Mesothelioma is an insidious mesothelial neoplasm originating in the pleura, pericardium, peritoneum, or tunica vaginalis, with approximately 80% of cases involving the thorax. The predominant cause of malignant mesothelioma is exposure to asbestos. The incidence of mesothelioma in the United States is estimated to be approximately 2,000–3,000 cases per year, with an increasing incidence worldwide, secondary to the proliferation and poor regulation of industrial and household utilization of asbestos.1–6
A nihilistic attitude regarding mesothelioma has persisted among many physicians because of significant associated morbidity and mortality, as well as poor response to standard therapeutic interventions. Novel treatment paradigms, however, offer hope for enhanced palliation, improved tumor responses, and prolonged survival.6,8,9 This review will focus on standard therapeutic interventions for malignant pleural mesothelioma (MPM) such surgery, chemotherapy, and radiation therapy, as well as experimental approaches such as targeted therapy, immunotherapy, and gene-based therapies.
Surgery for MPM
Surgery for malignant pleural mesothelioma (MPM) can be diagnostic, palliative, or cytoreductive, although potentially associated with significant morbidity and mortality. The development of thoracoscopy has allowed for earlier diagnosis of mesothelioma. The vast majority of patients with MPM however, have advanced disease at diagnosis, as well as co-morbid medical illnesses, which often preclude aggressive surgical intervention.
Dyspnea from the accumulation of a pleural effusion is the most common presenting symptom of MPM. For symptomatic effusions in MPM, the optimal palliative approach is maximal drainage of the effusion and subsequent pleurodesis. The most widely-used compound for pleurodesis in MPM is sterile talc, administered either as a powder (“poudrage”) via thoracoscopy or slurry via tube thoracostomy.10 The presence of bulky tumor in the pleural space, or entrapment of the lung by a thick visceral pleural peel, is a contraindication to pleurodesis in patients with MPM. Attempts at talc pleurodesis in the setting of lung entrapment can lead to a multiloculated pleural space with a high risk of empyema. In this setting of lung entrapment in MPM, the preferred intervention is insertion of a tunneled intrapleural catheter to drain recurrent effusions and provide effective palliation of dyspnea [Figure 1].11 The primary concern regarding the use of tunneled pleural catheters (TPCs) in mesothelioma is the development of tumor implants at the insertion site or along the subcutaneous tunnel.11, 12 Recent reports of TPCs for MPE show equivalent results for the control of effusions compared with talc slurry pleurodesis. Therefore, TPCs should be considered for management of symptomatic effusions in patients with MPM, even in those whose lungs are unable to expand.13 Pleuro-periotoneal shunting, an alternative approach for dealing with lung entrapment in pleural mesothelioma, carries the overt risk of malignant seeding of the peritoneal cavity, and is therefore infrequently utilized.
Figure 1.
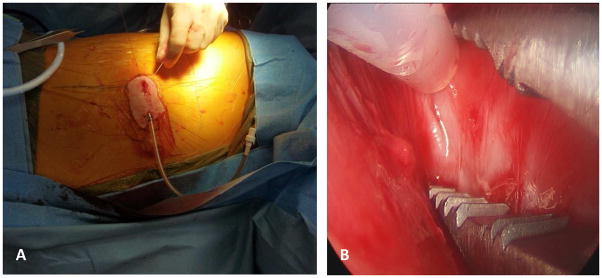
Panel A: Tunelled pleural catheters (TPC) can be an important method of palliation in patients with mesothelioma and recurrent symptomatic pleural effusions. Panel B: Thoracoscopic placement of TPC’s can be performed even in the setting of prior talc pleurodesis to facilitate intrapleural instillation of experimental therapies. Images courtesy of Dr. Joseph Friedberg, Division of Thoracic Surgery, Perelman School of Medicine of the University of Pennsylvania.
Thoracoscopic parietal pleurectomy is an alternative to talc pleurodesis in reducing the recurrence of pleural effusions in mesothelioma, and with less morbidity than open pleurectomy.14 Complete parietal and visceral pleurectomy (pleurectomy/decortication) may palliate dyspnea in mesothelioma patients with bulky intrapleural disease with or without lung entrapment, but by itself has not been shown to prolong survival.15
Extrapleural pneumonectomy (EPP) –en bloc resection of the lung, the parietal and visceral pleurae, and portions of the ipsilateral pericardium and diaphragm - provides maximal tumor cytoreduction and facilitates higher radiation dosage to the involved hemithorax. EPP alone has no influence on survival in the absence of adjuvant therapy. In most surgical series of EPP in MPM, median survival is less than two years, with average 5 year survival rates of 10–20%.15–18 There are, however, long-term survivors following EPP for maximal cytoreduction as a component of multimodality treatment involving adjuvant radiation therapy and postoperative chemotherapy.19–21 Unfortunately, the benefits of EPP with adjuvant chemotherapy +/− local radiotherapy are limited to otherwise healthy patients with early-stage disease, epithelial histology, and no mediastinal lymph node involvement. Patients with biphasic or sarcomatoid histology and/or mediastinal or hilar node positivity have an ominous prognosis.19
Several approaches for adjuvant therapy in conjunction with EPP have been studied. Investigators at Brigham and Women’s Hospital in Boston initially combined EPP with sequential postoperative chemotherapy and adjuvant external beam radiation therapy to the ipsilateral hemithorax.19,20,21 More recently, the Brigham group has investigated the role of hyperthermic intracavitary chemotherapy as an adjuvant to maximal cytoreductive surgery, in combination with hemithoracic irradiation and systemic chemotherapy. 22, 23 Other novel multi-center clinical trials combine maximal surgical debulking with adjuvant intensity-modulated radiation therapy (IMRT), or alternatively assess the role of neoadjuvant chemotherapy prior to cytoreductive surgery to improve long-term outcomes.24
Other investigators have evaluated the utility of post-resectional photodynamic therapy (PDT) [Figure 2]. The single randomized trial of this technology in MPM, conducted by Pass and colleagues at the National Cancer Institute in the early 1990’s, failed to confirm any benefit for adjuvant PDT compared to surgery alone with or without adjuvant chemo/immunotherapy. More recent data by Friedberg et al. showed improvements in overall survival compared with historical controls and improved outcomes with PDT after radical pleurectomy compared with outcomes post EPP.25, 26 Novel photosensitizers are currently under study that may provide better local control, decreased photosensitivity, and perhaps improved induction of systemic anti-tumor immune responses.
Figure 2.
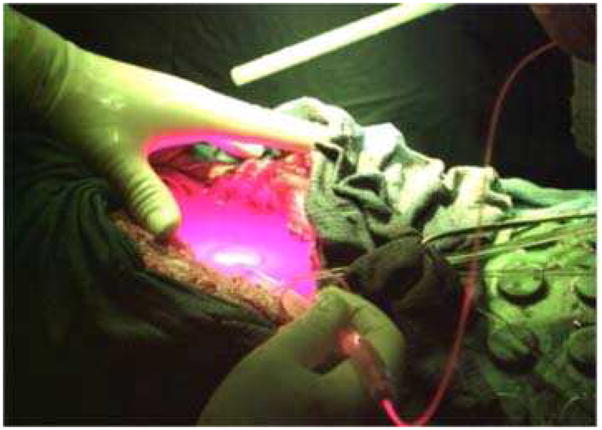
Intrathoracic photodynamic therapy (PDT) has demonstrated promise in clinical trials as an intra-operative adjunctive therapy after maximal cytoreductive surgery. PDT can improve local control by direct cell killing of microscopic residual disease in the post-operative hemithorax as well as induce systemic anti-tumor responses which may result in prolongation of median survival. Images courtesy of Dr. Joseph Friedberg, Division of Thoracic Surgery, Perelman School of Medicine of the University of Pennsylvania.
There have been several recent reports about the use of radical pleurectomy as a maximal debulking procedure in multimodality protocols that hold great promise [Figure 3], with various adjuvant intraoperative therapies such as intrapleural photodynamic therapy (PDT), intrapleural hyperthermic chemotherapy (Cisplatin, Gemcitabine), and hyperthermic perfusion with Povidone-Iodine.26,27 These have also been administered in association with IMRT in the presence of intact lung with demonstration of preserved/improved pulmonary function.28
Figure 3.
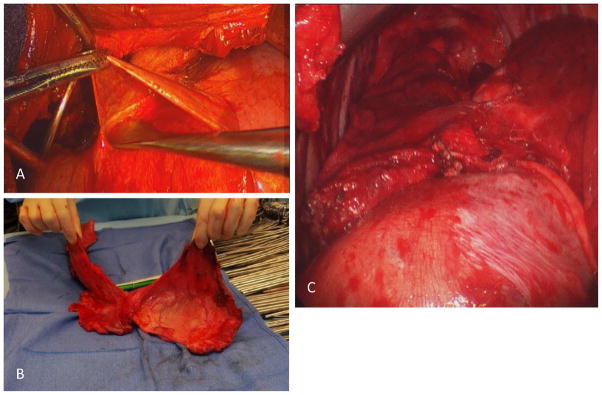
Radical Pleurectomy as lung-sparing modality of maximal surgical debulking in malignant pleural mesothelioma. Panel A: Dissection of visceral pleura off surface of lung. Panel B: Radical pleurectomy specimen in patient with early-stage mesothelioma. Panel C: View of right lower lobe after completion of radical pleurectomy with full re-expansion. Images courtesy of Dr. Joseph Friedberg, Division of Thoracic Surgery, Perelman School of Medicine of the University of Pennsylvania.
Radiation Therapy for MPM
Contrary to the prevailing wisdom that malignant pleural mesothelioma is a radio-resistant neoplasm, mesothelioma cell lines in vitro may be more responsive to ionizing radiation than non-small cell lung cancer cell lines. External-beam radiation therapy for mesothelioma is, however, limited by the large treatment volumes required and the radiation sensitivity of the surrounding organs (heart, lung, esophagus, spinal cord).
Although palliative radiotherapy with an attempt to treat the entire involved pleural surface is technically difficult and associated with a high risk of radiation pneumonitis, myelitis, hepatitis, and myocarditis, it can provide effective local palliation in up to 50 percent of patients.29 There are also anecdotal reports of long-term survivors following high-dose external beam irradiation and even intrapleural administration of radioactive isotopes.29. Furthermore, radiation therapy may play a role by preventing chest wall recurrences after thoracoscopy/thoracotomy and in improving local control after pleurectomy or extrapleural pneumonectomy. Mesothelioma frequently implants along the tracts of biopsies, chest tubes, thoracoscopy trocars, and surgical incisions, producing uncomfortable subcutaneous nodules. This can be prevented with prophylactic radiotherapy. In a small randomized trial, Boutin and colleagues demonstrated that 21 Gy administered in three daily fractions, 10 to 15 days after thoracoscopy, significantly decreased local recurrence at incision sites. These findings have been confirmed by other investigators as well.30, 31
Multimodality approaches commonly include adjuvant radiation following surgery, although there are no randomized trials that demonstrate its efficacy. Because the lung remains in place after pleurectomy, radiotherapy doses must be lower than when EPP is performed.29
The Radiation Oncology group at the University of Texas M.D. Anderson Cancer Center reported encouraging results using IMRT following EPP. Using careful treatment planning and IMRT, radiation doses of up to 50–60 Gy were possible without severe toxicity. With the combination of EPP and IMRT, local recurrences after surgery were virtually eliminated; however, novel distant disease patterns have begun to emerge. These data suggest that the combination of EPP and IMRT requires an additional treatment modality (i.e. chemotherapy or immunotherapy) to limit distant tumor growth. Although IMRT following EPP appeared to be more effective for local disease control in this initial series, a second series suggested there was a significant increase in severe toxicity (6 of 13 patients developed fatal pneumonitis).34 More recent studies have demonstrated safety of IMRT in MPM, even in the presence of an intact lung in the adjuvant setting.24,28 Novel forms of radiation therapy, including proton-beam therapy, are currently under investigation for treatment of MPM.
Chemotherapy for MPM
The current standard of care for first-line systemic therapy in good performance status patients with unresectable MPM is combination chemotherapy with Pemetrexed and Cisplatin. Pemetrexed (Alimta®, Eli Lilly and Company, Indianapolis, IN) is an multi-targeted anti-folate compound which blocks several enzymes in the folate metabolism pathway. Pemetrexed is a potent inhibitor of thymidylate synthase (TS), the rate-limiting enzyme in the synthesis of thymidylate, which is required for DNA synthesis. TS is also the enzyme inhibited by the cytotoxic agents 5-Fluorouracil and Raltitrexed. 9, 35
In 2003, Vogelzang and colleagues reported the results of a phase III randomized clinical trial in 456 chemotherapy-naive MPM patients comparing treatment with Pemetrexed and Cisplatin to Cisplatin monotherapy.9 Response rates were 41.3% in the Pemetrexed/Cisplatin arm versus 16.7% in the control arm (P <0.0001). Median time to progression was significantly longer in the Pemetrexed/Cisplatin arm: 5.7 months versus 3.9 months (P =0.001). Median survival time in the Pemetrexed/Cisplatin arm was 12.1 months versus 9.3 months in the Cisplatin-only arm (P =0.020, two-sided log-rank test). The hazard ratio for death of patients in the combination arm versus those in the control arm was 0.77. Another randomized Phase III study of Cisplatin and Raltitrexed in unresectable MPM showed similar increases in median survival.36
The combination of Gemcitabine and Carboplatin is also an acceptable first-line option for systemic therapy of MPM due to its acceptable toxicity profile, good response rate, and palliative effects. A single-arm Northern Italian Phase II study of Gemcitabine and Carboplatin in patients with pleural mesothelioma reported a 26% partial response rate, a median response duration of 55 weeks, and significant palliative benefits. Median survival for patients in this study was 66 weeks.37–39 A recent randomized clinical trial showed no benefit from the addition of Bevacizumab to this regimen40
There is, however, no current standard of care for second-line chemotherapy in mesothelioma following treatment with Cisplatin and Pemetrexed. The most commonly used second-line regimens include Gemcitabine or other drugs with single-agent activity such as Vinorelbine. There exists insufficient evidence to recommend second-line chemotherapy as a standard treatment. Patients with adequate performance status should be enrolled into clinical trials of second-line treatment.41–45 A large, double-blinded, randomized clinical trial of the histone deacetylase (HDAC) inhibitor Vorinistat in second line therapy for MPM showed no survival benefit for study drug over placebo.46
“Targeted” Therapy
The presence of active platelet derived growth factor (PDGF) and epidermal growth factor (EGF) pathways in some mesothelioma cell lines in vitro implied that novel inhibitors of these pathways might prove useful clinically, either as monotherapy, or in combination with chemotherapy. Unfortunately, early-phase clinical trials of imatinib mesylate and gefitinib (and erlotinib), inhibitors of the tyrosine kinases critical to the PDGF and EGF pathways, respectively, failed to demonstrate any significant clinical benefits in MPM.47–50 Clinical trials were conducted with other novel “targeted” agents, such as the anti-angiogenic agents, bevacizumab and thalidomide, and the copper-chelating agent, tetrathiomolybdate, which depletes copper, a key co-factor in tumor angiogenesis. Only the latter compound has demonstrated any benefit in human trials.51–53
NEW THERAPEUTIC APPROACHES
Despite the improvements in survival achieved with surgery-based multimodality therapy and combination chemotherapy for MPM, less morbid, more effective interventions are needed. Addressing the focality of the disease process within the involved hemithorax, many investigators have attempted to treat MPM by direct instillation of chemotherapeutic and other therapeutic agents into the pleural space, but without much success.54–56 Based upon case reports of spontaneous tumor remissions and associations of intratumoral lymphocytic infiltration with improved median survival rates, several groups have investigated immunotherapeutic approaches for MPM as an potential means of achieving better tumor response rates.2
Immunotherapy
The use of compounds to stimulate an antitumor immune response against pleural malignancy stemmed from the observation that patients who developed post-operative empyemas after lung cancer resection had improved survival rates.57,58 Subsequently, intrapleural bacille Calmette-Guérin (BCG) was studied as a surgical adjuvant, but no significant clinical benefits were noted.59 Several systemic immunotherapies have been administered to patients with MPM, including interleukin-2 (IL-2) and interferon-gamma (IFN-γ), both of which demonstrated limited efficacy and significant side effects. Subcutaneous IFN-α-2a was found to be somewhat efficacious and reasonably well-tolerated, with a 14% overall response rate as monotherapy for MPM.60 One European phase I–II study of intrapleural IL-2 administered by continuous infusion via an indwelling catheter revealed a 19 percent partial response rate, but with marked dose-related toxicity, primarily the development of empyemas.61
Boutin and colleagues in Marseilles, France pioneered the intrapleural administration of immunostimulatory cytokines to treat MPM, demonstrating significant local tumor responses with both intrapleural IL-2 and IFN-γ. Most impressive were the results of intrapleural IFN-γ in patients with early-stage mesothelioma (tumor localized to the parietal +/− visceral pleural surfaces), with an overall response rate of 20 percent. Furthermore, 17 of 89 patients treated had histologically-confirmed partial or complete responses on follow-up thoracoscopy. Overall, patients with stage I disease had a response rate of 45 percent.62–63
Other groups demonstrated only limited activity with intrapleural IL-2, and with the combination of intrapleural interferon-gamma and autologous activated macrophages. Immunotherapy trials in Australia demonstrated some significant tumor regression with repeated intratumoral injection of granulocyte-monocyte colony stimulating factor (GM-CSF), but with complications related to the catheters used for cytokine instillation.64, 65,66
Gene Therapy
In the absence of curative therapies for MPM, several groups have investigated the nascent technologies of gene transfer as a potential mediator of anti-tumor responses in MPM [Table 1].67 Intrapleural gene therapy for mesothelioma is attractive as the disease typically remains localized for the majority of its course, and access to the tumor in the pleural cavity is relatively easy and safe. Gene transfer delivery systems (“vectors”) utilized in pre-clinical and clinical studies were either liposomal/DNA complexes or modified viruses, included herpes, vaccinia, and adenoviruses. Therapeutic genes delivered by these vectors included so-called “suicide” genes, cytokines, tumor suppressor genes (ie. p53), and pro-apoptotic genes. Studies have also been conducted using replication-restricted, “tumor-selective” adeno- and herpes viruses, as well as carrier cells, such as modified ovarian carcinoma cells (OVCAR-3).67, 68 \
Table 1.
Intrapleural Gene Therapy Trials for Mesothelioma
| Study | Phase | Histology | Total No. (# evaluable) | Agent | Delivery | Best Clinical Response (%) | Additional Outcome Measures |
|---|---|---|---|---|---|---|---|
| Sterman 1998 | I | MPM | 21 | Ad.HSVtk/GCV | Intrapleural - single dose; GCV x 14 days | Gene transfer confirmed in 11 of 20 evaluable patients in a dose-related fashion | Strong anti-adenoviral immune responses generated, including high titers of neutralizing antibody and T- cell proliferative responses |
| Sterman 2000 | I | MPM | 8 | Ad.HSVtk/GCV + Corticosteroids | Intrapleural - single dose; GCV x 14 days | Two long-term survivors with stable disease for 6 years post treatment | Safety and toxicity without difference from initial clinical trial. |
| Sterman 2007 | I | MPM MPE |
MPM - 7 MPE – 3 |
Ad.IFNβ | Intrapleural - single dose | 1 (10%) with CR, 2 (20%) with PR, 4 (40%) with SD | Successful gene transfer, induction of humoral/innate immune response |
| Dong 2008 | I | MM MPE |
Treatment = 27; Control = 21 | Ad.wt-p53 +/− IP Cisplatin | Intrapleural/Intraperitoneal – weekly x 4 | Total effective rates for the treatment group (63.0%) and for the control group (42.9%) | Safety and toxicity |
| Sterman 2010 | I | MPM MPE |
MPM – 10 MPE – 7 |
Ad.IFNβ | Intrapleural – two doses | 3 (18%) with PR/MR 11 (61%) with SD |
Successful gene transfer with 1st dose but not 2nd, induction of humoral immune response |
| Sterman 2011 | I | MPM | 9 | Ad.IFNα–2b | Intrapleural - two doses | 2 (22%) PR, 4 (44%) with SD | Ad.IFNα induced much higher levels of gene transfer than Ad.INFβ Induction of humoral/innate immune response |
| Schwarzenberger 2011 | I | MPM | 15 | PA1-STK Cells/GCV | Multiple intrapleural infusion (every 4 weeks x3) followed by 7 days of intravenous GCV | CR (0), PR (0) and stable disease (9) and (3) at 3 and 6 months, respectively. | Median overall survival from the time of treatment initiation - 7.7 months |
| Sterman/Haas 2012- | IIA | MPM | Ongoing | Ad.IFNα–2b | Intrapleural - two doses with:
|
Ongoing | Gene transfer, immune response, safety and toxicity |
Abbreviations used:; Ad- adenovirus; MPM – malignant pleural mesothelioma; MPE – malignant pleural effusion; DCC-E1A – Liposomal E1A gene conjugate; IHC -immunohistochemical staining; RT-PCR - reverse transcriptase-polymerase chain reaction; IFN-β – Interferon beta; Ad.wt-p53 – Adenovirus wild-type p53 gene construct; IFN-α – Interferon alpha; CR – complete response; PR – partial response; MR – mixed response; SD – stable disease; PD – progressive disease
Clinical Investigations of Gene Therapy in MPM
“Suicide” Gene Therapy
Suicide gene therapy involves transduction of tumor cells with a gene encoding an enzyme that induces sensitivity to an otherwise benign therapeutic agent. In essence, a “prodrug” is transformed into a toxic metabolite by introduction of the enzyme into the malignant cells with subsequent accumulation leading to tumor cell death or “suicide.”69, 70 A major advantage of suicide gene therapy is the induction of a “bystander effect”— the killing of neighboring cells not transduced with the vector. A commonly studied suicide gene is the herpes simplex virus-1 thymidine kinase (HSVtk) gene which makes transduced cells sensitive to the nucleoside analog ganciclovir (GCV). GCV is metabolized poorly by mammalian cells and thus is usually non-toxic. However, after conversion to GCV-monophosphate by HSVtk, it is metabolized rapidly by endogenous kinases to GCV-triphosphate which acts as a potent inhibitor of DNA polymerase and competes with normal mammalian nucleosides for DNA replication.69,70
Based on data from extensive preclinical studies, our group at the University of Pennsylvania in 1995 initiated a series of Phase 1 clinical trials of adenoviral suicide gene therapy (Ad.HSVtk/GCV) in patients with advanced MPM to assess toxicity, gene transfer efficiency, and immune response induction.71–73 Subsequent to a single intrapleural administration of Ad.HSVtk vector, GCV was given intravenously twice daily for two weeks. Dose-related intratumoral HSVtk gene transfer was demonstrated in 23 of 30 patients with those treated at a dose ≥3.2×1011 plaque forming units (pfu) with evidence of HSVtk protein expression up to 30–50 cell layers deep by immunohistochemical assessment. Overall, the suicide gene therapy was well-tolerated with minimal side effects and no dose-limiting toxicity. Anti-tumor and anti-adenoviral vector immune responses, including induction of high titers of anti-adenoviral neutralizing antibody and proliferative T-cell responses, were generated in both serum and pleural fluid. A number of clinical responses were seen at the higher dose levels, with two patients showing long periods of survival (one seven years and one still alive after 14 years). One of the two surviving patients had demonstrable reduction of tumor metabolic activity as assessed by serial 18-fluorodeoxyglucose positron emission tomography (18FDG PET) scans over several months. This long response period was hypothesized to be due to induction of a secondary immune bystander effect of the Ad.HSVtk/GCV instillation.71–73
Schwarzenberger and colleagues at Louisiana State University conducted a Phase 1 trial using irradiated ovarian carcinoma cells (OVCAR-3) retrovirally-transfected with HSVtk (PA1-STK cells) that were instilled intrapleurally followed by GCV for 7 days. Minimal side effects were seen, although there were some post-treatment increases in the percentage of CD8+ T lymphocytes in the pleural fluid. However, no significant clinical responses were documented.74,75
Cytokine Gene Therapy
The rationale for cytokine gene therapy is that high level expression of immunostimulatory cytokines (such as interleukin 2 [IL-2], IL-12, tumor necrosis factor [TNF], GM-CSF, or interferons) from tumor cells will activate the immune system in situ, resulting in a more effective anti-tumor immune response without having to target specific antigens. The advantages of cytokine gene delivery over systemic administration of these agents included lower toxicity, higher local concentrations, and longer persistence of the cytokine.67
Robinson and colleagues conducted the first clinical trial of intratumoral cytokine gene delivery in MPM patients using a replication-restricted vaccinia virus (VV) expressing the human IL-2 gene. Serial VV-IL-2 vector injections over a period of 12 weeks into chest wall lesions of six patients with advanced MPM resulted in minimal toxicity, but no significant tumor regression. Modest intratumoral T-cell infiltration was detected on post-treatment biopsy specimens. V-IL-2 mRNA was detected in biopsy specimens for up to six days post-injection despite the generation of significant levels of anti-VV-neutralizing antibodies.68, 76
Based upon success of in vivo experiments77, 78 our group at the Hospital of the University of Pennsylvania conducted the first human trial of intrapleural interferon gene therapy for MPM and malignant pleural effusions (MPE).79 The study evaluated the safety and feasibility of a single-dose intrapleural IFN-beta gene transfer using an adenoviral vector (Ad.IFN-β) in patients with MPM and MPE. Ad.IFN-β was administered via an indwelling pleural catheter in escalating doses in two cohorts of patients - MPM (7 patients) and MPE (3 patients). Subjects were evaluated for toxicity, gene transfer, immune responses, and anti-tumor responses via 18FDG PET scans and chest computed tomography (CT) scans. Intrapleural Ad.IFN-beta was well tolerated with transient lymphopenia as the most common side effect. Other side effects included hypoxia and liver function abnormalities. Gene transfer was documented in 7 of the 10 patients by demonstration of IFN-β mRNA or protein expression in pleural fluid. Antitumor immune responses were demonstrated in seven of the 10 patients and included the detection of cytotoxic T cells, activation of circulating natural killer cells, and humoral responses to known tumor associated antigens as well as to allogenic mesothelioma cell lines. Four of 10 patients showed meaningful clinical responses defined as disease stability and/or regression on PET and CT scans at day 60 after vector instillation.79 This study demonstrated that administration of intrapleural Ad.IFN-β was feasible and well-tolerated, and resulted in successful gene transfer. Lastly, the study also demonstrated that a single intrapleural dose of IFN-β vector induced demonstrable antitumor immune responses as well as anecdotal clinical responses in a heavily- pretreated MPM patient population.79
A second Phase I trial was then conducted to determine whether using two doses of Ad.IFN-β vector would prove superior to a single dose.80 Ten patients with MPM and seven with MPE received two doses of Ad.IFN-β through an indwelling pleural catheter. Repeated doses were generally well tolerated. The most common adverse events were lymphopenia, hypoalbuminemia, hypotension, anemia, hypocalcemia, and mild cytokine release syndrome (CRS). One patient developed pericardial tamponade but pericardial fluid analysis did not reveal tumor cells or elevated IFN-β levels.80
In this repeat dose gene transfer study, high levels of IFN-β were detected in pleural fluid after the first dose, however, absent intrapleural IFN-β expression after the second dose correlated with the rapid induction of neutralizing Ad antibodies (Nabs). Antibody responses against tumor antigens were induced in most patients. At 2 month follow-up imaging, 1 MPM patient had a partial response, 2 had stable disease, and 9 had progressive disease. On PET scanning, 2 patients had mixed responses and 11 had stable disease. There were 7 patients with survival times longer than 18 months. Overall, repeated intrapleural instillation of Ad.IFN-β vector was safe, induced immune responses, and some evidence of clinical responses. However, rapid development of Nabs prevented effective gene transfer after the second dose, even with a shortened dose interval of 7 days.80
We then designed another Phase I trial to evaluate a shortened dosing interval by administering a second dose of intrapleural Ad-IFN vector 3 days after the first dose, prior to the expected peak of Nab production.81 For this trial, our group utilized a recombinant, replication-incompetent adenovirus vector expressing the human interferon-α2b gene (Ad.IFN-α2b) obtained from Schering-Plough/Merck (SCH721015). Ad.IFN-α2b was instilled on study days 1 and 4 via a tunneled pleural catheter. The starting vector dose was 1×1012 viral particles, but this dose was reduced to 3×1011 after the first 3 patients developed significant CRS symptoms. Subjects were assessed for anti-tumor responses at day 60 day using CT and PET scans. Pleural fluid and serum IFN-α2b levels, mesothelin-related protein (SMRP) levels and Nabs were measured. In general, although most patients developed some CRS symptoms, Ad.IFN-α2b vector instillation was well tolerated. Elevated and sustained serum IFN-α levels were occasionally associated with protracted “flu-like symptoms” lasting 1–2 weeks. Pleural catheter-related infections occurred in two patients and both were treated successfully with antibiotics. Successful gene transfer and high IFN-α levels in pleural fluid were demonstrated even in patients who received a lower dose of vector. Furthermore, there was evidence that the second Ad.IFN-α2b dose resulted in successful gene transfer. There were encouraging immunologic responses, such as new or increased intensity bands on immunoblots containing extracts of mesothelioma cell lines, in seven of 8 patients, as well as up-regulation of the activation marker CD69 on circulating NK cells.81
At initial radiographic assessment using modified RECIST criteria (Response Evaluation Criteria in Solid Tumors) on day 60, 3 subjects had progressive disease, 4 had stable disease, and 2 had partial responses. Two patients had sufficient improvement that they were subsequently able to undergo successful radical pleurectomy (RP), with no signs of recurrence now 21 and 33 months post-surgery. One patient, who has been previously treated with radical pleurectomy and chemotherapy, had an impressive radiographic and metabolic tumor response in that many of the pleural-based malignant foci had regressed on PET/CT by two months after vector instillations. On six month follow-up PET/CT post Ad.IFN-α2b, many lesions had completely resolved, most at sites distant from vector instillation. The crucial result of the study was the recognition of low levels of Nabs at the shortened dosing interval with prolonged intrapleural interferon expression.81 The combination of a better dosing strategy as above as well as the higher potency and sustained levels of IFN-α may result in better anti-tumor response in future clinical trials.
Thus, although these strategies seem to be successful in initiating anti-tumor immunes responses, they are limited by large tumor volumes and significant immuno-inhibitory networks, even beyond Nabs. These networks, created by the tumors, involve cytokines such as TGF-beta, interleukin-10, prostaglandin E2, vascular-endothelial cell growth factor, and additionally by inhibitor cells such as T-regulatory cells and myeloid derived suppressor cells. Ongoing clinical trials with the Ad.IFN-α2b vector involve combination with front-line and second line chemotherapy, as well as a brief course of high-dose cyclo-oxygenase-2 (COX-2) inhibitor (Celecoxib) for modification of the tumor microenvironment and these inhibitory networks. [Figure 4] Future trials are going to likely require combination approaches that stimulate the immune system, reduce tumor burden (surgery and/or chemotherapy) and “inhibit the inhibitors” (with agents such as COX-2 inhibitors or anti-TGF-beta antibodies).
Figure 4.
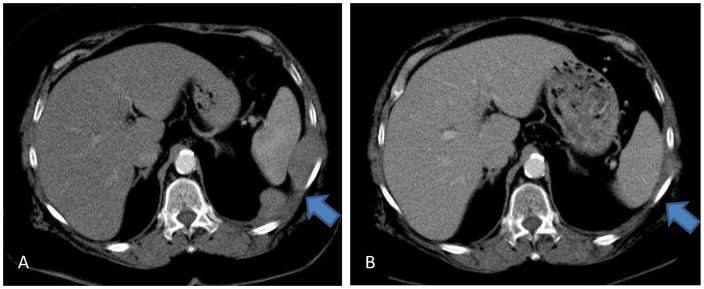
Panel A: Chest CT scan of patient newly diagnosed with biphasic mesothelioma involving the left pleural space and extending through left chest wall and left hemidiaphragm. Panel B: Chest CT scan status post 2 weeks of high-dose cyclo-oxygenase-2 (COX-2) inhibitor (Celecoxib) and 2 doses of intrapleural Ad.IFN-α2b vector and 4 cycles of combination chemotherapy with Pemetrexed and Cisplatin. Near-complete response of intrathoracic and chest wall tumor demonstrated.
Another major direction of the field is to use adoptive transfer of gene-modified autologous lymphocytes that have been altered ex vivo by using retroviruses or lentiviruses to augment their ability to attack mesothelioma cells. This can be done by transfection of T-cell receptors with altered specificity or by the introduction of totally artificial chimeric T-cell antigen receptors (CARs) that use single chain antibody fragments to define antigen specificity and intracellular fragments of both the T-cell receptor and accessory molecules (such as CD28 or 4-1BB) to enhance activation.82 Our group at Penn, and others at Memorial Sloan Kettering Cancer Center and the National Cancer Institute, are designing CARs to target T-cells to the tumor antigen mesothelin for use in the treatment of mesothelioma. The approach has worked well in preclinical models,82 and clinical trials utilizing this approach have been initiated at several centers over the past two years.
Novel treatments such as gene therapy for MPM have not yet reached routine clinical practice. An appropriate analogy may be the development of monoclonal antibodies where it took more than 20 years from discovery to actual clinical applications. Despite what some perceive as a slow start, we feel that progress in clearly being made and novel therapeutic tool will find their place in the armamentarium against MPM in the next decade.
Summary
Over the past decade, advances have been made that have improved our ability to treat malignant pleural mesothelioma. We have evidence that these treatments are increasing the quality and quantity of life for patients with mesothelioma. Multimodality treatment programs that combine maximal surgical cytoreduction with novel forms of radiation therapy and more effective chemotherapy combinations may offer significant increases in survival for certain subgroups of mesothelioma patients. Lung-sparing surgery may allow for improvements in pulmonary function after surgery-based multimodality therapy, and potential longer overall survival than that seen with EPP. Experimental treatments such as immunotherapy and gene therapy present a window of hope for all mesothelioma patients, and in the future, may be combined with “standard therapy” in multimodality protocols.
Over the past decade, advances have been made that have improved our ability to treat malignant pleural mesothelioma.
We have evidence that these treatments are increasing the quality and quantity of life for patients with mesothelioma.
Multimodality treatment programs that combine maximal surgical cytoreduction with novel forms of radiation therapy and more effective chemotherapy combinations may offer significant increases in survival for certain subgroups of mesothelioma patients.
Lung-sparing surgery may allow for improvements in pulmonary function after surgery-based multimodality therapy, and potential longer overall survival than that seen with EPP.
Experimental treatments such as immunotherapy and gene therapy present a window of hope for all mesothelioma patients, and in the future, may be combined with “standard therapy” in multimodality protocols.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antman KH, Pass HI, Li FP, et al. Principles and Practice of Oncology. 4. 1993. Benign and Malignant Mesothelioma, Cancer; pp. 1489–1508. [Google Scholar]
- 2.Antman KH. Natural history and epidemiology of malignant mesothelioma. Chest. 1993;103:373S. doi: 10.1378/chest.103.4_supplement.373s. [DOI] [PubMed] [Google Scholar]
- 3.Pisani RJ, Colby TV, Williams DE. Malignant mesothelioma of the pleura. Mayo Clin Proc. 1988;63:1234. doi: 10.1016/s0025-6196(12)65411-1. [DOI] [PubMed] [Google Scholar]
- 4.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 5.Peto J, Hodgson JT, Matthews FE, Jones JR. Continuing increase in mesothelioma mortality in Britain. Lancet. 1995;345:535. doi: 10.1016/s0140-6736(95)90462-x. [DOI] [PubMed] [Google Scholar]
- 6.Statement on malignant mesothelioma in the United Kingdom. British Thoracic Society Standards of Care Committee. Thorax. 2001;56:250. doi: 10.1136/thorax.56.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterman DH, Kaiser LR, Albelda SM. Advances in the treatment of malignant pleural mesothelioma. Chest. 1999;116:504–520. doi: 10.1378/chest.116.2.504. [DOI] [PubMed] [Google Scholar]
- 8.Sterman DH, Albelda SM. Advances in the Diagnosis, Evaluation and Management of Malignant Pleural Mesothelioma. Respirology. 2005;10:266–283. doi: 10.1111/j.1440-1843.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- 9.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003 Jul 15;21:2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 10.Haas AR, Sterman DH, Musani AI. Malignant pleural effusions: management options with consideration of coding, billing, and a decision approach. Chest. 2007 Sep;132(3):1036–41. doi: 10.1378/chest.06-1757. [DOI] [PubMed] [Google Scholar]
- 11.Pien GW, Gant M, Washam C, Sterman DH. Use of an Implantable Pleural Catheter For “Trapped Lung” Syndrome In Patients With Malignant Pleural Effusion. Chest. 2001;119:1641–6. doi: 10.1378/chest.119.6.1641. [DOI] [PubMed] [Google Scholar]
- 12.Lee YC, Fysh ET. Indwelling pleural catheter: changing the paradigm of malignant effusion management. J Thorac Oncol. 2011;6(4):655–7. doi: 10.1097/JTO.0b013e3182114aa0. [DOI] [PubMed] [Google Scholar]
- 13.Davies HE, Mishra EK, Kahan BC, Wrightson JM, Stanton AE, Guhan A, Davies CW, Grayez J, Harrison R, Prasad A, Crosthwaite N, Lee YC, Davies RJ, Miller RF, Rahman NM. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383–9. doi: 10.1001/jama.2012.5535. [DOI] [PubMed] [Google Scholar]
- 14.Waller DA, Morritt GN, Forty J. Video-assisted thoracoscopic pleurectomy in the management of malignant pleural effusion. Chest. 1995;107:1454. doi: 10.1378/chest.107.5.1454. [DOI] [PubMed] [Google Scholar]
- 15.van Ruth S, Baas P, Zoetmulder FA. Surgical treatment of malignant pleural mesothelioma: a review. Chest. 2003;123:551. doi: 10.1378/chest.123.2.551. [DOI] [PubMed] [Google Scholar]
- 16.Rusch VW, Piantadosi S, Holmes EC. The role of extrapleural pneumonectomy in malignant pleural mesothelioma. A Lung Cancer Study Group trial. J Thorac Cardiovasc Surg. 1991;102:1. [PubMed] [Google Scholar]
- 17.Sugarbaker DJ. Extrapleural pneumonectomy, chemotherapy and radiotherapy in the treatment of diffuse malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1991;102:10. [PubMed] [Google Scholar]
- 18.Grondin SC, Sugarbaker DJ. Pleuropneumonectomy in the treatment of malignant pleural mesothelioma. Chest. 1999;116:450S. doi: 10.1378/chest.116.suppl_3.450s. [DOI] [PubMed] [Google Scholar]
- 19.Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg. 1999;117:54. doi: 10.1016/s0022-5223(99)70469-1. [DOI] [PubMed] [Google Scholar]
- 20.Maggi G, Casadi C, Cianci R, et al. Trimodality management of malignant pleural mesothelioma. Eur J Cardio Thorac Surg. 2001;19:346. doi: 10.1016/s1010-7940(01)00594-2. [DOI] [PubMed] [Google Scholar]
- 21.Sugarbaker DJ. Multimodality management of malignant pleural mesothelioma: introduction. Semin Thorac Cardiovasc Surg. 2009;21(2):95–6. doi: 10.1053/j.semtcvs.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Chang MY, Sugarbaker DJ. Innovative therapies: intraoperative intracavitary chemotherapy. Thorac Surg Clin. 2004;14(4):549–56. doi: 10.1016/S1547-4127(04)00109-4. [DOI] [PubMed] [Google Scholar]
- 23.Tilleman TR, Richards WG, Zellos L, Johnson BE, Jaklitsch MT, Mueller J, Yeap BY, Mujoomdar AA, Ducko CT, Bueno R, Sugarbaker DJ. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: a phase II prospective study. J Thorac Cardiovasc Surg. 2009 Aug;138(2):405–11. doi: 10.1016/j.jtcvs.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 24.Bölükbas S, Manegold C, Eberlein M, Bergmann T, Fisseler-Eckhoff A, Schirren J. Survival after trimodality therapy for malignant pleural mesothelioma: Radical Pleurectomy, chemotherapy with Cisplatin/Pemetrexed and radiotherapy. Lung Cancer. 2011;71:75–81. doi: 10.1016/j.lungcan.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Friedberg JS, Mick R, Stevenson J, Metz J, Zhu T, Buyske J, Sterman DH, Pass HI, Glatstein E, Hahn SM. A Phase I study of Foscan-mediated photodynamic therapy and surgery in patients with mesothelioma. Ann Thorac Surg. 2003;75:952–959. doi: 10.1016/s0003-4975(02)04474-0. [DOI] [PubMed] [Google Scholar]
- 26.Friedberg JS, Culligan MJ, Mick R, Stevenson J, Hahn SM, Sterman D, Punekar S, Glatstein E, Cengel K. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2012 May;93(5):1658–65. doi: 10.1016/j.athoracsur.2012.02.009. discussion 1665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang-Lazdunski L, Bille A, Belcher E, Cane P, Landau D, Steele J, Taylor H, Spicer J. Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine followed by adjuvant chemotherapy in patients with malignant pleural mesothelioma. J Thorac Oncol. 2011;6(10):1746–52. doi: 10.1097/JTO.0b013e3182288af9. [DOI] [PubMed] [Google Scholar]
- 28.Bölükbas S, Eberlein M, Schirren J. Prospective study on functional results after lung-sparing radical pleurectomy in the management of malignant pleural mesothelioma. J Thorac Oncol. 2012;7(5):900–5. doi: 10.1097/JTO.0b013e31824de2dc. [DOI] [PubMed] [Google Scholar]
- 29.de Graaf-Strukowska L, van der Zee J, van Putten W, Senan S. Factors influencing the outcome of radiotherapy in malignant mesothelioma of the pleura--a single institution experience with 189 patients. Int J Radiat Oncol Biol Phys. 1999;43:511. doi: 10.1016/s0360-3016(98)00409-x. [DOI] [PubMed] [Google Scholar]
- 30.Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. Chest. 1995;108:754. doi: 10.1378/chest.108.3.754. [DOI] [PubMed] [Google Scholar]
- 31.Bydder S, Phillips M, Joseph DJ, et al. A randomised trial of single-dose radiotherapy to prevent procedure tract metastasis by malignant mesothelioma. Br J Cancer. 2004;91:9. doi: 10.1038/sj.bjc.6601957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahamad A, Stevens C, Smythe R, et al. Intensity-modulated radiation therapy: a novel approach to the management of malignant pleural mesothelioma. Int J Radiation Oncololgy Biol Phys. 2003;55:768. doi: 10.1016/s0360-3016(02)04151-2. [DOI] [PubMed] [Google Scholar]
- 33.Ahamad A, Stevens C, Smythe R, et al. Promising early local control of malignant pleural mesothelioma following postoperative intensity modulated radiotherapy (IMTR) to the chest. Cancer J. 2003;9:476. doi: 10.1097/00130404-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Allen AM, Czerminska M, Jänne PA, Sugarbaker DJ, Bueno R, Harris JR, Court L, Baldini EH. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys. 2006;65(3):640–5. doi: 10.1016/j.ijrobp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Janne PA. Chemotherapy for malignant pleural mesothelioma. Clin Lung Cancer. 2003;5:98–106. doi: 10.3816/CLC.2003.n.023. [DOI] [PubMed] [Google Scholar]
- 36.van Meerbeeck JP, Gaafar R, Manegold C, Van Klaveren RJ, Van Marck EA, Vincent M, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an inter group study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada 2005. Journal of Clinical Oncology. 23:6881–6889. doi: 10.1200/JCO.20005.14.589. [DOI] [PubMed] [Google Scholar]
- 37.Lee CW, Murray N, Anderson H, Rao SC, Bishop W. Outcomes with first-line platinum-based combination chemotherapy for malignant pleural mesothelioma: a review of practice in British Columbia. Lung Cancer. 2009;64:308–313. doi: 10.1016/j.lungcan.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Ceresoli GL, Zucali PA, Favaretto AG, Grossi F, Bidoli P, Del Conte G, et al. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. Journal of Clinical Oncology. 2006;24:1443–1448. doi: 10.1200/JCO.2005.04.3190. [DOI] [PubMed] [Google Scholar]
- 39.Favaretto AG, Aversa SM, Paccagnella A, de Manzini VP, Palmisano V, Oniga F, et al. Gemcitabine combined with carboplatin in patients with malignant pleural mesothelioma: a multicentric phase II study. Cancer. 2003;97:2791–7. doi: 10.1002/cncr.11405. [DOI] [PubMed] [Google Scholar]
- 40.Kindler HL, Karrison TG, Gandara DR, Lu C, Krug LM, Stevenson JP, Jänne PA, Quinn DI, Koczywas MN, Brahmer JR, Albain KS, Taber DA, Armato SG, 3rd, Vogelzang NJ, Chen HX, Stadler WM, Vokes EE. Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo inpatients with malignant mesothelioma. J Clin Oncol. 2012;30(20):2509–15. doi: 10.1200/JCO.2011.41.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zucali PA, Ceresoli GL, Garassino I, De Vincenzo F, Cavina R, Campagnoli E, Cappuzzo F, Salamina S, Soto Parra HJ, Santoro A. Gemcitabine and vinorelbine in pemetrexed-pretreated patients with malignant pleural mesothelioma. Cancer. 2008 Apr 1;112(7):1555–61. doi: 10.1002/cncr.23337. [DOI] [PubMed] [Google Scholar]
- 42.Xanthopoulos A, Bauer TT, Blum TG, Kollmeier J, Schonfeld N, Serke M. Gemcitabine combined with oxaliplatin in pretreated patients with malignant pleural mesothelioma: an observational study. Journal of Occupational Medicine & Toxicology. 2008;3:34. doi: 10.1186/1745-6673-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stebbing J, Powles T, McPherson K, Shamash J, Wells P, Sheaff MT, et al. The efficacy and safety of weekly vinorelbine in relapsed malignant pleural mesothelioma. Lung Cancer. 2009;63:94–97. doi: 10.1016/j.lungcan.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Pasello G, Nicotra S, Marulli G, Rea F, Bonanno L, Carli P, et al. Platinum-based doublet chemotherapy in pre-treated malignant pleural mesothelioma (MPM) patients: A mono-institutional experience. Lung Cancer. 2011 doi: 10.1016/j.lungcan.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Ceresoli GL, Zucali PA, De Vincenzo F, Gianoncelli L, Simonelli M, Lorenzi E, et al. Retreatment with pemetrexed-based chemotherapy in patients with malignant pleural mesothelioma. Lung Cancer. 2011;72:73–77. doi: 10.1016/j.lungcan.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Zauderer MG, Krug LM. Novel therapies in phase II and III trials for malignant pleural mesothelioma. J Natl Compr Canc Netw. 2012;10(1):42–7. doi: 10.6004/jnccn.2012.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathy A, Baas P, Dalesio O, van Zandwijk N. Limited efficacy of imatinib mesylate in malignant mesothelioma: a phase II trial. Lung Cancer. 2005;50:83. doi: 10.1016/j.lungcan.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 48.Porta C, Mutti L, Tassi G. Negative results of an Italian Group for Mesothelioma (G.I.Me) pilot study of single-agent imatinib mesylate in malignant pleural mesothelioma. Cancer Chemother Pharmacol. 2007;59:149. doi: 10.1007/s00280-006-0243-4. [DOI] [PubMed] [Google Scholar]
- 49.Govindan R, Kratzke RA, Herndon JE, 2nd, et al. Gefitinib in patients with malignant mesothelioma: a phase II study by the Cancer and Leukemia Group B. Clin Cancer Res. 2005;11:2300. doi: 10.1158/1078-0432.CCR-04-1940. [DOI] [PubMed] [Google Scholar]
- 50.Garland LL, Rankin C, Gandara DR, et al. Phase II study of erlotinib in patients with malignant pleural mesothelioma: a Southwest Oncology Group Study. J Clin Oncol. 2007;25:2406. doi: 10.1200/JCO.2006.09.7634. [DOI] [PubMed] [Google Scholar]
- 51.Baas P, Boogerd W, Dalesio O, et al. Thalidomide in patients with malignant pleural mesothelioma. Lung Cancer. 2005;48:291. doi: 10.1016/j.lungcan.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Dubey S, Janne PA, Krug L, Pang H, Wang X, Heinze R, et al. A phase II study of sorafenib in malignant mesothelioma: results of Cancer and Leukemia Group B 30307. Journal of Thoracic Oncology. 2010;5:1655–1661. doi: 10.1097/JTO.0b013e3181ec18db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pass HI, Brewer GJ, Dick R, Carbone M, Merajver S. A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: final results. Ann Thorac Surg. 86(2):383–9. doi: 10.1016/j.athoracsur.2008.03.016. discussion 390, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Ike O, Shimuzu V, Hitomi S, et al. Treatment of malignant pleural effusions with doxorubicin hydrochloride-containing ply (L-lactic acid) microspheres. Chest. 1991;99:911. doi: 10.1378/chest.99.4.911. [DOI] [PubMed] [Google Scholar]
- 55.Monneuse O, Beaujard AC, Guibert B, et al. Long-term results of intrathoracic chemohyperthermia (ITCH) for the treatment of pleural malignancies. Br J Cancer. 2003;88:1839. doi: 10.1038/sj.bjc.6601000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Ruth S, Baas P, Haas RL, et al. Cytoreductive surgery combined with intraoperative hyperthermic intrathoracic chemotherapy for stage I malignant pleural mesothelioma. Ann Surg Oncol. 2003;10:176. doi: 10.1245/aso.2003.03.022. [DOI] [PubMed] [Google Scholar]
- 57.Bone G. Postoperative empyema and survival in lung cancer. Br Med J. 1973;2(5859):178. doi: 10.1136/bmj.2.5859.178-a. [DOI] [PMC free article] [PubMed] [Google Scholar]; Minasian H, Lewis CT, Evans SJ. Influence of postoperative empyema on survival after pulmonary resection for bronchogenic carcinoma. Br Med J. 1978;2(6148):1329–31. doi: 10.1136/bmj.2.6148.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawaetz O, Halkier E. The relationship between postoperative empyema and long-term survival after pneumonectomy. Results of surgical treatment of bronchogenic carcinoma. Scand J Thorac Cardiovasc Surg. 1980;14(1):113–7. doi: 10.3109/14017438009109865. [DOI] [PubMed] [Google Scholar]
- 59.Bakker W, Nijhuis-Heddes JM, van der Velde EA. Post-operative intrapleural BCG in lung cancer: a 5-year follow-up report. Cancer Immunol Immunother. 1986;22(2):155–9. doi: 10.1007/BF00199131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson BW, Manning LS, Bowman RV, et al. The scientific basis for the immunotherapy of human malignant mesothelioma. Eur Respir Rev. 1993;3:195. [Google Scholar]
- 61.Astoul P, Picat-Joossen D, Viallat JR, Boutin C. Intrapleural administration of interleukin-2 for the treatment of patients with malignant pleural mesothelioma: a phase II study. Cancer. 1998;83:2099. doi: 10.1002/(sici)1097-0142(19981115)83:10<2099::aid-cncr8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 62.Boutin C, Nussbaum E, Monnet I, et al. Intrapleural treatment with recombinant gamma - interferon in early stage malignant mesothelioma. Cancer. 1994;74:2460. doi: 10.1002/1097-0142(19941101)74:9<2460::aid-cncr2820740912>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 63.Boutin C, Viallat JR, Van Zandwijk N, et al. Activity of intrapleural recombinant gamma-interferon in malignant mesothelioma. Cancer. 1991;67:2033. doi: 10.1002/1097-0142(19910415)67:8<2033::aid-cncr2820670804>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 64.Goey SH, Eggermont AM, Punt CJ, et al. Intrapleural adminstration of interleukin 2 in pleural mesothelioma: a phase I-II study. Br J Cancer. 1995;72:1283. doi: 10.1038/bjc.1995.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nowak AK, Lake RA, Kindler HL, Robinson BW. New approaches for mesothelioma: biologics, vaccines, gene therapy, and other novel agents. Semin Oncol. 2002;29:82. doi: 10.1053/sonc.2002.30234. [DOI] [PubMed] [Google Scholar]
- 66.Davidson JA, Musk AW, Wood BR, Morey S, Ilton M, Yu LL, et al. Intralesional cytokine therapy in cancer: a pilot study of GM-CSF infusion in mesothelioma. J Immunother. 1998;21 (5):389–98. doi: 10.1097/00002371-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 67.Vachani A, Moon E, Wakeam E, Albelda SM. Gene therapy for mesothelioma and lung cancer. Am J Respir Cell Mol Biol. 2010 Apr;42(4):385–393. doi: 10.1165/rcmb.2010-0026RT. [DOI] [PubMed] [Google Scholar]
- 68.Robinson BW, Mukherjee SA, Davidson A, et al. Cytokine gene therapy or infusion as treatment for solid human cancer. J Immunother. 1998;21:211. doi: 10.1097/00002371-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 69.Hwang HC, Smythe WR, Elshami AA, et al. Gene therapy using adenovirus carrying the herpes simplex-thymidine kinase gene to treat in vivo models of human malignant mesothelioma and lung cancer. Am J Respir Cell Mol Biol. 1995;13:7. doi: 10.1165/ajrcmb.13.1.7598939. [DOI] [PubMed] [Google Scholar]
- 70.Smythe WR, Hwang HC, Amin KM, et al. Successful treatment of experimental human mesothelioma using adenovirus transfer of the herpes simplex-thymidine kinase gene. Ann Surg. 1995;222:78. doi: 10.1097/00000658-199507000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sterman D, Treat J, Litzky LA, et al. Adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir gene therapy in patients with localized malignancy: results of a phase I clinical trial in malignant mesothelioma. Hum Gene Ther. 1998;9:1083. doi: 10.1089/hum.1998.9.7-1083. [DOI] [PubMed] [Google Scholar]
- 72.Sterman DH, Molnar-Kimber K, Iyengar T, et al. A pilot study of systemic corticosteroid administration in conjunction with intrapleural adenoviral vector administration in patients with malignant pleural mesothelioma. Cancer Gene Ther. 2000;7:1511. doi: 10.1038/sj.cgt.7700269. [DOI] [PubMed] [Google Scholar]
- 73.Sterman DH, Recio A, Vachani A, Sun J, Cheung L, DeLong P, Amin KM, Litzky LA, Wilson JM, Kaiser LR, Albelda SM. Long-term follow-up of patients with malignant pleural mesothelioma receiving high-dose adenovirus herpes simplex thymidine kinase/ganciclovir suicide gene therapy. Clin Cancer Res. 2005;15; 11(20):7444–53. doi: 10.1158/1078-0432.CCR-05-0405. [DOI] [PubMed] [Google Scholar]
- 74.Schwarzenberger P, Lei DH, Freeman SM, et al. Antitumor activity with the HSV-tk-gene-modified cell line PA-1-STK in malignant mesothelioma. Am J Respir Cell Mol Biol. 1998;19:333. doi: 10.1165/ajrcmb.19.2.3123. [DOI] [PubMed] [Google Scholar]
- 75.Schwarzenberger P, Byrne P, Gaumer R, Norton J, Harrison L, Marrogi A, Kolls JK. Treatment of mesothelioma with gene-modified PA1STK cells and ganciclovir: aphase I study. Cancer Gene Ther. 2011;18(12):906–12. doi: 10.1038/cgt.2011.60. [DOI] [PubMed] [Google Scholar]
- 76.Mukherjee S, Haenel T, Himbeck R, et al. Replication-restricted vaccinia as a cytokine gene therapy vector in cancer: persistent transgene expression despite antibody generation. Cancer Gene Ther. 2000;7:663. doi: 10.1038/sj.cgt.7700133. [DOI] [PubMed] [Google Scholar]
- 77.Odaka M, Sterman D, Wiewrodt R, Zhang Y, Kiefer M, Amin K, et al. Eradication of intraperitoneal and distant tumor by adenovirus-mediated interferon-beta gene therapy due to induction of systemic immunity. Cancer Research. 2001;61:6201–6212. [PubMed] [Google Scholar]
- 78.Vachani A, Sterman DH, Albelda SM. Cytokine Gene Therapy for Malignant Pleural Mesothelioma. Journal of Thoracic Oncology. 2007;2(4):265–267. doi: 10.1097/01.JTO.0000263706.23579.35. [DOI] [PubMed] [Google Scholar]
- 79.Sterman DH, Recio A, Carroll RG, Gillespie CT, Haas A, Vachani A, Kapoor V, Sun J, Hodinka R, Brown JL, Corbley MJ, Parr M, Ho M, Pastan I, Machuzak M, Benedict W, Zhang XQ, Lord EM, Litzky LA, Heitjan DF, June CH, Kaiser LR, Vonderheide RH, Albelda SM. A phase I clinical trial of single-dose intrapleural IFN-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin Cancer Res. 2007;13:4456–66. doi: 10.1158/1078-0432.CCR-07-0403. [DOI] [PubMed] [Google Scholar]
- 80.Sterman DH, Recio A, Haas AR, Vachani A, Katz SI, Gillespie CT, Cheng G, Sun J, Moon E, Pereira L, Wang X, Heitjan DF, Litzky L, June CH, Vonderheide RH, Carroll RG, Albelda SM. A phase I trial of repeated intrapleural adenoviral-mediated interferon-beta gene transfer for mesothelioma and metastatic pleural effusions. Mol Ther. 2010;18(4):852–60. doi: 10.1038/mt.2009.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sterman DH, Haas AR, Moon E, Recio A, Schwed D, Vachani A, Katz S, Gillespie C, Cheng GJ, Sun J, Papasavvas E, Montaner LJ, Heitjan DF, Litzky L, Friedberg J, Culligan M, June CH, Carroll RG, Albelda SM. A Trial of Intrapleural Adenoviral-mediated Interferon-alpha2b Gene Transfer for Malignant Pleural Mesothelioma. Am J Respir Crit Care Med. 2011;184:1395–1399. doi: 10.1164/rccm.201103-0554CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, Chew A, Carroll RG, Scholler J, Levine BL, Albelda SM, June CH. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70(22):9053–61. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]


