Abstract
Based on our theory, main triplets of amino acid residues have been discovered in cell-adhesion receptors (integrins) of marine sponges, which participate as homologies in the interface between two major immune molecules, MHC class I (MHCI) and CD8αβ. They appear as homologies also in several human neural receptor heteromers and subunits. The obtained results probably mean that neural and immune receptors also utilize these structural integrin triplets to form heteromers and ion channels, which are required for a tuned and integrated intracellular and intercellular communication and a communication between cells and the extracellular matrix with an origin in sponges, the oldest multicellular animals.
Keywords: Neural receptor-receptor interactions, Receptor interface, Marine sponges, Triplet homologies
Introduction
Based on a mathematical approach, Tarakanov and Fuxe (2010, 2011) have deduced a set of triplet homologies (so called ‘triplet puzzle’) that may be responsible for protein-protein interactions, including receptor heteromers and human immunodeficiency virus (HIV) entry. For example, the triplet of amino acid residues ITL (Ile-Thr-Leu) appears in both receptors of any of six receptor heteromers: GABAB1-GABAB2 (GABAB receptor), GABAB1-mGluR1, GABAB1-CXCR4, CXCR4-CCR2, 5HT1B-5HT1D, and MHC class I MHCI-CD8. At the same time, this triplet ITL does not appear in both receptors of any of known non-heteromers (GABAB2-A2A, A2A-D1, A1-D2, NTSR1-D1, TSHR-D2, and CD4-D2; see Tarakanov and Fuxe 2010). According to recent biochemical studies (Borroto-Escuela et al. 2010, 2011, 2012a,b; Romero-Fernandez et al. 2011), such triplets exist in the interacting domains forming the receptor interface. Furthermore, a ‘guide-and-clasp’ manner of receptor-receptor interactions has been proposed where the ‘adhesive guides’ may be the triplet homologies (Tarakanov and Fuxe, 2010). According to recent bioinformatic studies (Tarakanov et al. 2012 a,b,c,d), several triplet homologies of such receptor heteromers in human brain may be the same as in cell-adhesion receptors of marine sponges, known to be highly conserved from the lowest metazoa to vertebrates (Gamulin et al. 1994; Muller 1997; Pancer et al. 1997; Buljan and Bateman 2009). Interactions between such triplets probably represent a general molecular mechanism for receptor-receptor interactions (Fuxe et al 2012) and may play an important role in human learning (Agnati et al. 2003) and some diseases (Tarakanov et al. 2009).
In the current paper, many of such triplets have been found in integrins of marine sponges together with human alpha and beta integrins. This means that such triplet homologies may play a role in alpha-beta heterodimeric complexes forming integrin receptors and interact with extracellular matrix proteins (Barczyk et al. 2010). Of especial interest is that the same integrin triplets exist also in the murine and human MHCI interface with CD8, in human neural receptors and in the interface of both protomers of several receptor heteromers. The presence of such triplet homologies in several receptor subunits building up the neuromuscular nicotinic cholinergic receptors has also been demonstrated. At least one of the homologies may have a role in the intermolecular subunit interactions of this ion channel receptor.
Methods
Amino acid codes of receptors and other proteins have been obtained from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov) and the Universal Protein Resource (http://www.uniprot.org). Table 1 summarizes data on proteins used. In abstract mathematical terms, any protein is just a word coded by a 20-letter alphabet where triplet is any 3-letter subword. Thus, triplet homology is any triplet which exists in both given words. Our theory of triplet puzzle supposes some basic set of triplets as a code that determines whether two receptors bind or not (Tarakanov and Fuxe 2010). None of the widely used software like Clustal (http://www.clustal.org/), AGGRESCAN (http://bioinf.uab.es/aggrescan/), accelrys (http://accelrys.com/), and so on seems to be able to deal with so specific and complicated combinatorial puzzle. Our original software has been developed to determine such basic set of triplet homologies from two given sets of protein-protein pairs (which bind and do not bind). The core of this software is the computing of all triplet homologies between two given words (but not only their alignment like in the above mentioned Clustal). The method consists in forming the binary matrix of all one-letter homologies (which element is 1 if there is homology and 0 otherwise) and then filtering this matrix using rather specific rules of so called cellular automata (for example, see Tarakanov and Prokaev 2007; http://youtu.be/1DevThU5fyM).
Table 1.
Data on proteins used
| Protein | Species | Type | Accession code |
|---|---|---|---|
| ITGA | Sponge (Geodia cydonium) | Metazoan adhesion receptor subunit Integrin-α | CAA65943 |
| ITGB | Sponge (Geodia cydonium) | Metazoan adhesion receptor subunit Integrin-β | CAA77071 |
| ITGB4 | Sponge (Marichromatium purpuratum) | Metazoan adhesion receptor subunit Integrin-β4 | ZP_08774040 |
| MHCI | Mouse (Mus musculus) | H-2 class I histocompatibility antigen | NP_001001892 |
| CD8a | Mouse | T-cell surface glycoprotein chain CD8α | NP_001074579 |
| CD8b | Mouse | T-cell surface glycoprotein chain CD8β | NP_033988 |
| MHCI | Human (Homo sapiens) | H-2 class I histocompatibility antigen | AAA59599 |
| CD8a | Human | T-cell surface glycoprotein chain CD8α | NP_001139345 |
| CD8b | Human | T-cell surface glycoprotein chain CD8β | NP_757362) |
| CXCR4 | Human | Chemokine receptor | P61073 |
| TSHR | Human | Thyroid stimulating hormone receptor | NP_000360 |
| FGFR1 | Human | Fibroblast growth factor receptor | NP_075598 |
| 5HT1A | Human | Serotonin receptor | AAH69159 |
| Collagen | Human | Matrix protein | P02452 |
| ITGAIIB | Human | Integrin receptor subunit-αIIb | P08514 |
| ITGAL | Human | Integrin receptor subunit-αL | P20701 |
| ITGAM | Human | Integrin receptor subunit-αM | NP_001139280 |
| ITGAV | Human | Integrin receptor subunit-αV | EAX10934 |
| ITGAX | Human | Integrin receptor subunit-αX | NP_000878 |
| ITGB2 | Human | Integrin receptor subunit-β2 | NP_000202 |
| ITGB3 | Human | Integrin receptor subunit-β3 | NP_000203 |
| ITGB4 | Human | Integrin receptor subunit-β4 | NP_000204 |
| ITGB5 | Human | Integrin receptor subunit-β5 | NP_000205 |
| ITGB6 | Human | Integrin receptor subunit-β6 | P18564 |
| ITGB8 | Human | Integrin receptor subunit-β8 | P26012 |
| ACHA | Human | Acetylcholine receptor subunit-α | P02708 |
| ACHB | Human | Acetylcholine receptor subunit-β | P11230 |
| ACHD | Human | Acetylcholine receptor subunit-δ | Q07001 |
| ACHE | Human | Acetylcholine receptor subunit-ε | Q04844 |
| mGluR1 | Human | Metabotropic glutamate receptor | NP_000829 |
| GABAB2 | Human | γ-aminobutyric acid receptor subunit-2 | O75899 |
| GABAB1 | Human (Homo sapiens) | γ-aminobutyric acid receptor subunit-1 | NP_001461 |
| GABAB1 | Mouse (Mus musculus) | " | NP_062312 |
| GABAB1 | Norway rat (Rattus norvegicus) | " | NP_112290 |
| GABAB1 | Western clawed frog (Xenopus (Silurana) tropicalis) | " | NP_001107291 |
| GABAB1 | Green puffer (Tetraodon nigroviridis) | " | uniprot/Q4S9D9 |
| GABAB1 | Zebrafish (Danio rerio) | " | NP_001070794 |
| GABAB1 | African malaria mosquito (Anopheles gambiae) | " | uniprot/Q7PME5 |
| GABAB1 | Drosophila pseudoobscura | " | XP_001357356 |
| GABAB1 | Human body louse (Pediculus humanus corporis) | " | XP_002430445 |
| GABAB1 | Caenorhabditis elegans | " | ACE63490 |
No experimental research has been performed on humans and/or animals.
Results
The triplets ITL (Ile-Thr-Leu), RPA (Arg-Pro-Ala), DGR (Asp-Gly-Arg), LLG (Leu-Leu-Gly), and GLL (Gly-Leu-Leu) of the integrin receptors of marine sponges appear as homologies in murine and human MHCI, GABAB1, and human integrin receptor heteromers (see Tables 2 and 3, Figures 1 and 2). The triplets ITL (Ile-Thr-Leu) and DGR (Asp-Gly-Arg) are particularly interesting. For example, the triplet ITL is in the interface providing the binding between MHCI and CD8αβ (Wang et al. 2009). This triplet homology exists also in three GABAB1 receptor heteromers of human brain: GABAB1-GABAB2 forming the GABAB receptor (Marshall et al. 2001), GABAB1-mGluR1, and GABAB1-CXCR4 and may mediate the interaction in two of them (see Table 3 and Figure 1). In the first two heteromers also triplet GLL (Gly-Leu-Leu) may participate in the interaction (see Table 3 and Figure 2).
Table 2.
Example of integrin triplets of marine sponges in murine and human proteins
| Protein | Species | Type | LLG | GLL | ITL | RPA | GDR | RDG | DGR |
|---|---|---|---|---|---|---|---|---|---|
| ITGA | Sponge | Integrin-α | - | - | + | + | + | - | - |
| ITGB | Sponge | Integrin-β | + | + | - | - | - | - | - |
| ITGB4 | Sponge | Integrin-β | - | - | - | - | - | + | + |
| MHC Class I | Mouse | Immune receptor | + | - | + | + | - | - | + |
| CD8a | Mouse | Immune receptor | + | - | + | - | - | - | - |
| CD8b | Mouse | Immune receptor | - | - | - | - | - | - | - |
| MHC Class I | Human | Immune receptor | + | - | + | + | - | + | + |
| CD8a | Human | Immune receptor | - | - | + | + | - | - | - |
| CD8b | Human | Immune receptor | - | + | + | - | - | - | - |
| CXCR4 | Human | Immune receptor | - | - | + | - | - | - | - |
| TSHR | Human | Endocrine receptor | - | - | - | + | - | - | - |
| FGFR1 | Human | Receptor tyrosine kinase | - | - | - | + | - | - | - |
| 5HT1A | Human | Neural receptor | + | - | - | - | - | - | - |
| Collagen | Human | Matrix protein | - | - | - | - | + | + | + |
| ITGAIIB | Human | Integrin-α | + | + | - | - | - | + | + |
| ITGAL | Human | Integrin-α | - | + | - | - | - | - | - |
| ITGAM | Human | Integrin-α | + | + | - | - | - | - | - |
| ITGAV | Human | Integrin-α | + | + | - | - | - | - | - |
| ITGAX | Human | Integrin-α | + | + | + | - | + | - | |
| ITGB2 | Human | Integrin-β | - | + | - | - | - | - | + |
| ITGB3 | Human | Integrin-β | - | + | - | - | - | - | + |
| ITGB4 | Human | Integrin-β | + | - | - | - | - | - | - |
| ITGB5 | Human | Integrin-β | + | - | - | - | - | + | - |
| ITGB6 | Human | Integrin-β | - | + | - | - | - | - | - |
| ITGB8 | Human | Integrin-β | - | + | - | - | + | - | - |
| ACHA | Human | Neural receptor subunit | + | - | - | - | - | - | - |
| ACHB | Human | Neural receptor subunit | + | - | + | + | + | - | - |
| ACHD | Human | Neural receptor subunit | - | + | + | + | - | - | - |
| ACHE | Human | Neural receptor subunit | + | + | - | - | - | - | - |
| GABAB1 | Human | Neural receptor | + | + | + | - | - | - | - |
| GABAB2 | Human | Neural receptor | - | + | + | - | - | - | - |
| mGluR1 | Human | Neural receptor | - | + | + | - | - | - | - |
(+ yes, - no).
Table 3.
Example of integrin triplets of marine sponges in the protomers of human receptor heteromers and in subunits of the neuromuscular nicotinic receptor
| Receptor heteromer | Reference | Function | LLG | GLL | ITL | RPA | DGR |
|---|---|---|---|---|---|---|---|
| MHCI-CD8a | Gao et al. (1997) | Adaptive immune response | - | - | # | + | - |
| Wang et al. (2009) | |||||||
| MHC1-CD8b | Wang et al. (2009) | Adaptive immune response | - | - | # | - | - |
| CD8a-CD8b | Wang et al. (2009) | Coreceptor of T cells | - | - | + | - | - |
| ITGAIIB-ITGB3 | Barczyk et al. (2010) | RGD (Arg-Gly-Asp) receptor | - | # | - | - | # |
| ITGAV-ITGB3 | Barczyk et al. (2010) | RGD receptor | - | # | - | - | - |
| ITGAV-ITGB5 | Barczyk et al. (2010) | RGD receptor | # | - | - | - | - |
| ITGAV-ITGB6 | Barczyk et al. (2010) | RGD receptor | - | # | - | - | - |
| ITGAV-ITGB8 | Barczyk et al. (2010) | RGD receptor | - | # | - | - | - |
| ITGAL-ITGB2 | Barczyk et al. (2010) | Leukocyte receptor | - | + | - | - | - |
| ITGAM-ITGB2 | Barczyk et al. (2010) | Leukocyte receptor | - | + | - | - | - |
| ITGAX-ITGB2 | Barczyk et al. (2010) | Leukocyte receptor | - | + | - | - | - |
| GABAB1-GABAB2 | Marshall et al. (2001) | Activation of the potassium channels and regulation of receptor trafficking | - | # | # | - | - |
| GABAB1-mGluR1 | Hirono et al. (2001) | Modulation of excitatory transmission | - | # | + | - | - |
| GABAB1-CXCR4 | Guyon and Nahon (2007) | Modulation of neuroendocrine systems | - | - | # | - | - |
| ACHA-ACHB | Changeux et al. 1984 | Part of the neuromuscular nicotinic receptor | + | - | - | - | - |
| ACHA-ACHE | Changeux et al. 1984 | Part of the neuromuscular nicotinic receptor | + | - | - | - | - |
| ACHB-ACHD | Changeux et al. 1984 | Part of the neuromuscular nicotinic receptor | - | - | + | # | - |
(+ yes in both receptors, # may mediate their interaction, - no in any receptor).
Figure 1.
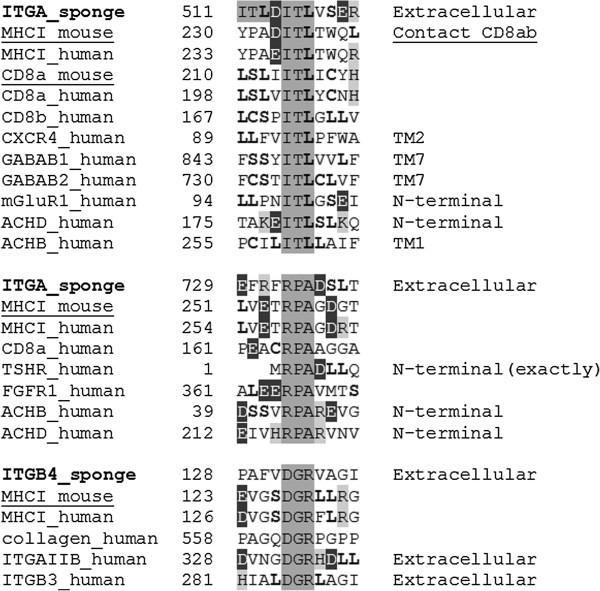
Example of the triplets ITL, RPA, and DGR (dark-shaded letters) in the integrins of marine sponges existing in the murine (underlined) and human MHCI-CD8 complex, human collagen (DGR triplet), and human receptor heteromers: TM1, TM2 and TM7 are the first, the second and the seventh transmembrane α-helices of ACHB, CXCR4, and GABAB (GABAB1-GABAB2 heteromer) receptors, respectively, and contain the ITL triplet. The RPA triplet is also found in the TSHR and FGFR1; the RPA but not the ITL triplet homologies are in a position to contribute to the physical interaction between the beta and delta subunits of the neuromuscular nicotinic receptor (ACHB-ACHD); light-shaded letters are positively charged amino acids (R, K, and H), whereas dark-shaded white letters are negatively charged amino acids (D and E); bold letters are main players of leucine-rich motifs (L, S, and C).
Figure 2.
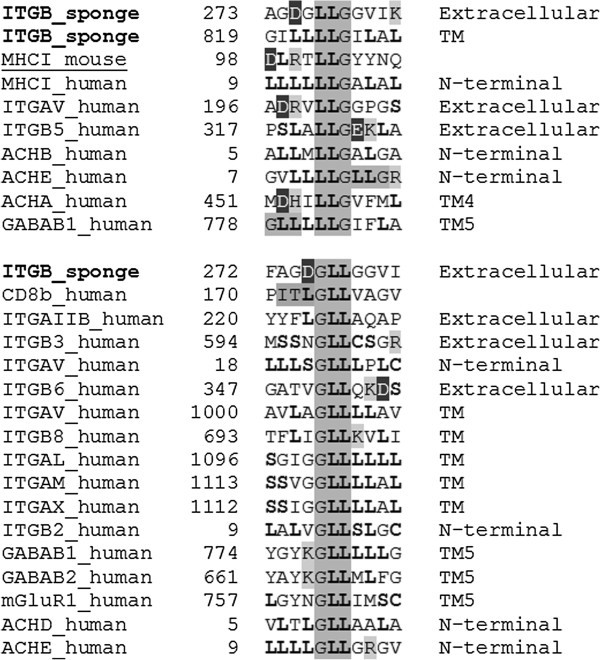
Example of the triplets LLG and GLL (dark-shaded letters) in the integrins of marine sponges, murine (underlined) and human MHC Class I and human receptor heteromers.
The triplet DGR (Asp-Gly-Arg) is in fact the inverse triplet of RGD (Arg-Gly-Asp) that provides the binding site for integrin RGD-binding receptors (see Table 3). Moreover, a small peptide ligand RGD (Arg-Gly-Asp) that mimics extracellular matrix protein binding to integrins also causes impairments in plasticity at glutamatergic synapses (Wiggins et al. 2011).
The evolution of the ITL triplet in the GABAB1 receptor subunit is displayed in Figure 3. In phylogeny, it appears to begin in fish (Tetraodon) and then continues to man, while it is missing in zebrafish (Danio rerio). Thus, the usefulness of the ITL triplet in recognition is rediscovered in the fish GABAB1 receptor.
Figure 3.
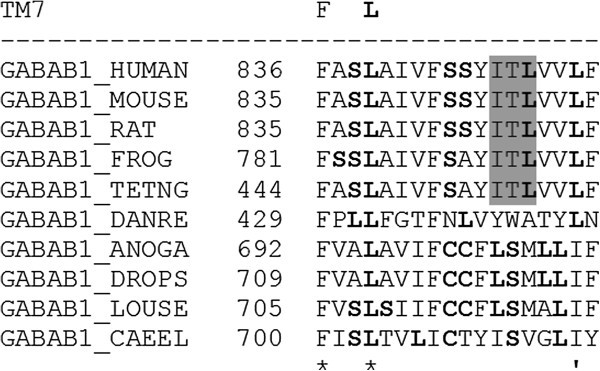
The triplet ITL (dark-shaded letters) during the evolution of GABAB1 subunit: CAEEL (Caenorhabditis elegans), LOUSE (Pediculus humanus corporis), DROPS (Drosophila pseudoobscura), ANOGA (Anopheles gambiae), DANRE (Danio rerio), TETNG (Tetraodon nigroviridis), FROG (Xenopus tropicalis), RAT (Rattus norvegicus), MOUSE (Mus musculus), and HUNAN (Homo sapiens); asterisk (*) marks homologies (F and L); quote (') marks leucine-like homologies (L and I); bold letters are main players of leucine-rich motifs (L, S, and C).
Furthermore, the RPA triplet homology in the beta and delta interacting nicotinic subunits of the neuromuscular nicotinic receptor (see Changeux et al. 1984) is in a location (N-terminal parts of ACHB and ACHD) where it may participate in forming part of their interface (see Figure 1 and Table 3).
Discussion
The triplet ITL (Ile-Thr-Leu) found in integrins of marine sponges is presented as a homology in the interface between MHC Class I and CD8αβ heterodimer (coreceptor in T cells). It is postulated that this triplet homology can contribute to the formation of the MHCI-CD8 heteromeric complex which leads to a strong activation of the T cell by guiding the T-cell receptor into relevant self-MHC recognition (see Wang et al. 2009). Thus, it seems possible that the ITL triplet may have a critical role in the interaction between these two immune receptors which is necessary for appropriate T cell function. A mutation of the ITL triplet in these immune receptors will be of value to test this hypothesis. The indications have also been obtained that triplet homology ITL in the N-terminal of beta and delta nicotinic receptor subunits of the neuromuscular nicotinic receptor may help mediate their interaction in the subunit interface.
Conclusion
Integrin triplets of marine sponges found in the interface of human receptor heteromers and even in the interface between two major immune molecules MHCI-CD8 seem to confirm once more our theory. This triplet puzzle arose as a surprising merger of pure mathematics and most recent biochemical studies of receptor-receptor interactions. As a result, it appears that neural and immune receptor heteromers in humans may also utilize these structural elements originating in sponges, the oldest multicellular animals. Thus, the triplet puzzle may be an ancient and general mechanism for protein-protein recognition.
Acknowledgement
The authors have not received any support for this work.
Footnotes
Competing interests
Both authors declare that they have no competing interests.
Authors’ contributions
AT carried out the mathematical studies and computations. KF carried out the biomedical interpretation of the results. All authors read and approved the final manuscript.
Contributor Information
Alexander O Tarakanov, Email: tar@iias.spb.su.
Kjell G Fuxe, Email: kjell.fuxe@ki.se.
References
- Agnati LF, Franzen O, Ferre S, Leo G, Franco R, Fuxe K. Possible role of intramembrane receptor-receptor interactions in memory and learning via formation of long-lived heteromeric complexes: focus on motor learning in the basal ganglia. J Neural Transm Suppl. 2003;65:1–28. doi: 10.1007/978-3-7091-0643-3_1. [DOI] [PubMed] [Google Scholar]
- Barczyk M, Carracedo S, Gullbeerg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Narvaez M, Marcellino D, Parrado C, Narvaez JA, Tarakanov AO, Agnati LF, Diaz-Cabiale Z, Fuxe K. Galanin receptor-1 modulates 5-hydroxtryptamine-1A signaling via heterodimerization. Bioch Biophys Res Commun. 2010;393:767–772. doi: 10.1016/j.bbrc.2010.02.078. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Tarakanov AO, Guidolin D, Ciruela F, Agnati LF, Fuxe K. Moonlight characteristics of G protein-coupled receptors: focus on receptor heteromers and relevance for neurodegeneration. IUBMB Life. 2011;63:463–472. doi: 10.1002/iub.473. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Romero-Fernandez W, Mudo G, Perez-Alea M, Ciruela F, Tarakanov AO, Narvaez M, Di Liberto V, Agnati LF, Belluardo N, Fuxe K. FGFR1-5-HT1A heteroreceptor complexes and their enhacement of hippocampal plasticity. Biol Psych. 2012a;71:84–91. doi: 10.1016/j.biopsych.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Romero-Fernandez W, Perez-Alea M, Narvaez M, Tarakanov AO, Mudo G, Agnati LF, Ciruela F, Belluardo N, Fuxe K. The existence of FGFR1-5-HT1A receptor heterocomplexes in midbrain 5-HT neurons of the rat: relevance for neuroplasticity. J Neurosci. 2012b;32:6295–6303. doi: 10.1523/JNEUROSCI.4203-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Buljan M, Bateman A. The evolution of protein domain families. Biochem Soc Trans. 2009;37:751–755. doi: 10.1042/BST0370751. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Devillers-Thiéry A, Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984;225:1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Marcellino D, Romero-Fernandez W, Frankowska M, Guidolin D, Filip M, Ferraro L, Woods AS, Tarakanov A, Ciruela F, Agnati LF, Tanganelli S. GPCR heteromers and their allosteric receptor-receptor interactions. Curr Med Chem. 2012;19:356–363. doi: 10.2174/092986712803414259. [DOI] [PubMed] [Google Scholar]
- Gamulin V, Rinkevich B, Schäcke H, Kruse M, Müller IM, Müller WE. Cell adhesion receptors and nuclear receptors are highly conserved from the lowest metazoa (marine sponges) to vertebrates. Biol Chem Hoppe Seyler. 1994;375:583–588. doi: 10.1515/bchm3.1994.375.9.583. [DOI] [PubMed] [Google Scholar]
- Gao GF, Tormo J, Gerth UC, Wyer JR, McMichael AJ, Stuart DI, Jakobsen NK. Crystal structure of the complex between human CD8α and HLA-A2. Nature. 1997;387:630–634. doi: 10.1038/42523. [DOI] [PubMed] [Google Scholar]
- Guyon A, Nahon JL. Multiple actions of the chemokine stromal cell-derived factor 1a on neuronal activity. J Mol Endocrinol. 2007;38:365–376. doi: 10.1677/JME-06-0013. [DOI] [PubMed] [Google Scholar]
- Hirono M, Yoshioka T, Konishi S. GABA(B) receptor activation enhances mGluR-mediated responses at cerebellar excitatory synapses. Nat Neurosci. 2001;4:1207–1216. doi: 10.1038/nn764. [DOI] [PubMed] [Google Scholar]
- Marshall FH, Jones KA, Kaupmann K, Bettler B. GABAB receptors – the first 7TM heterodimers. Trends Pharmacol Sci. 2001;20:396–399. doi: 10.1016/S0165-6147(99)01383-8. [DOI] [PubMed] [Google Scholar]
- Muller WEG. Origin of metazoan adhesion molecules and adhesion receptors as deduced from cDNA analyses in the marine sponge Geodia cydonium: a review. Cell Tissue Res. 1997;289:383–395. doi: 10.1007/s004410050885. [DOI] [PubMed] [Google Scholar]
- Pancer Z, Kruse M, Muller I, Muller WEG. On the origin of metazoan adhesion receptors: Cloning of integrin α subunit from the sponge Geodia cydonium. Mol Biol Evol. 1997;14:391–398. doi: 10.1093/oxfordjournals.molbev.a025775. [DOI] [PubMed] [Google Scholar]
- Romero-Fernandez W, Borroto-Escuela DO, Tarakanov AO, Mudo G, Narvaez M, Perez-Alea M, Agnati LF, Ciruela F, Belluardo N, Fuxe K. Agonist-induced formation of FGFR1 homodimers and signaling differ among members of the FGF family. Biochem Biophys Res Commun. 2011;409:764–768. doi: 10.1016/j.bbrc.2011.05.085. [DOI] [PubMed] [Google Scholar]
- Tarakanov AO, Fuxe KG. Triplet puzzle: homologies of receptor heteromers. J Mol Neurosci. 2010;41:294–303. doi: 10.1007/s12031-009-9313-5. [DOI] [PubMed] [Google Scholar]
- Tarakanov AO, Fuxe KG. The triplet puzzle of homologies in receptor heteromers exists also in other types of protein-protein interactions. J Mol Neurosci. 2011;44:173–177. doi: 10.1007/s12031-011-9511-9. [DOI] [PubMed] [Google Scholar]
- Tarakanov A, Prokaev A. Identification of cellular automata by immunocomputing. J Cellular Automata. 2007;2:39–45. [Google Scholar]
- Tarakanov AO, Fuxe KG, Agnati LF, Goncharova LB. Possible role of receptor heteromers in multiple sclerosis. J Neural Transm. 2009;116:989–994. doi: 10.1007/s00702-009-0197-x. [DOI] [PubMed] [Google Scholar]
- Tarakanov AO, Fuxe KG, Borroto-Escuela DO. On the origin of the triplet puzzle of homologies in receptor heteromers: Immunoglobulin triplets in different types of receptors. J Mol Neurosci. 2012a;46:616–621. doi: 10.1007/s12031-011-9649-5. [DOI] [PubMed] [Google Scholar]
- Tarakanov AO, Fuxe KG, Borroto-Escuela DO. On the origin of the triplet puzzle of homologies in receptor heteromers: toll-like receptor triplets in different types of receptors. J Neural Transm. 2012b;119:517–523. doi: 10.1007/s00702-011-0734-2. [DOI] [PubMed] [Google Scholar]
- Tarakanov AO, Fuxe KG, Borroto-Escuela DO. Integrin triplets of marine sponges in human brain receptor heteromers. J Mol Neurosci. 2012c;48:154–160. doi: 10.1007/s12031-012-9793-6. [DOI] [PubMed] [Google Scholar]
- Tarakanov AO, Fuxe KG, Borroto-Escuela DO. Integrin triplets of marine sponges in human D2 receptor heteromers. J Recept Sig Transd. 2012d;32:202–208. doi: 10.3109/10799893.2012.692119. [DOI] [PubMed] [Google Scholar]
- Wang R, Natarajan K, Margulies DH. Structural basis of the CD8ab/MHCI interaction: focused recognition orients CD8b to a T cell proximal position. J Immunol. 2009;183:2554–2564. doi: 10.4049/jimmunol.0901276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins A, Smith RJ, Shen HW, Kalivas PW. Integrins modulate relapse to cocain-seeking. J Neurosci. 2011;31:16177–16184. doi: 10.1523/JNEUROSCI.3816-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


