Abstract
Background:
The purpose of this in vitro study was to evaluate surface discoloration of three microhybrid composite resins (Esthet•X HD, Clearfil AP-X, Gradia Direct) and five nanohybrid composite resins (Ceram•X, GC Kalore, G-aenial, Grandio, GrandioSO), after staining and bleaching procedures.
Materials and Methods:
The composite resins were polymerized with a curing light (Celalux II, Voco, Cuxhaven, Germany) into 160 silicon molds (6,4 mm in diameter and 2 mm in thickness) to obtain identical specimens. Twenty samples for each composite resin were prepared. The specimens were polished using an automated polishing machine with the sequence of 600-, 800-, 1000-grit abrasive paper under water irrigation. The specimens were immersed in tea and distilled water: the specimens were dipped for 20 min, once a day (every 24 h), for 14 days into the drinks. The specimens were then bleached with carbamide peroxide at 17% (Perfect Bleach-Voco). The color of specimens was measured with a spectrophotometer according to the CIE L*a*b* system after light-polymerization of composite resin specimens, after 7 days, after 14 days, and after bleaching. The color difference h index (DEab*) between each measurement was calculated. Statistical analysis was made using analysis of variance (ANOVA).
Results:
All specimens showed a significant increase in staining with a similar trend and no significant differences between microhybrid and nanohybrid composite resins. After whitening procedures, materials tested showed both significant and unsignificant differences of the h index.
Conclusions:
Microhybrid and nanohybrid composite resins had similar in vitro surface discoloration in tea. After bleaching, discoloration was removed from some composite resins tested.
Keywords: Bleaching, composite resin, surface discoloration
INTRODUCTION
A crucial property of esthetic restorative materials is their long-term stability in the oral environment: although great improvements have been achieved during recent years, color stability is still a problem.[1] Composite resins are susceptible to various degrees of discoloration after prolonged exposure to the oral environment.[2–4] Three types of discolorations are generally described:[5] external discoloration due to the accumulation of plaque and surface stains (extrinsic stain), surface or sub-surface color alteration implying superficial degradation or slight penetration and reaction of staining agents within the superficial layer of composite resins (absorption), body, or intrinsic discoloration due to physical–chemical reactions in the deeper portion of the restoration. The structure of the composite resin and the characteristics of its particles have a direct impact on susceptibility to extrinsic staining. Moreover, composite resins undergo superficial and microstructural changes resultant from mastication and from finishing and polishing procedures.[6–8] The external and the surface types of discolorations are also closely related to hygiene, dietary, and smoking habits.[9,10] Every component of composite resins may be involved in sub-surface or intrinsic discolorations. The affinity of composite resin for stains is modulated by its conversion rate and its chemical characteristics, water sorption rate being particularly important. An insufficient resin conversion rate will favour the absorption of some colorants and the color stability of composite resins depends on various factors like curing time, curing mode, aging conditions, and composition of the materials.[11] The intrinsic color of composite resins can also be altered with the passage of time as a result of various influences such as visible and ultra-violet irradiation, thermal changes, and humidity. The lighter shades of composite resins are likely to be subject to higher color degradation through the effects of environmental exposure to ultraviolet light.[12] The staining susceptibility of a composite resin may also be attributed to its filler type: Nanohybrid absorbs staining substances more easily than microhybrid. The purpose of this in vitro study was to evaluate the effects on the surface discoloration of eight composite resins, three microhybrid, and five nanohybrid, after staining and bleaching procedures. There were two working hypothesis: The type of composite does not influence the surface discoloration; the bleaching process removes staining from the surface of composite resins.
MATERIALS AND METHODS
Three microhybrid composite resins and five nanohybrid composite resins were evaluated in this study. For each brand, the A2 shade was selected. Table 1 shows details concerning the restorative materials used in this study. Twenty disk-shaped specimens of each composite resin were prepared in accordance with the manufacturer's instructions.
Table 1.
Composite resins used in this study
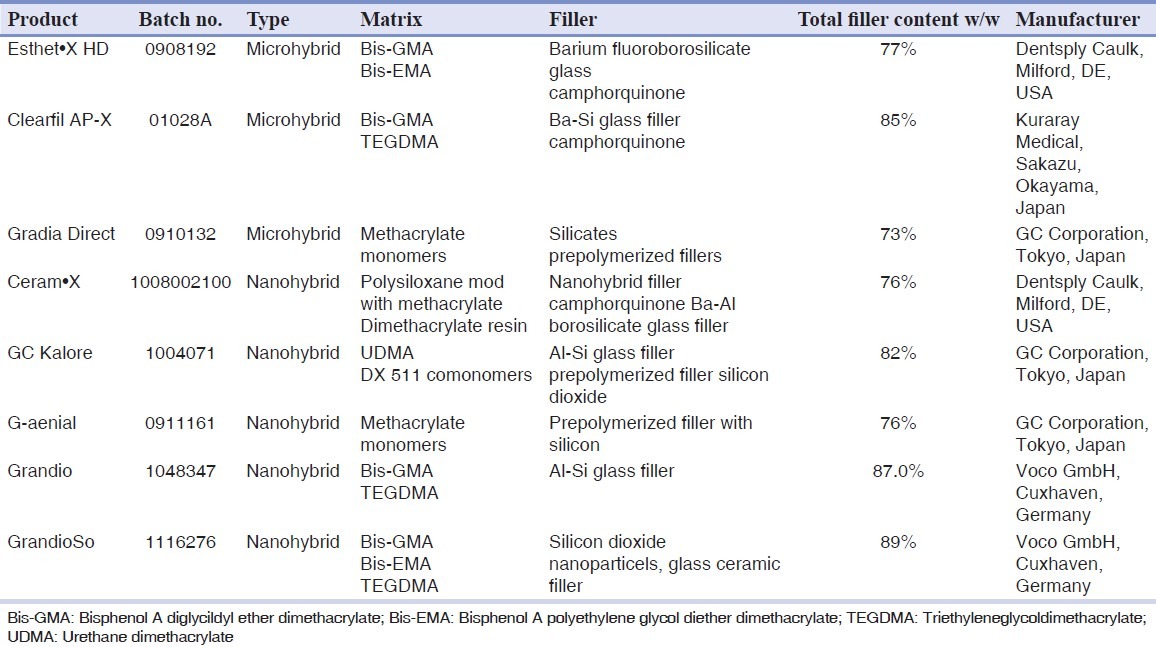
Group 1: Microhybrid composite resin – Esthet•X HD (Dentsply Caulk, Milford, DE, USA).
Group 2: Microhybrid composite resin – Clearfil AP-X (Kuraray Medical, Sakazu, Okayama, Japan).
Group 3: Microhybrid composite resin – Gradia Direct (GC Corporation, Tokyo, Japan).
Group 4: Nanohybrid composite resin – Ceram•X (Dentsply Caulk, Milford, DE, USA).
Group 5: Nanohybrid composite resin – GC Kalore (GC Corporation, Tokyo, Japan).
Group 6: Nanohybrid composite resin – G-aenial (GC Corporation, Tokyo, Japan).
Group 7: Nanohybrid composite resin – Grandio (Voco GmbH, Cuxhaven, Germany).
Group 8: Nanohybrid composite resin – GrandioSo (Voco GmbH, Cuxhaven, Germany).
Specimens were prepared by condensing the material into a silicon mold (6,4 mm in diameter and 2 mm in thickness), placed on a white opaque paper background covered by a Mylar strip (Henry Schein; Melville, NY). The mold was filled with the composite resin and a second Mylar strip was placed on the top of the filled mold. A glass slide was pressed against the upper Mylar strip to extrude the excess composite resin and to form a flat surface. The distal end of the light guide was placed against the surface of the matrix strip and positioned concentrically with the cavity in the mold; the material was then light-cured from the top with the curing light Celalux II (Voco, Cuxhaven, Germany). One light polymerization mode was used for each material – standard: 1000 mW/cm2 for 40 s. The cordless curing unit was maintained at full charge before use, and irradiance was monitored periodically by using a radiometer (SDS Kerr, Orange, CA). The specimens were polished using an automated polishing machine (APL-4; Arotec S.A. Ind Com, Cotia, SP, Brazil) with the sequence of 600-, 800-, 1000-grit abrasive paper under water irrigation. The specimen's bottom surface was identified with a scalpel blade. After that the specimens were stored in distilled water for 24 h in complete darkness at 37°C as baseline measurement.[13]
Staining process
For each composite, specimens were subdivided into two subgroups according to storage solutions (distilled water/subgroup W and tea/subgroup T); every subgroup of 10 samples of composite resin was immersed in staining solution at room temperature over a 14-day test period:
-
(i)
subgroup W (control): 10 specimens immersed in vials containing distilled water at 37°C,
-
(ii)
subgroup T: 10 specimens immersed in vials containing 500 ml of tea (Twinings, English breakfast, Andover, Hampshire, UK).
Tea solution was prepared by immersing two prefabricated tea bags (2 . 2 g) into 500 ml of boiling distilled water for 10 min. The specimens were dipped for 20 min, once a day (every 24 h) for 14 days into the tea to simulate a period of clinical exposure. After the immersion period, the specimens were washed and stored in distilled water. The controls were left in distilled water for all the time of the study. Solutions were changed daily and put in vials with a cover that prevents evaporation of staining solutions.
Bleaching process
Every sample was submitted to bleaching procedures with a bleaching agent based on carbamide peroxide at 17% (Perfect Bleach, Voco, Germany). It was painted on the top surface of the specimen according to the manufacturer's instructions and stored in vials sealed with parafilm (Parafilm M, Sigma-Aldrich, St. Louis, MO, USA). All bleaching agents were applied at room temperature for 2 h per day for 14 days according to manufacturer's instruction to simulate the bleaching process. After bleaching the specimens were rinsed with tap water for 1 min to remove the bleaching agents, blotted dry, and stored in distilled water at 37°C.
Color testing
A colorimetric evaluation according to the CIE L*a*b* system, relative to standard illuminant A against a white background, was performed at four experimental periods, after 24 hours’ immersion in distilled water, at 7 days, at 14 days and after the bleaching process. Color of the specimens was measured with a spectrophotometer (SP820λ; Techkon Gmbh, Konig-Stein, Germany) against a white background. Before each measurement session, the colorimeter was calibrated according to the manufacturer's recommendations by using the supplied white calibration standard. All specimens were chromatically measured four times and the average values were calculated; then, each color parameter for each specimen of the same shade was averaged. The CIE 1976 L* a* b* color system is used for the determination of color differences.[14,15] The L* value refers to “lightness”; the higher is the L value, the higher the lightness (a value of 100 corresponds to perfect white and that of zero to black). CIE L* a* b* values are called the “chromaticity coordinates”; “a*” shows red color on positive values and green color on negative values (+a* = red; –a* = green); “b*” shows yellow color on positive values and blue color on negative values (+b* = yellow; –b* = blue). The total color differences (DEab*) were calculated as follows:
DEab* = [(DL*)2 + (Da*)2 + (Db*)2]1/2 = h.
A value of DEab* < 3.3 was considered clinically acceptable.[1,16]
Statistical analysis
The color measurements of the experimental specimens (subgroup T) were compared with control specimens (subgroup W). Differences in color change by the immersion protocols were calculated and a statistical analysis was performed using statistical software (Stata 7; College Station, TX, USA). Descriptive statistics including the mean, standard deviation, minimum, median, and maximum for each group were calculated. The distributions were assessed and found to be normal (Kolmogorov–Smirnov Test). A one-way analysis of variance test (ANOVA) was applied to determine whether significant differences existed among the groups. Tukey's test was applied as post hoc to evaluate pairwise comparisons. Significance was predetermined at P < 0.05.
RESULTS
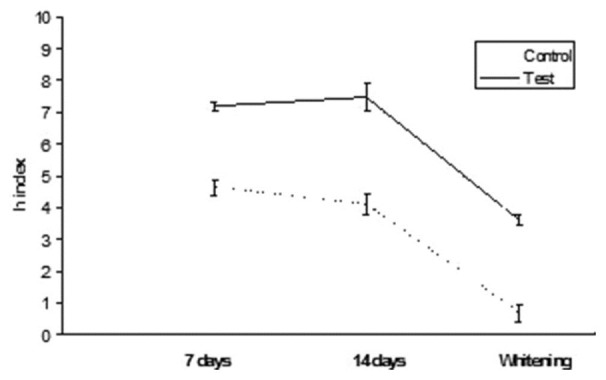
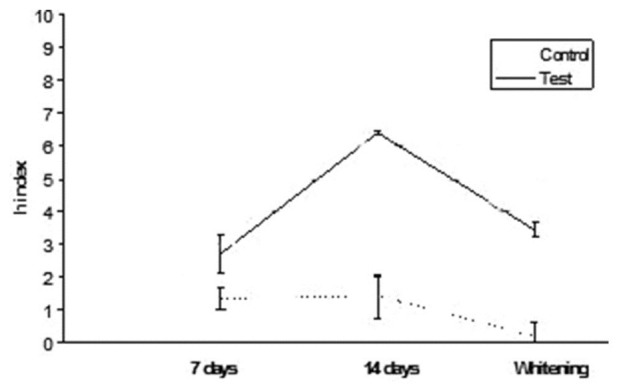
Results are summarized in Table 2. ANOVA found significant differences among the various groups. Post hoc Tukey's test showed that test subgroups presented significantly higher h index values (DEab*) than control subgroups for all the three different conditions tested (P < 0.05). Esthet•X HD exhibited significantly higher h values than all other groups tested (P < 0.05). No significant differences were found when comparing 7 and 14 days immersion in distilled water (P > 0.05). On the contrary, when analyzing tea immersion, two materials (Ceram•X and GrandioSo) showed significantly higher h index values after 14 days immersion than after 7 days (P < 0.05). All other materials did not show significant differences between 7 and 14 days of tea immersion (P < 0.05). After whitening procedure, materials tested showed significant reduction (Esthet•X HD, Ceram•X, GC Kalore, GrandioSo) (P < 0.05) or unsignificant differences (Clearfil AP-X, Gradia Direct, G-aenial, Grandio) (P > 0.05) of h index values than 14 days of tea immersion. All results obtained in function of time are shown in Figures 1–8.
Table 2.
Mean values and standard deviations of color change (DEab) of specimens
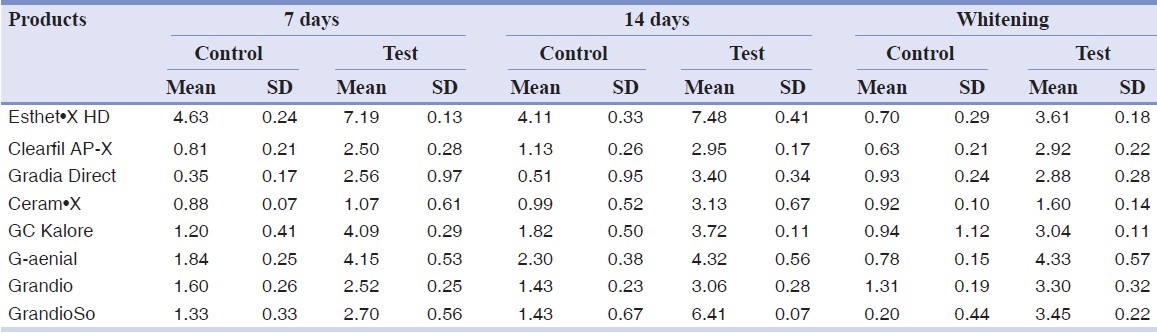
Figure 1.
Esthet•X HD: h index values after staining in tea and whitening
Figure 8.
GrandioSo: h index values after staining in tea and whitening
Figure 2.
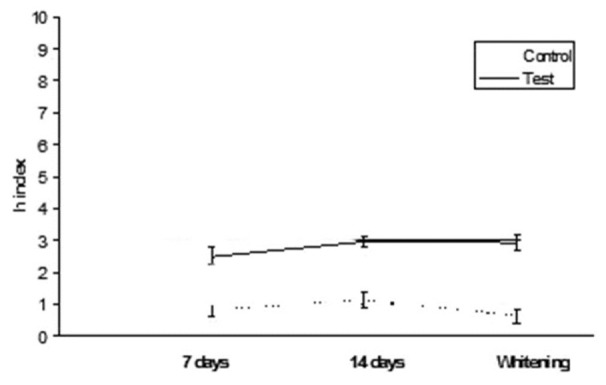
Clearfil AP-X: h index values after staining in tea and whitening
Figure 3.
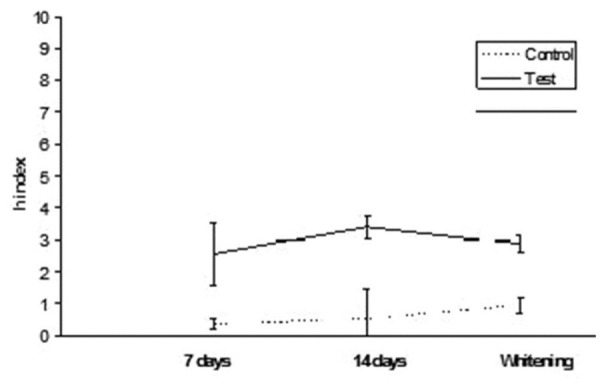
Gradia Direct: h index values after staining in tea and whitening
Figure 4.
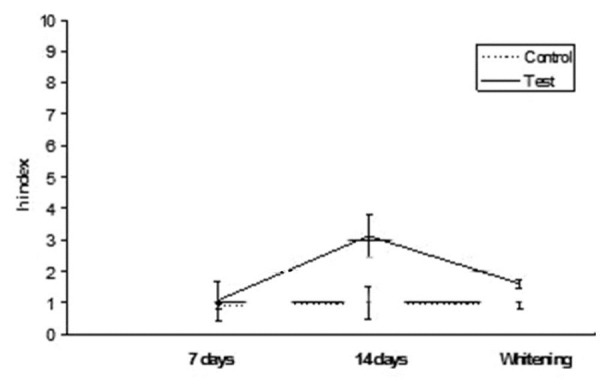
Ceram•X: h index values after staining in tea and whitening
Figure 5.
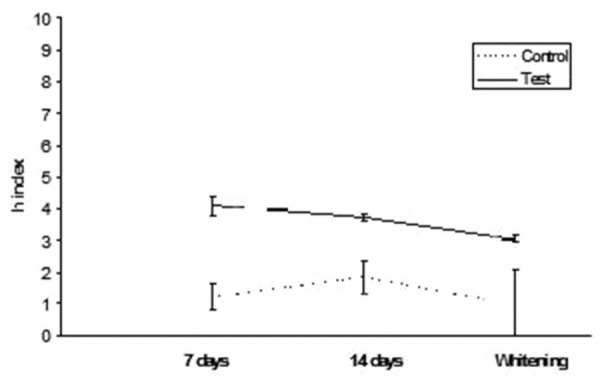
GC Kalore: h index values after staining in tea and whitening
Figure 6.
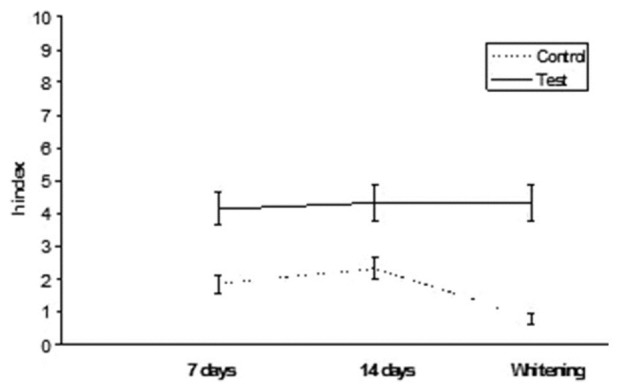
G-aenial: h index values after staining in tea and whitening
Figure 7.
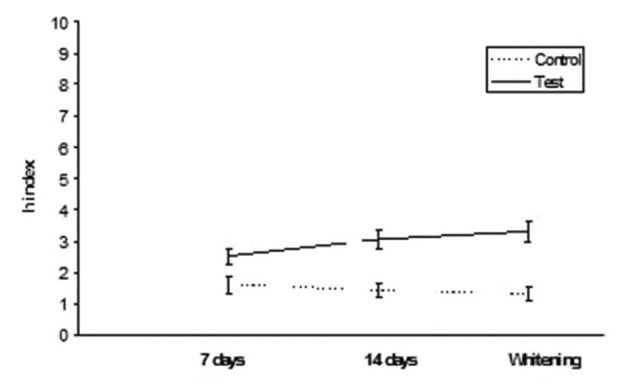
Grandio: h index values after staining in tea and whitening
DISCUSSION
This study focused on the problem of surface discoloration of modern composites by investigating their susceptibility to being stained by normal diet and food dyes as well as by assessing reactions to various physical–chemical conditions of clinical relevance. The primary hypothesis that drinks staining of nanohybrid composite resins would not be higher than microhybrid composite resins was accepted. Villalta et al.[1] and Lee et al.[16] obtained varying results when comparing in vitro staining of microhybrid and nanohybrid composite resins submitted to various solutions. Villalta et al.[1] assessed that staining susceptibility of a material may be attributed to the type of resin or filler. The authors demonstrated that nanohybrid composite resins absorb stains such as coffee or red wine more easily than microhybrid composite resins. Lee et al.[16] obtained analogous results using chlorhexidine and other staining substances, concluding that the smoothest surfaces were not necessarily the most stain resistant, and staining ability was influenced by each composite monomer and filler composition. In contrast with the results of these authors, in the present study, nanohybrid and microhybrid composite resins reacted in the similar way to the action of tea, with no significant differences after 7 days or 14 days of treatment. Surface discoloration was observed in each specimen after 7 days and was significantly higher after 14 days (Ceram•X and Grandioso). The color differences of the control subgroups after 7 days and after 14 days were not significant (P > 0.05). This result can be described to the composite resins susceptibility to chemical erosion of the resin matrix, hydrolytic breakdown of filler particles, and chemical degradation of silane agent, which are responsible processes for discoloration and staining of the composite resin. Therefore, to explain the present results, it is suggested that the greater susceptibility of the composite resin tested to staining by tea may be due to the high temperature employed in the immersion cycle (±€70°C), which probably heated the surface of the material, thus affecting the staining process. As this process appears to involve the degradation of the organic matrix which facilitates pigmentation, the material's aesthetic properties may be impaired.[17] The color of stained specimens of four groups (Esthet•X HD, Ceram•X, GC Kalore, GrandioSo) returned to baseline after bleaching. The whitening process regarded both the stained specimens, which returned to baseline even after a large discoloration due to staining, both the control subgroups of each product, which partially lost superficial stain. Therefore, the conclusion of the study was that carbamide peroxide was responsible of removing stain from the surface of specimens. The bleaching mechanism for the teeth is that the active agents (peroxide solutions) can flow freely through the enamel and dentin and oxidize the pigments in the teeth. The results of this study indicated that the surface discoloration of composite resins after bleaching was probably due to superficial cleansing of the specimens, not intrinsic color change.[1] However, Clearfil AP-X, Gradia Direct, G-aenial, and Grandio were not bleached by Perfect Bleach. Although bleaching agents can partially remove the exterior staining from some composite resins, they will not bleach them, whereas they can effectively bleach teeth.[18,19] Therefore, after bleaching, the composite resin restoration may not always match the surrounding bleached tooth structure. In fact, more researches should be conducted in order to confirm the results of this paper.
CONCLUSION
Under conditions and within the limitations of the present in vitro study, it can be concluded that staining procedures used in this study affected the color stability of tested composite resins. Different composite resins (microhybrid and nanohybrid) reacted in the same way when exposed in vitro to tea for 7 days and 14 days. After bleaching, discoloration was differently removed from the surface of composite resins tested.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Villalta P, Lu H, Okte Z, Garcia-Godoy F, Powers J. Effects of staining and bleaching on color change of dental composite resins. J Prosthet Dent. 2006;95:137–42. doi: 10.1016/j.prosdent.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Uchida H, Vaidyanathan J, Viswanadhan T, Vaidyanathan TK. Color stability of dental composites as a function of shade. J Prosthet Dent. 1998;79:372–7. doi: 10.1016/s0022-3913(98)70147-7. [DOI] [PubMed] [Google Scholar]
- 3.Vargas MA, Kirchner HL, Diaz-Arnold AM, Beck VL. Color stability of ionomer and resin composite restoratives. Oper Dent. 2001;26:166–71. [Google Scholar]
- 4.Janda R, Roulet JF, Latta M, Steffin G, Ruttermann S. Color stability of resin-based filling materials after aging when cured with plasma or halogen light. Eur J Oral Sci. 2005;113:251–7. doi: 10.1111/j.1600-0722.2005.00217.x. [DOI] [PubMed] [Google Scholar]
- 5.Dietschi D, Campanile G, Holz J, Meyer JM. Comparison of the color stability of ten new-generation composites: An in vitro study. Dent Mater. 1994;10:353–62. doi: 10.1016/0109-5641(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 6.Petromilli Nordi Sasso Garcia P, Neto ER, Aleixo Dos Santos P, Duarte Bonini Campos JA, Palma Dibb RG. Influence of surface sealant on the traslucency of composite resins: Effect of immersion time and immersion media. Mater Res. 2008;11:193–7. [Google Scholar]
- 7.Ramos RP, Chimello DT, Chinellatti MA, Palma Dibb RG, Mondelli J. Effect of three surface sealants on marginal sealing of class V composite resin restorations. Oper Dent. 2000;25:448–52. [PubMed] [Google Scholar]
- 8.Nasim I, Neelakantan P, Sujeer R, Subbarao CV. Color stability of microfilled, microhybrid and nano composite resins-An in vitro study. J Dent. 2010;38:137–42. doi: 10.1016/j.jdent.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Gupta G, Gupta T. Evaluation of the effect of various beverages and food material on the color stability of provisional materials – An in vitro study. J Cons Dent. 2011;14:287–92. doi: 10.4103/0972-0707.85818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asmussen E, Hansen EK. Surface discoloration of restorative resins in relation to surface softening and oral hygiene. Scand J Dent Res. 1986;94:174–7. doi: 10.1111/j.1600-0722.1986.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 11.Janda R, Roulet JF, Latta M, Kaminsky M, Ruttermann ST. Effect of exponential polymerization on color stability of resin-based filling materials. Dent Mater. 2007;23:696–704. doi: 10.1016/j.dental.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Kolbeck C, Rosentritt M, Lang R, Handel G. Discoloration of facing and restorative composites by UV-irradiation and staining food. Dent Mater. 2006;22:63–8. doi: 10.1016/j.dental.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Sarac D, Sarac YS, Kulunk S, Ural C, Kulunk T. The effect of polishing techniques on the surface roughness and color change of composite-based resins. J Prosthet Dent. 2006;96:33–40. doi: 10.1016/j.prosdent.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Lee YK, Powers M. Influence of a series of organic and chemical substances on the translucency of resin composites. J Biomed Mater Res Part B: Appl Biomater. 2006;77:21–7. doi: 10.1002/jbm.b.30401. [DOI] [PubMed] [Google Scholar]
- 15.Lee WK, Lu H, Oguri M, Powers JM. Changes in color and staining of dental composite resins after wear simulation. J Biomed Mater Res Part B: Appl Biomater. 2007;82:313–9. doi: 10.1002/jbm.b.30735. [DOI] [PubMed] [Google Scholar]
- 16.Lee YK, Powers JM. Discoloration of dental resins composites after immersion in a series of organic and chemical solutions. J Biomed Mater Res Part B: Appl Biomater. 2005;73:361–7. doi: 10.1002/jbm.b.30216. [DOI] [PubMed] [Google Scholar]
- 17.Veeramachaneni C, Pramod Reddy L, Jaya Prakash T, Anitha Rao G, Pradeep M. Spectrophotometric and colorimetric evaluation of staining of the light cure composite after exposure with different intensities of light curing units. J Cons Dent. 2011;14:391–4. doi: 10.4103/0972-0707.87208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerlach RW, Gibb RD, Sagel PA. Initial color change and color retention with a hydrogen peroxide bleaching strip. Am J Dent. 2002;15:3–7. [PubMed] [Google Scholar]
- 19.Turker SB, Biskin T. Effect of three bleaching agents on the surface properties of three different esthetic restorative materials. J Prosthet Dent. 2003;89:466–73. doi: 10.1016/s0022-3913(03)00105-7. [DOI] [PubMed] [Google Scholar]


