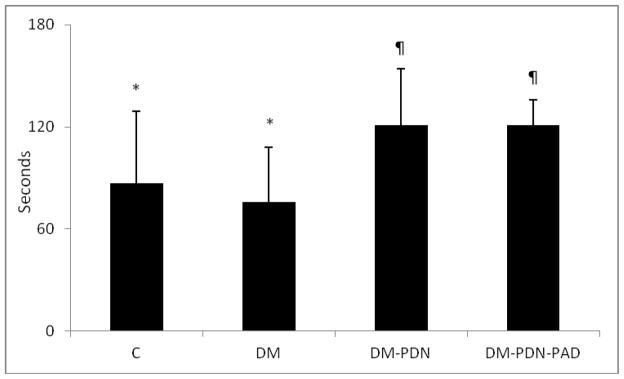
Time to PCr post exercise recovery was increased in T2DM patients with peripheral neuropathy (DM-DPN) and T2DM patients with peripheral neuropathy and PAD (DM-DPN-PAD) when compared to the healthy subjects (C) and T2DM patients without complications (DM), (* vs ¶: p <0.01).
When all subjects were studied as one group, significant correlations were observed between the Pi/PCr ratio recovery time and age (r=0.33, p<0.05) NDS (r=0.51, p<0.001), OPG (r=0.59, p <0.0001) and marginally with TNFα (r=0.30, p=0.059). Multiple regression analysis showed that after adjusting for age, BMI, gender and group participation of study subjects, only G-CSF, OPG and TNFα were significant contributing factors in the variation of the Pi/PCr ratio recovery time. The PCr recovery time correlated with age (r=0.35, p<0.05), NDS (r=0.50, p<0.001), OPG (r=0.41, p <0.01) and OPN (r=0.41, p <0.01). A second multiple regression model showed that after adjusting for age, BMI, gender and group participation there was no significant contributing factor in the variation of the Pi/PCR ratio or PCr ratio recovery times. Finally, no correlations were observed between insulin resistance, assessed by the HOMA model, and the Pi/PCR ratio or PCr recovery times.
We also studied seven T1DM patients with lower extremity complications, four with neuropathy alone and three with both neuropathy and PAD. As in the case of T2DM patients, there were no difference between patients with neuropathy alone and patients with both neuropathy and PAD (data not shown) and they were all considered as one group. The comparisons with the control group, the T2DM patients with lower extremity complications are shown in Table 3.
Table 3.
Comparison between T1DM and T2DM patients
| Controls | T2DM Patients, With No Complications (T2DM) | T2DM Patients, With complications (T2DM-Compl) | T1DM Patients, With complications (T1DM-Compl) | |
|---|---|---|---|---|
| No | 14 | 11 | 17 | 7 |
| Age (years) | 55 ± 11 | 63 ± 9 | 64 ± 9 | 57 ± 9 |
| Males (%) | 8 (57) | 6 (55) | 9 (53) | 6 (86) |
| DM duration (years) a | - | 12 ± 8 | 21 ± 13 | 39 ± 17 |
| BMI (kg.m−2) | 26.9 ± 6.4 | 30.7 ± 4.5 | 32.5 ± 7.1 | 28.4 ± 6.0 |
| HbA1c b | 5.8 ± 0.5 | 7.1 ± 0.7 | 7.3 ± 1.2 | 8.0 ± 0.8 |
| OPG (pg/ml) c | 617 (390:1442) | 573 (440:713) | 887 (686:2863) | 1435 (870:2388) |
| OPN (ng/ml) d | 26.1 (18.4:39.3) | 19.3 (11.3:23.2) | 33.4 (17.7:67.7) | 60.4 (38.3:69.6) |
| G-CSF (pg/ml) e | 18.9 (13.2:31.5) | 23.1 (17.4:32.0) | 38.2 (28.2:54.3) | 20.8 (14.5:34.8) |
| MCP-1 (pg/ml) f | 343 (208:437) | 575 (450:706) | 435 (298:602) | 370 (297:409) |
| TNFa (pg/ml) g | 4.41 (3.69:8.10) | 6.02 (4.97:7.79) | 9.95 (7.78:15.89) | 9.92 (5.23:18.7) |
| CRP (μg/ml) | 5.53 (1.35: 11.97) | 2.13 (1.08:4.04) | 10.91 (4.54:33.94) | 2.59 (1.59:19.03) |
| Average Applied Force | 15.8 ± 4.4 | 14.4 ± 5.9 | 13.1 ± 4.3 | 11.9 ± 3.2 |
| Pi/PCr Ratio Resting levels | 0.14 ± 0.04 | 0.14 ± 0.03 | 0.13 ± 0.05 | 0.12 ± 0.03 |
| Pi/PCr Ratio Exercise Maximal Level | 0.50 ± 0.27 | 0.49 ± 0.25 | 0.56 ± 0.25 | 0.49 ± 0.25 |
| Pi/PCr Ratio Post-Exercise Level | 0.13 ± 0.05 | 0.13 ± 0.03 | 0.12 ± 0.04 | 0.11 ± 0.04 |
| Pi/PCr Ratio Post-exercise Recovery Time (sec) h | 58 ± 25 | 61 ± 15 | 87 ± 25 | 86 ± 13 |
| PCr Resting level | 41488 ± 14907 | 48863 ± 25069 | 46846 ± 20050 | 35570 ± 19243 |
| PCr Exercise Minimal Level | 22313 ± 7687 | 30473 ± 17707 | 27822 ± 15240 | 24214 ± 17190 |
| PCr Post-Exercise Level | 41704 ± 14895 | 48508 ± 24591 | 48036 ± 20599 | 36484 ± 19022 |
| PCr Post-exercise Recovery Time (sec) i | 87 ± 42 | 76 ± 32 | 121 ± 27 | 104 ± 16 |
Data are Mean ± SD
T1DM-Compl vs T2DM, T2DM-Compl: p<0.001
C vs T2DM, T2DM-Compl, T1DM-Compl; T2DM vs T1DM-Compl: p<0.0001
C vs T1DM-Compl; T2DM vs T2DM-Compl, T1DM-Compl: p<0.02
C vs T1DM-Compl; T2DM vs T2DM-Compl, T1DM-Compl: p<0.01
C, T2DM, T1DM-Compl vs T2DM-Compl: p<0.01
C vs T2DM: p<0.02
C vs T2DM-Compl, T1DM-Compl; T2DM vs T2DM-Compl: p<0.01
C, T2DM vs T2DM-Compl, T1DM-Compl: p<0.001
C, T2DM vs T2DM-Compl: p<0.01
DISCUSSION
The main findings of the present study are that the post-exercise time of recovery of the Pi/PCr ratio and PCr levels, a measurement of mitochondrial oxidative phosphorylation, was equally present in T2DM patients with peripheral neuropathy and patients with both peripheral neuropathy and mild PAD. In contrast, no differences were observed between the healthy controls and type 2 diabetic subjects without long-term complications. In addition, the two diabetic groups with complications had increased inflammatory cytokines and the observed increases were strongly associated with the observed mitochondrial dysfunction.
PAD has been shown to affect mitochondrial oxidative phosphorylation assessed by MRS but these abnormalities are uncoupled from tissue perfusion suggesting an intrinsic mitochondrial problem 1, 6. In the present study, we first reported that T2DM patients with peripheral neuropathy in the absence of PAD had similar impairment with those with both peripheral neuropathy and mild PAD. In addition, no associations were observed between mitochondrial oxidative phosphorylation and the skin blood flow and oxygenation. No associations also existed with the endothelium dependent vasodilation in the macrocirculation, assessed by flow mediated vasodilation (FMD), which, as would be expected from previous studies, was impaired in all three diabetic groups 23. These data are in agreement with the previously observed uncoupling between tissue perfusion and mitochondrial oxidative phosphorylation and indicate that impaired mitochondrial oxidative phosphorylation is not associated with changes in vascular reactivity 6.
The causality in the observed association between mitochondrial oxidative phosphorylation and peripheral neuropathy is not clear. One possible explanation can be the well known direct effects of neuropathy on muscle function that leads to muscle atrophy and, therefore, mitochondrial oxidative phosphorylation. Animal studies have indicated that dorsal root ganglia (DRG) neuron mitochondrial dysfunction and imbalance between mitochondrial biogenesis and fission are involved in the development of sensory neuropathy 11–13. However, there are no previous human studies that have specifically explored this issue. Human studies have also shown that different mitochondrial haplogroups are significantly associated with an increased risk of specific diabetes complications and that the H3 haplogroup is specifically associated with the development of neuropathy 24. Therefore, the possibility that impaired mitochondrial function in various tissues and cells, such as muscle and neurons, is the primary event that causes diabetes complications cannot be excluded and needs further investigation. If this hypothesis proves correct, it can lead to new therapeutic approaches for the management of diabetic lower extremity problems.
Previous studies that have employed this technique have reported impaired mitochondrial oxidative phosphorylation in the vastus lateralis muscle at the thigh of subjects with extreme insulin resistance and type 2 diabetic patients 7–9. However, another study reported no impairment at the same muscle in patients with short or long duration of T2DM without any serious diabetic complications and subjects with pre-diabetes 10. In the present study, no difference was also observed between the diabetic patients without complications, all of whom had type 2 diabetes of long duration, and the healthy controls. However, the inflammatory status of both groups was similar while it was considerably increased in the two groups with complications, which also had impaired mitochondrial oxidative phosphorylation. In addition, TNFα, G-CSF and osteoprotegerin (OPG) were the only independent variables associated with the post-exercise time of recovery for the Pi/PCr ratio. These results indicate that the diabetes complications-related proinflammatory state was the main factor that may have influenced mitochondrial oxidative phosphorylation. To our knowledge, there are no previous studies that have directly examined the interaction between inflammation and mitochondrial function in the past. However, previous studies have indicated that local inflammation, mainly due to infiltration by activated macrophages, impairs oxidative phosphorylation through PPARγ signaling 25.
The finding of the present study regarding the lack of pro-inflammatory status in the diabetic patients without complications despite the fact that they all had type 2 diabetes for an average period of twelve years is intriguing. Similar results, namely lack of a pro-inflammatory status in diabetic patients without complications and long duration of diabetes, while it is present in patients with peripheral neuropathy has also been observed in a previous study in our unit that employed similar techniques and included 212 subjects 26. Elevated serum TNFα levels have also been found in another study to be associated with decreased renal function in type 1 DM patients without proteinuria 27. These findings raise the hypothesis that the coexistence of diabetes and proinflammatory state leads to the development of mitochondrial oxidative phosphorylation and long term complications while in the absence of inflammation no complications develop. The factors that are associated to the high inflammatory in the patients with complication are not clear and may be related to genetic factors, diet, life style and environmental. Further studies will be required explore this hypothesis that can lead to targeting inflammation for the management of diabetic complications.
Osteoprotegerin, a member of the tumor necrosis factor receptor superfamily 11B that acts as a decoy receptor activator of nuclear factor kappaB ligand (RANKL) and osteopontin were initially thought to be mainly involved in bone remodeling. However, recent studies have indicated that these cytokines are also involved in muscle function. Thus, osteoprotegerin has been associated with left ventricular hypertrophy and the development of coronary artery disease 28, 29. Osteopontin has been reported to play a role in the pro-inflammatory state and fibrosis that is present in dystrophic mouse muscle and is increased in muscle biopsies form subjects with Duchenne muscular dystrophy 30. In addition, osteopontin is greatly upregulated in regenerating muscle and promotes macrophage infiltration in necrotic muscle 31. The present study is the first to describe an association between these two cytokines and impaired mitochondrial oxidative phosphorylation in diabetic patients with neuropathy. Further studies will be required to establish the mechanisms that are involved and whether manipulation of the expression of these cytokines can have an effect on muscle mitochondrial function.
Previous studies in our unit have shown foot muscle atrophy and reduced PCr levels in diabetic patients with diabetic neuropathy and increased Pi/PCr ratio at resting conditions in both non-neuropathic and neuropathic diabetic patients 18, 20, 32. In the present study, no differences were observed at resting conditions among the four tested groups and this finding is in agreement with previous studies that included patients with PAD 1, 6. The main reason for this difference between the foot and calf muscles is probably the fact that neuropathy and severe tissue hypoxia mainly affect the foot muscles, causing considerably more severe muscle atrophy and dysfunction. However, no studies are available regarding the mitochondrial oxidative phosphorylation of the foot muscles. No differences in the resting levels of PCr of healthy controls and type 2 diabetes have also been previously reported 8 while a study that included insulin-resistant offspring of patients with type 2 diabetes reported a 20% reduction in the Pi/PCr ratio when compared to healthy controls 33. It is not clear whether this reduction in the Pi/PCr ratio is restricted in the prediabetic state and what factors may be associated with this finding.
In the present study, we evaluated both the Pi/PCr ratio and the PCr levels changes during exercise. The main reason for this was that our previous studies that involved foot muscles showed a reduction in resting total 31P concentration, which is mainly comprised of PCr, due to muscle atrophy 18, 20, 32. The fact that both Pi/PCr ratio and the PCr measurements showed similar results, especially regarding the changes in post-exercise recovery, clearly strengthens the validity of our results. Of note, the Pi/PCr ratio post-exercise recovery time tented to be shorter than the PCr one in all groups. This finding is compatible with the finding of previous studies that have indicated different kinetics between Pi and PCr and have attributed it to a Pi “undershoot” due to its intracellular redistribution into different compartments 34, 35.
The HOMA model was employed to measure insulin resistance and as a result, these measurements were restricted in patients not treated with insulin. Nonetheless, our results indicate that there were no differences between diabetic patients without complications and with peripheral neuropathy. In addition, no correlations were observed between insulin resistance and the Pi/PCR ratio and PCr recovery times. Furthermore, we also included a group of T1DM with similar demographics and peripheral neuropathy with or without PAD and we observed no differences between T1DM and T2DM regarding their mitochondrial oxidative phosphorylation. These results strongly indicate that the type of diabetes, and therefore the presence and severity of insulin resistance, do not affect mitochondrial oxidative phosphorylation once lower extremity complications are present. Our findings are also in agreement with previous studies that have reported impaired mitochondrial function in T1DM 36. It is also of interest that both T1DM and T2DM patients with lower extremity complications had similar increases in osteoprotegerin, osteopontin and TNFα, another indication of the association of these cytokines to the mitochondrial oxidative phosphorylation.
The current study has its limitations. Firstly, although the groups were matched for age, the control group was slightly younger. However, it would be expected that this could have resulted in better mitochondrial oxidative phosphorylation when compared to the DM patients without complication and the lack of such a finding, if anything, strengthens the conclusion that there are no differences between these two groups. Furthermore, the most interesting finding of the present study is the observed differences between the group with T2DM without complications and the two T2DM groups with complications. The fact that all three groups were matched for all important parameters that could affect the results, such as treatment with statins, antihypertensives and insulin, further validates our findings.
Another limitation is that no patients with diabetes and PAD but without neuropathy were investigated. The main reason for this is that it is almost impossible to identify such patients as neuropathy usually precedes the development of PAD. However, the fact that patients with neuropathy alone and both neuropathy and PAD had similar impairment of the mitochondrial oxidative phosphorylation and proinflammatory state indicate that the two conditions do not have any additive effects. Furthermore, previous studies that have studied patients with PAD included both diabetic and non-diabetic patients and did not find any association between diabetes and PCr recovery time 6, 37.
In summary, our results indicate that that mitochondrial oxidative phosphorylation is impaired only in diabetic patients with neuropathy with or without PAD and is associated with the increased proinflammatory state that was observed in these groups. Further studies will be required to examine the role of inflammation in the development of impaired mitochondrial oxidative phosphorylation and lower extremity complications.
Acknowledgments
This work in part is funded by NIH 1R21DK082987 to A. Veves. CSZ is supported in part by NIH R21MH081076. The project described was supported by the Clinical Translational Science Award UL1RR025758 to Harvard University and Beth Israel Deaconess Medical Center from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institute of Health.
Footnotes
DUALITY OF INTERESTS: None
AUTHORS CONTRIBUTION
AV was responsible for the study concept, design and initial data analysis. FT and CS performed all vascular reactivity evaluations and cytokine measurements, TD, TL and JG were responsible for subject recruitment and clinical evaluation, CZ and RV were responsible for design of the MRS study and protocols and 31P data collection and analysis, CG was responsible for the statistical analysis. FT and AV wrote the manuscript. All authors participated in the data discussion, read the manuscript and approved the final version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pipinos, Shepard AD, Anagnostopoulos PV, Katsamouris A, Boska MD. Phosphorus 31 nuclear magnetic resonance spectroscopy suggests a mitochondrial defect in claudicating skeletal muscle. J Vasc Surg. 2000;31(5):944–952. doi: 10.1067/mva.2000.106421. [DOI] [PubMed] [Google Scholar]
- 2.Greiner A, Esterhammer R, Pilav S, Arnold W, Santner W, Neuhauser B, Fraedrich G, Jaschke WR, Schocke MF. High-energy phosphate metabolism in the calf muscle during moderate isotonic exercise under different degrees of cuff compression: a phosphorus 31 magnetic resonance spectroscopy study. J Vasc Surg. 2005;42(2):259–267. doi: 10.1016/j.jvs.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 3.Greiner A, Esterhammer R, Messner H, Biebl M, Muhlthaler H, Fraedrich G, Jaschke WR, Schocke MF. High-energy phosphate metabolism during incremental calf exercise in patients with unilaterally symptomatic peripheral arterial disease measured by phosphor 31 magnetic resonance spectroscopy. J Vasc Surg. 2006;43(5):978–986. doi: 10.1016/j.jvs.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Pipinos, Judge AR, Zhu Z, Selsby JT, Swanson SA, Johanning JM, Baxter BT, Lynch TG, Dodd SL. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic Biol Med. 2006;41(2):262–269. doi: 10.1016/j.freeradbiomed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Pipinos, Sharov VG, Shepard AD, Anagnostopoulos PV, Katsamouris A, Todor A, Filis KA, Sabbah HN. Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease. J Vasc Surg. 2003;38(4):827–832. doi: 10.1016/s0741-5214(03)00602-5. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JD, Epstein FH, Meyer CH, Hagspiel KD, Wang H, Berr SS, Harthun NL, Weltman A, Dimaria JM, West AM, Kramer CM. Multifactorial determinants of functional capacity in peripheral arterial disease: uncoupling of calf muscle perfusion and metabolism. J Am Coll Cardiol. 2009;54(7):628–635. doi: 10.1016/j.jacc.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sleigh A, Raymond-Barker P, Thackray K, Porter D, Hatunic M, Vottero A, Burren C, Mitchell C, McIntyre M, Brage S, Carpenter TA, Murgatroyd PR, Brindle KM, Kemp GJ, O’Rahilly S, Semple RK, Savage DB. Mitochondrial dysfunction in patients with primary congenital insulin resistance. J Clin Invest. 2011;121(6):2457–2461. doi: 10.1172/JCI46405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Jeneson JA, Backes WH, van Echteld CJ, van Engelshoven JM, Mensink M, Schrauwen P. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007;50(1):113–120. doi: 10.1007/s00125-006-0475-1. [DOI] [PubMed] [Google Scholar]
- 9.Sleigh A, Stears A, Thackray K, Watson L, Gambineri A, Nag S, Campi VI, Schoenmakers N, Brage S, Carpenter TA, Murgatroyd PR, O’Rahilly S, Kemp GJ, Savage DB. Mitochondrial Oxidative Phosphorylation Is Impaired in Patients with Congenital Lipodystrophy. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2011-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Feyter HM, van den Broek NM, Praet SF, Nicolay K, van Loon LJ, Prompers JJ. Early or advanced stage type 2 diabetes is not accompanied by in vivo skeletal muscle mitochondrial dysfunction. Eur J Endocrinol. 2008;158(5):643–653. doi: 10.1530/EJE-07-0756. [DOI] [PubMed] [Google Scholar]
- 11.Fernyhough P, Roy Chowdhury SK, Schmidt RE. Mitochondrial stress and the pathogenesis of diabetic neuropathy. Expert Rev Endocrinol Metab. 2010;5(1):39–49. doi: 10.1586/eem.09.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akude E, Zherebitskaya E, Chowdhury SK, Smith DR, Dobrowsky RT, Fernyhough P. Diminished superoxide generation is associated with respiratory chain dysfunction and changes in the mitochondrial proteome of sensory neurons from diabetic rats. Diabetes. 2011;60(1):288–297. doi: 10.2337/db10-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent AM, Edwards JL, McLean LL, Hong Y, Cerri F, Lopez I, Quattrini A, Feldman EL. Mitochondrial biogenesis and fission in axons in cell culture and animal models of diabetic neuropathy. Acta Neuropathol. 2010;120(4):477–489. doi: 10.1007/s00401-010-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao H, Bowers JL, Holtzman D, Mulkern RV. RARE imaging of PCr in human forearm muscles. J Magn Reson Imaging. 1997;7(6):1048–1055. doi: 10.1002/jmri.1880070617. [DOI] [PubMed] [Google Scholar]
- 15.Kemp GJ, Taylor DJ, Radda GK. Control of phosphocreatine resynthesis during recovery from exercise in human skeletal muscle. NMR Biomed. 1993;6(1):66–72. doi: 10.1002/nbm.1940060111. [DOI] [PubMed] [Google Scholar]
- 16.Arora S, Smakowski P, Frykberg RG, Simeone LR, Freeman R, LoGerfo FW, Veves A. Differences in foot and forearm skin microcirculation in diabetic patients with and without neuropathy. Diabetes Care. 1998;21(8):1339–1344. doi: 10.2337/diacare.21.8.1339. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 18.Greenman RL, Panasyuk S, Wang X, Lyons TE, Dinh T, Longoria L, Giurini JM, Freeman J, Khaodhiar L, Veves A. Early changes in the skin microcirculation and muscle metabolism of the diabetic foot. Lancet. 2005;366(9498):1711–1717. doi: 10.1016/S0140-6736(05)67696-9. [DOI] [PubMed] [Google Scholar]
- 19.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 20.Dinh T, Doupis J, Lyons TE, Kuchibhotla S, Julliard W, Gnardellis C, Rosenblum BI, Wang X, Giurini JM, Greenman RL, Veves A. Foot muscle energy reserves in diabetic patients without and with clinical peripheral neuropathy. Diabetes Care. 2009;32(8):1521–1524. doi: 10.2337/dc09-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Economides PA, Caselli A, Tiani E, Khaodhiar L, Horton ES, Veves A. The effects of atorvastatin on endothelial function in diabetic patients and subjects at risk for type 2 diabetes. J Clin Endocrinol Metab. 2004;89(2):740–747. doi: 10.1210/jc.2003-031116. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, King GL, LoGerfo FW, Horton ES, Veves A. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48(9):1856–1862. doi: 10.2337/diabetes.48.9.1856. [DOI] [PubMed] [Google Scholar]
- 24.Achilli A, Olivieri A, Pala M, Hooshiar Kashani B, Carossa V, Perego UA, Gandini F, Santoro A, Battaglia V, Grugni V, Lancioni H, Sirolla C, Bonfigli AR, Cormio A, Boemi M, Testa I, Semino O, Ceriello A, Spazzafumo L, Gadaleta MN, Marra M, Testa R, Franceschi C, Torroni A. Mitochondrial DNA backgrounds might modulate diabetes complications rather than T2DM as a whole. PLoS One. 2011;6(6):e21029. doi: 10.1371/journal.pone.0021029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. 2009;94(6):2157–2163. doi: 10.1210/jc.2008-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niewczas MA, Ficociello LH, Johnson AC, Walker W, Rosolowsky ET, Roshan B, Warram JH, Krolewski AS. Serum concentrations of markers of TNFalpha and Fas-mediated pathways and renal function in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2009;4(1):62–70. doi: 10.2215/CJN.03010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coutinho T, Al-Omari M, Mosley TH, Jr, Kullo IJ. Biomarkers of left ventricular hypertrophy and remodeling in blacks. Hypertension. 2011;58(5):920–925. doi: 10.1161/HYPERTENSIONAHA.111.178095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venuraju SM, Yerramasu A, Corder R, Lahiri A. Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J Am Coll Cardiol. 2010;55(19):2049–2061. doi: 10.1016/j.jacc.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Vetrone SA, Montecino-Rodriguez E, Kudryashova E, Kramerova I, Hoffman EP, Liu SD, Miceli MC, Spencer MJ. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. J Clin Invest. 2009;119(6):1583–1594. doi: 10.1172/JCI37662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirata A, Masuda S, Tamura T, Kai K, Ojima K, Fukase A, Motoyoshi K, Kamakura K, Miyagoe-Suzuki Y, Takeda S. Expression profiling of cytokines and related genes in regenerating skeletal muscle after cardiotoxin injection: a role for osteopontin. Am J Pathol. 2003;163(1):203–215. doi: 10.1016/S0002-9440(10)63644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenman RL, Khaodhiar L, Lima C, Dinh T, Giurini JM, Veves A. Foot small muscle atrophy is present before the detection of clinical neuropathy. Diabetes Care. 2005;28(6):1425–1430. doi: 10.2337/diacare.28.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350(7):664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983;1(1):77–94. [PubMed] [Google Scholar]
- 35.Schocke MF, Esterhammer R, Arnold W, Kammerlander C, Burtscher M, Fraedrich G, Jaschke WR, Greiner A. High-energy phosphate metabolism during two bouts of progressive calf exercise in humans measured by phosphorus-31 magnetic resonance spectroscopy. Eur J Appl Physiol. 2005;93(4):469–479. doi: 10.1007/s00421-004-1233-z. [DOI] [PubMed] [Google Scholar]
- 36.Karakelides H, Asmann YW, Bigelow ML, Short KR, Dhatariya K, Coenen-Schimke J, Kahl J, Mukhopadhyay D, Nair KS. Effect of insulin deprivation on muscle mitochondrial ATP production and gene transcript levels in type 1 diabetic subjects. Diabetes. 2007;56(11):2683–2689. doi: 10.2337/db07-0378. [DOI] [PubMed] [Google Scholar]
- 37.Isbell DC, Berr SS, Toledano AY, Epstein FH, Meyer CH, Rogers WJ, Harthun NL, Hagspiel KD, Weltman A, Kramer CM. Delayed calf muscle phosphocreatine recovery after exercise identifies peripheral arterial disease. J Am Coll Cardiol. 2006;47(11):2289–2295. doi: 10.1016/j.jacc.2005.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]


