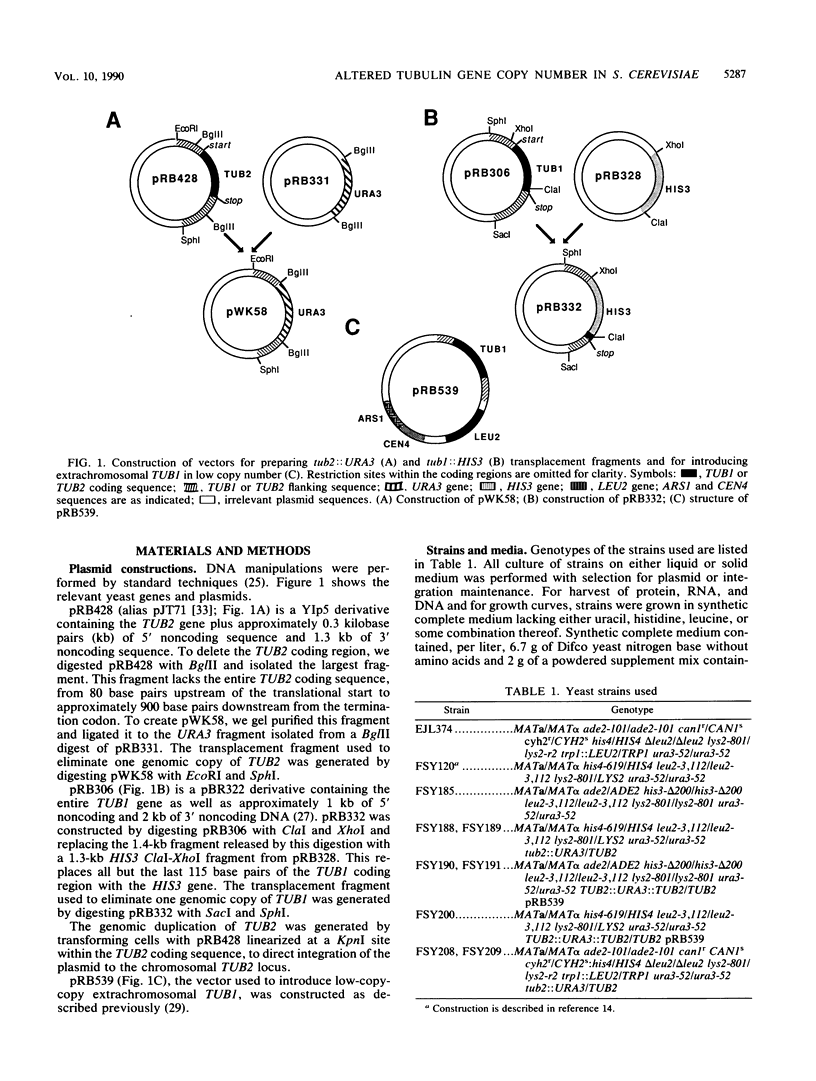
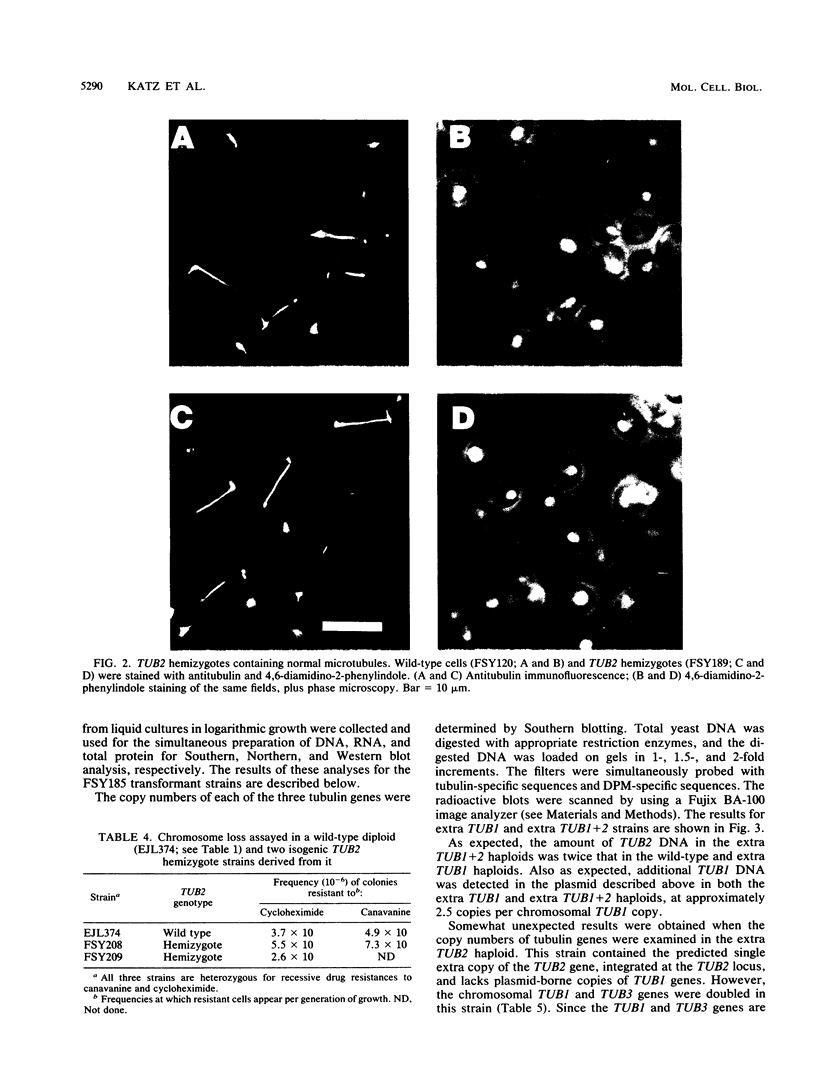
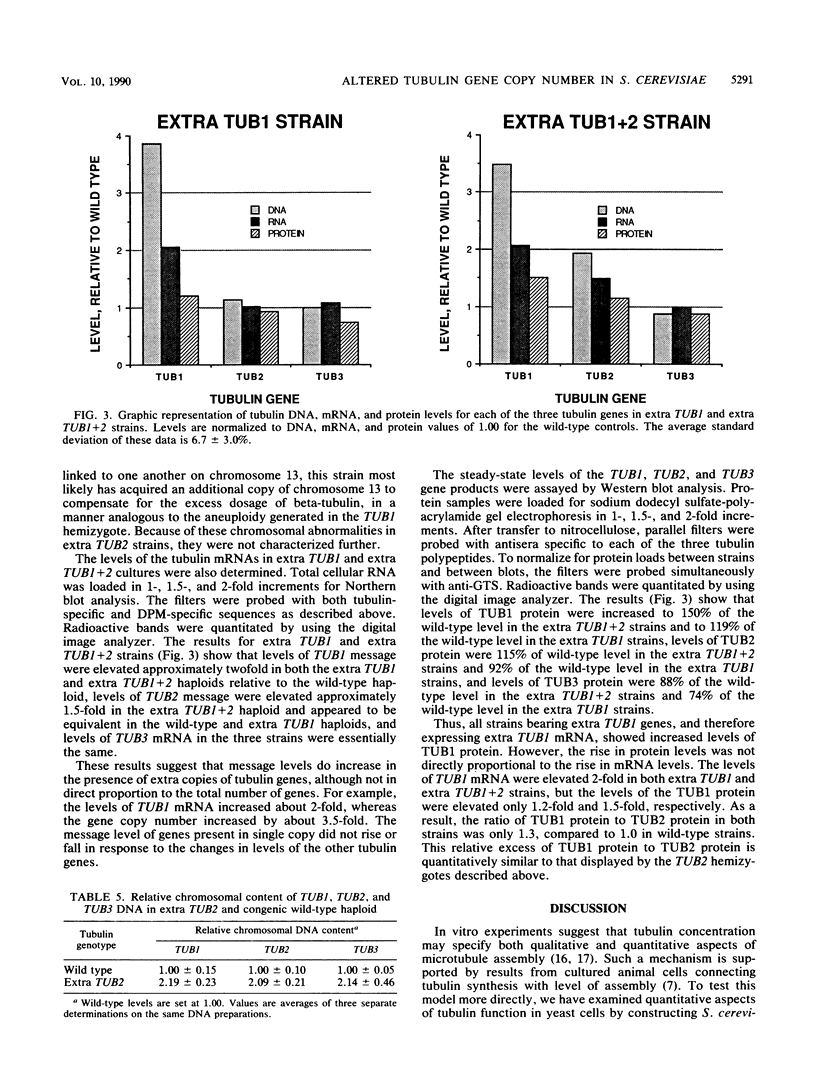
Abstract
Microtubule organization in the cytoplasm is in part a function of the number and length of the assembled polymers. The intracellular concentration of tubulin could specify those parameters. Saccharomyces cerevisiae strains constructed with moderately decreased or increased copy numbers of tubulin genes provide an opportunity to study the cellular response to a steady-state change in tubulin concentration. We found no evidence of a mechanism for adjusting tubulin concentrations upward from a deficit, nor did we find a need for such a mechanism: cells with no more than 50% of the wild-type tubulin level were normal with respect to a series of microtubule-dependent properties. Strains with increased copies of both alpha- and beta-tubulin genes, or of alpha-tubulin genes alone, apparently did down regulate their tubulin levels. As a result, they contained greater than normal concentrations of tubulin but much less than predicted from the increase in gene number. Some of this down regulation occurred at the level of protein. These strains were also phenotypically normal. Cells could contain excess alpha-tubulin protein without detectable consequences, but perturbations resulting in excess beta-tubulin genes may have affected microtubule-dependent functions. All of the observed regulation of levels of tubulin can be explained as a response to toxicity associated with excess tubulin proteins, especially if beta-tubulin is much more toxic than alpha-tubulin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum P., Thorner J., Honig L. Identification of tubulin from the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4962–4966. doi: 10.1073/pnas.75.10.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Boggs B., Cabral F. Mutations affecting assembly and stability of tubulin: evidence for a nonessential beta-tubulin in CHO cells. Mol Cell Biol. 1987 Aug;7(8):2700–2707. doi: 10.1128/mcb.7.8.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Gasdaska P., Hartwell L. Dominant effects of tubulin overexpression in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Mar;9(3):1049–1059. doi: 10.1128/mcb.9.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F., Hill T. L., Chen Y. Interference of GTP hydrolysis in the mechanism of microtubule assembly: an experimental study. Proc Natl Acad Sci U S A. 1984 Feb;81(3):771–775. doi: 10.1073/pnas.81.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W. Autoregulated instability of tubulin mRNAs: a novel eukaryotic regulatory mechanism. Trends Biochem Sci. 1988 Sep;13(9):339–343. doi: 10.1016/0968-0004(88)90103-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W. The multitubulin hypothesis revisited: what have we learned? J Cell Biol. 1987 Mar;104(3):381–383. doi: 10.1083/jcb.104.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Futcher B., Carbon J. Toxic effects of excess cloned centromeres. Mol Cell Biol. 1986 Jun;6(6):2213–2222. doi: 10.1128/mcb.6.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm C., Meeks-Wagner D. W., Fangman W. L., Botstein D. A rapid, efficient method for isolating DNA from yeast. Gene. 1986;42(2):169–173. doi: 10.1016/0378-1119(86)90293-3. [DOI] [PubMed] [Google Scholar]
- Huffaker T. C., Thomas J. H., Botstein D. Diverse effects of beta-tubulin mutations on microtubule formation and function. J Cell Biol. 1988 Jun;106(6):1997–2010. doi: 10.1083/jcb.106.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A. O., Subrahmanyan L., Turnbull C., Kalnins V. I. Localization of the neurofilament protein in neuroblastoma cells by immunofluorescent staining. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3192–3196. doi: 10.1073/pnas.73.9.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz W. S., Solomon F. Diversity among beta-tubulins: a carboxy-terminal domain of yeast beta-tubulin is not essential in vivo. Mol Cell Biol. 1988 Jul;8(7):2730–2736. doi: 10.1128/mcb.8.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. M., Hyams J. S., Luba A. Absence of microtubule sliding and an analysis of spindle formation and elongation in isolated mitotic spindles from the yeast Saccharomyces cerevisiae. J Cell Biol. 1982 Aug;94(2):341–349. doi: 10.1083/jcb.94.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M. W. Implications of treadmilling for the stability and polarity of actin and tubulin polymers in vivo. J Cell Biol. 1980 Jul;86(1):330–334. doi: 10.1083/jcb.86.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M. W., Mitchison T. Microtubule dynamics. Nature. 1986 Dec 18;324(6098):621–621. doi: 10.1038/324621a0. [DOI] [PubMed] [Google Scholar]
- Mitchison T. J., Kirschner M. W. Some thoughts on the partitioning of tubulin between monomer and polymer under conditions of dynamic instability. Cell Biophys. 1987 Dec;11:35–55. doi: 10.1007/BF02797111. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 1984 Nov 15;312(5991):232–237. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]
- Neff N. F., Thomas J. H., Grisafi P., Botstein D. Isolation of the beta-tubulin gene from yeast and demonstration of its essential function in vivo. Cell. 1983 May;33(1):211–219. doi: 10.1016/0092-8674(83)90350-1. [DOI] [PubMed] [Google Scholar]
- Orlean P., Albright C., Robbins P. W. Cloning and sequencing of the yeast gene for dolichol phosphate mannose synthase, an essential protein. J Biol Chem. 1988 Nov 25;263(33):17499–17507. [PubMed] [Google Scholar]
- Pillus L., Solomon F. Components of microtubular structures in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2468–2472. doi: 10.1073/pnas.83.8.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Georges G. E., Solomon F., Botstein D. Insertions of up to 17 amino acids into a region of alpha-tubulin do not disrupt function in vivo. Mol Cell Biol. 1987 Oct;7(10):3799–3805. doi: 10.1128/mcb.7.10.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Pillus L., Grisafi P., Solomon F., Botstein D. Two functional alpha-tubulin genes of the yeast Saccharomyces cerevisiae encode divergent proteins. Mol Cell Biol. 1986 Nov;6(11):3711–3721. doi: 10.1128/mcb.6.11.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Solomon F., Botstein D. Genetically essential and nonessential alpha-tubulin genes specify functionally interchangeable proteins. Mol Cell Biol. 1986 Nov;6(11):3722–3733. doi: 10.1128/mcb.6.11.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz P. J., Solomon F., Botstein D. Isolation and characterization of conditional-lethal mutations in the TUB1 alpha-tubulin gene of the yeast Saccharomyces cerevisiae. Genetics. 1988 Nov;120(3):681–695. doi: 10.1093/genetics/120.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds N. W., Gilman A. G., Amano T., Nirenberg M. W. Regulation of axon formation by clonal lines of a neural tumor. Proc Natl Acad Sci U S A. 1970 May;66(1):160–167. doi: 10.1073/pnas.66.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M., Chen L. B., Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol. 1986 Oct;103(4):1557–1568. doi: 10.1083/jcb.103.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. H., Neff N. F., Botstein D. Isolation and characterization of mutations in the beta-tubulin gene of Saccharomyces cerevisiae. Genetics. 1985 Dec;111(4):715–734. doi: 10.1093/genetics/111.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein B., Solomon F. Phenotypic consequences of tubulin overproduction in Saccharomyces cerevisiae: differences between alpha-tubulin and beta-tubulin. Mol Cell Biol. 1990 Oct;10(10):5295–5304. doi: 10.1128/mcb.10.10.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]