Abstract
Background:
Cigarette smoking is a worldwide social epidemic and it is one of the main causes of preventable death and disability. Gingivitis, periodontitis, pocket depth, attachment loss, alveolar bone loss, and tooth loss are some of oral pathologies commonly found in cigarette smokers. The aim of this study was to explore, for the first time among Nigerians, the interplay between components of cigarette smoke and salivary levels of immunoglobulin classes so as to provide oral immunological based reasons for oral diseases in cigarette smokers.
Materials and Methods:
In this case-control study, 5 mL of unstimulated saliva was collected in plain sample bottles from 24 active smokers who smoke at least 6 sticks of cigarette per day and 21 sex and age-matched non-smokers who were apparently healthy. The samples were spun and supernatant stored at -20°C until assayed. The immunoglobulin levels of the samples were estimated using enzyme-linked immunosorbent assay (ELISA). Student's t-test (unpaired) was used to determine significant differences between the two groups. P values less than 0.05 was considered significant.
Results:
No significant differences were observed in the mean salivary levels of IgG, IgA, and IgE. Only IgM was significantly lower in smokers compared with non-smokers (P = 0.038). The proportion of smokers with detectable level of salivary IgE was lower compared with controls.
Conclusion:
Our study showed that there is decreased salivary IgM in smokers. This observation suggests that reduced salivary immunoglobulin level of IgM might be involved in the pathogenesis of oral diseases in cigarette smokers.
Keywords: Cigarette, immunoglobulin, nicotine, pan-hypogammaglobulin, saliva
INTRODUCTION
Tobacco smoke is a complex mixture of more than 4500 chemicals, many of which have toxic and/or carcinogenic activity.[1] Some of the components, which could be in the form of gases, vapors, and particulates, include carbon monoxide, hydrogen cyanide, phenols, acrolein, ammonia, formaldehyde, nicotine, nitrosamine, tar, heavy metals, and at least 48 known cancer-producing substances.[2]
Cigarette smoking is a worldwide social epidemic and it is one of the main causes of preventable death and disability.[1] It is an established risk factor for premature mortality due to cancer, cardiovascular disease, and chronic obstructive pulmonary disease.[3] The increased susceptibility of cigarette smokers to infections reflects multifunctional alteration of their innate and adaptive immune responses.[1,3]
Acrolein, a toxic unsaturated aldehyde, affects neutrophil functions and thus increases susceptibility to lung infections.[4] Leukocytosis is a well-known effect of cigarette smoking though the function of these cells is greatly reduced.[5,6] Eichel et al.[7] reported that a single cigarette provided enough toxic material that completely inhibited the function of oral salivary neutrophils in situ. Reduced phagocytic activity of neutrophils was also reported in smokers, which could be responsible for decreased defence of the gingival against bacterial attack.[8,9]
Continued exposure to cigarette smoking has been shown to affect both humoral and cellular immune responses. Initially (hours to days), there is an acute depression of the immune response followed by stimulation (weeks to months), and finally depression of the immune system sets in. This causes a decreased response to antigens and reduced serum concentration of IgG, IgM and IgA,[10] and also increased levels of autoantibodies notably; anti-nuclear rheumatoid factors.[11,12]
Although several reports have shown that cigarette smoking significantly reduces serum levels of immunoglobin classes in humans,[10,13] a recent report by Arinola et al.[14] showed significantly elevated serum levels IgG and IgM in active smokers. Similarly, conflicting observations have been reported on levels of salivary immunoglobulin classes in smokers. Bennet and Read[15] reported significantly reduced level of salivary IgA in smokers while EngstrÃm and EngstrÃm[16] reported increased levels of salivary IgA in smokers.
Studies on serum levels of immunoglobulin classes in smokers are conflicting. More so, research on salivary levels of immunoglobulin classes in cigarette smokers has been rarely explored. To explore, for the first time among Nigerians, the interplay between components of cigarette smoke and salivary levels of immunoglobulin classes, our study estimated salivary immunoglobulin classes (IgG, IgA, IgM, IgE) in Nigerian smokers to provide oral immunological based reasons for oral diseases in cigarette smokers.
MATERIALS AND METHODS
Forty-five (45) subjects were recruited for this study after obtaining informed consent from each subject and an ethical approval from the University of Ibadan/University College Hospital (U.I/U.C.H) Joint Ethics Review Committee. The test group consisted of 24 active smokers who smoke at least 6 sticks of cigarette per day while the control group comprised 21 sex- and age-matched non-smokers who were apparently healthy. All subjects were screened of periodontitis and other oral diseases. Those with oral diseases were excluded from the study. Also excluded were individuals with pregnancy, diabetes, and human immunodeficiency virus (HIV) infection. A short structured questionnaire was administered on each subject to obtain information on age, sex, occupation, cigarette smoking, and drug consumption.
About 5 mL of unstimulated saliva was collected from each subject using the spitting method into plain sample bottles. The samples were collected between 9 am and 11 am, at least 1 h after eating or washing of mouth. The samples were centrifuged at 3000× g for 5 min and the clear supernatant gently pipetted out into another clean plain bottle and stored at -20°C until analysed.
Immunoglobulin levels were estimated using enzyme-linked immunosorbent assay (ELISA) supplied by Immunology Consultant Laboratory, USA. IgE kit was supplied by Leinco Technologies, USA. The assay was carried out following manufacturer's instructions.
Statistical analysis
The data were presented as mean and standard deviation. Student's t-test (unpaired) was used to determine significant difference between the means. The 5% (P < 0.05) level of significance was considered significant.
RESULTS
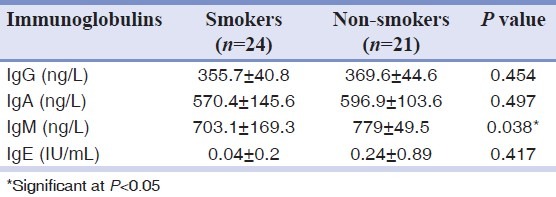
The mean ages of smokers and non-smokers were 39.9 and 39.5 years, respectively. No significant differences were observed in the mean salivary levels of IgG, IgA and IgE. Only IgM was significantly low in smokers compared with non-smokers (P = 0.038).
The mean level of salivary IgE was lower in smokers compared with control. Only 1 smoker (4.17%) had a detectable level of salivary IgE (0.04) while two non-smokers (9.52%) had detectable levels of IgE (0.24). Also the proportion of smokers with detectable level of salivary IgE was lower compared with controls [Table 1].
Table 1.
Levels of salivary immunoglobulin classes in active smokers and non-smokers
DISCUSSION
Cigarette smoking is among social practices commonly found in some Nigerian youth, despite its adverse health consequences.[6] Gingivitis, periodontitis, pocket depth, attachment loss, alveolar bone loss, and tooth loss are some of oral pathologies commonly found in cigarette smokers.[9] Tobacco smoking predisposes to infection, emphysema, and lung cancer. Herr et al.[17] reported that current or former smoking is associated with reduced levels of human β-defensins 2 (hBD2) in pharyngeal washes and sputum of patients with acute pneumonia. Of note, smoking is associated with reduced levels of surfactant proteins A and D (SP-A and SP-D).[18,19]
IgA is the predominant immunoglobulin secreted into external secretions including saliva and tears. Its increase could be due to increased local infection, increased antigenic inflammatory stimulus, increased local synthesis, and local host reaction against the presence of disease.[20] Levels of other immunoglobulin classes (IgG, IgM and IgE) have also been reported to decrease in oral diseases as observed in gingival fluid exudates.[10]
The mean levels of all the immunoglobulin classes were reduced in the saliva of smokers compared with non-smokers. However, only the salivary IgM mean level was significantly reduced in smokers (P = 0.038). This observation contradicts the report of EngstrÃm and EngstrÃm[16] who observed increased salivary IgA only. They suggested that their observation could be a reflection of protection of the oral mucosa. Earlier report by Bennet and Read[15] who reported a significantly low salivary level of IgA only partially supports our observed low salivary IgA level in smokers. In our study, the exclusion of subjects with oral diseases could be responsible for the observed differences in salivary IgA as there could be upsurge in oral antibodies production consequent to oral infection or diseases. More so, our observation could be as a result of nicotine contained in cigarette as nicotine affects the exocrine glands by an initial increase in salivary secretions followed by inhibition of the secretions.[9]
A study carried out on patients with oral mucosal disease showed higher level of salivary IgG. The causative effect was suggested to be increased permeability of oral mucosa which made it easy for the passage of IgG from vascular and extra vascular compartment into saliva by passive transmucosal diffusion.[20,21] Ferson[13] had earlier reported reduced serum immunoglobulin classes in smokers. Experimental studies also showed that mice that were chronically exposed to cigarette smoke were more susceptible to influenza and murine sarcoma viruses.[22,23] Similarly, enhanced replication of influenza virus and Legionella pneumophila was observed in the lungs of nicotine-treated animals and cells lines, respectively.[24,25]
Based on this observation, therefore, it could be suggested that reduced salivary immunoglobulin levels, especially IgM, could play an important role in the pathogenesis of oral diseases in cigarette smokers and thus could have potential benefit in screening smokers at risk of developing oral diseases.
Although only salivary IgM was significantly low in smokers, the observed non-significant reduction in all the classes of salivary immunoglobulin suggests pan-hypogammaglobulin in them. This supports the widely accepted view that cigarette smoke suppresses the immune system.
Further study is required to provide explanation for the reported blood polyclonal B cells activation[14] and the significantly reduced salivary IgM levels observed in these cigarette smokers.
Small sample size (due to strict inclusion criteria), unsuitable samples (occasioned by stimulation or collected after 11am), and exclusion of Ziehl-Neelson positive patients were some of the limitations of this study.
CONCLUSION
Our study showed that there is decreased salivary IgM in smokers. This observation suggests that reduced salivary immunoglobulin level of IgM might be involved in the pathogenesis of oral diseases in cigarette smokers.
ACKNOWLEDGEMENT
The authors wish to appreciate the contribution of Dr. Joseph Ijeboime Obafemi of the Department of Oral and Maxillofacial Surgery, Lagos University Teaching Hospital (LUTH), Lagos, Nigeria. He helped in screening out patients with periodontitis and other oral pathologies.
Footnotes
Source of Support: The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
Conflict of Interest: Nil.
REFERENCES
- 1.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–7. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 2.Howard G, Wagenknecht LE, Burke GL, Dies-Roux A, Evans GW. Cigarette smoking and progression of Atherosclerosis. JAMA. 1998;279:119–29. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- 3.Arcavi L. Benowitz NL.Cigarette Smoking and Infection. Arch Intern Med. 2004;164:2206–16. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstain EI, Nardini M, van der Vilet A. Inhibition of neutrophil apoptosis by acrolein; a mechanism of tobacco-related lung disease? Am J Physiol Lung Cell Mol Physiol. 2001;281:L732–9. doi: 10.1152/ajplung.2001.281.3.L732. [DOI] [PubMed] [Google Scholar]
- 5.Holt PG, Keast D. Environmentally induced changes in immunological function: Acute and chronic effects of inhalation of tobacco smoke and other atmospheric contaminants in man and experimental animals. Bacteriol Rev. 1977;41:205–16. doi: 10.1128/br.41.1.205-216.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arinola OG, Obikoya MA. Differential Effects of Alcoholic Beverages and Cigarette Smoke on Humoral Immunity. Afr J Biomed Res. 2009;12:241–4. [Google Scholar]
- 7.Eichel B, Sharik H. Tobacco smoke toxicity: Loss of human oral leukocyte function and fluid cell metabolism. Science. 1969;166:1424. doi: 10.1126/science.166.3911.1424. [DOI] [PubMed] [Google Scholar]
- 8.Kenny EB, Kraal JH, Saxe SR, Jones J. The effect of cigarette smoke on human oral polymorph nuclear leukocyte. J Periodont Res. 1977;12:227–34. doi: 10.1111/j.1600-0765.1977.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 9.Ramli J, Taiyeb ATB. Association between smoking and periodontal disease. Annal Dent Univ Malaya. 1999;6:21–6. [Google Scholar]
- 10.McGuire J, McQuade M, van Dyke T. Cotinine in saliva and gingival crevicular fluid of smokers with periodontal disease. J Periodontol. 1989;60:176–81. doi: 10.1902/jop.1989.60.4.176. [DOI] [PubMed] [Google Scholar]
- 11.Matthews JD, Whittingham S, Hooper BM, MacKay IR, Stenhouse NS. Association of antibodies with smoking, cardiovascular morbidity and death in the Busselton population. Lancet. 1973;2:754–9. doi: 10.1016/s0140-6736(73)91037-4. [DOI] [PubMed] [Google Scholar]
- 12.Masdotirr B, Jónsson T, Manfreðsdóttir A, Vikingsson A, Brekkan A, Valdimarson H. Smoking, rheumatoid factor isotypes and severity of rheumatoid arthritis. Rheumatology. 2000;39:1202–5. doi: 10.1093/rheumatology/39.11.1202. [DOI] [PubMed] [Google Scholar]
- 13.Ferson M, Edwards A, Lind A, Milton GW, Hersey P. Low natural killer-cell activity and immunoglobulin levels associated with smoking in human subjects. Int J Cancer. 1979;23:603–9. doi: 10.1002/ijc.2910230504. [DOI] [PubMed] [Google Scholar]
- 14.Arinola OG, Akinosun OM, Olaniyi JA. Passive and active cigarette smoking: Effects on the levels of antioxidant vitamins, immunoglobulin classes and acute phase reactants. Afr J Biotechnol. 2011;10:6130–2. [Google Scholar]
- 15.Bennet KR, Reade PC. Salivary immunoglobulin A levels in normal subjects, tobacco smokers, and patients with minor aphtous ulceration. Oral Surg Oral Med Oral Pathol. 1982;53:461–5. doi: 10.1016/0030-4220(82)90457-1. [DOI] [PubMed] [Google Scholar]
- 16.EngstrÃm EN, EngstrÃm PE. Effects of tobacco smoking on salivary immunoglobulin levels in immunodeficiency. Eur J Oral Sci. 1998;106:986–91. doi: 10.1046/j.0909-8836.1998.eos106602.x. [DOI] [PubMed] [Google Scholar]
- 17.Herr C, Beisswenger C, Hess C, Kandler K, Suttorp N, Welte T, et al. Suppression of pulmonary innate host defence in smokers. Thorax. 2009;64:144–9. doi: 10.1136/thx.2008.102681. [DOI] [PubMed] [Google Scholar]
- 18.Honda Y, Takahashi H, Kuroki Y, Akino T, Abe S. Decreased contents of superfactant protein A and D in BAL fluids of healthy smokers. Chest. 1996;109:1006–9. doi: 10.1378/chest.109.4.1006. [DOI] [PubMed] [Google Scholar]
- 19.Sims MW, Tal-Singer RM, Kiersten S, Musani AI, Beers MF, Panettieri RA, et al. Chronic obstructive pulmonary disease and inhaled steroids alter superfactant protein D (SP-D) levels: A cross-sectional study. Respir Res. 2008;9:13. doi: 10.1186/1465-9921-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patidar KA, Parwani RN, Wanjari SP. Correlation of salivary and serum IgG, IgA levels with total protein in oral submucous fibrosis. J Oral Sci. 2011;53:97–102. doi: 10.2334/josnusd.53.97. [DOI] [PubMed] [Google Scholar]
- 21.Gupta SC, Singh PA, Shukla HS, Sinha SN, Mehrotra TN, Kumar S. Serum immunoglobulins in carcinoma of various organs. Indian J Cancer. 1981;18:277–81. [PubMed] [Google Scholar]
- 22.Holt PG. Immune and inflammatory function in cigarette smokers. Thorax. 1987;42:241–9. doi: 10.1136/thx.42.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sopori ML, Goud NS, Kaplan AM. in Immunotoxicology and Immunopharmacology. In: Dean J H, editor. New York: Raven; 1994. pp. 413–33. [Google Scholar]
- 24.Sopori ML, Kozak W, Savage SM, Geng Y, Kluger MJ. Nicotine-induced modulation of T-cell function: Implications for inflammation and infection. Adv Exp Med Biol. 1998;402:279–89. doi: 10.1007/978-1-4615-5347-2_31. [DOI] [PubMed] [Google Scholar]
- 25.Matsunaga K, Klein TW, Friedman H, Yamamoto Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol. 2001;167:6518–24. doi: 10.4049/jimmunol.167.11.6518. [DOI] [PubMed] [Google Scholar]


