Abstract
Background:
Diabetes has become the next most widespread disease after cancer. Recent studies have found that diabetes and moderate to severe vitamin D deficiency are associated with reduced bone mineral content; therefore administration of vitamin D may correct these conditions. The purpose of this research is to compare the effect of vitamin D administration on bone to implant contact in diabetic rats with control group.
Materials and Methods:
In this randomized placebo-controlled trial, 48 Wistar rats were rendered diabetic (130≤ blood sugar ≤200 mg/dl) by IV injection of 35 mg/kg Alloxan. Implants were inserted in tibial bone; Then rats were divided into study and control groups and received oral vitamin D3 (160 IU) or placebo respectively for one week. Bone to implant contact value was measured under light microscope at 3 and 6 weeks.
Results:
Analysis of data indicated that vitamin D had no significant effect on bone to implant contact (BIC). At 3 weeks, the control group (n = 5) reported BIC level at 44 ± 19 and study group (n = 7) at 57 ± 20. At 6 weeks, the control group (n = 5) reported BIC level at 70 ± 29, and study group (n = 10) at 65 ± 22. Twenty one samples were missed because of death or incorrect lab processes.
Conclusion:
It seems that vitamin D supplement has no significant effect on BIC in 130 mg/dL ≤ blood sugar ≤200 mg/dL (P = 0.703) andwas also not time dependent (P = 0.074).
Keywords: Aloxan, bone-implant contact, type 2 diabetes, vitamin D
INTRODUCTION
The number of people with diabetes is expected to rise to 300 million in 2025 in the world. Type 2 diabetes is the predominant form worldwide, accounting for 90% of cases.[1]
Hyperglycemia results from lack of insulin or inadequate insulin secretion following increased insulin resistance. In vivo and in vitro studies have found that deficiency of vitamin D results in reduction in insulin secretion which leads to hyperglycemia, increased hemoglobin A1C and insulin resistance.[2–3]
Insulin-dependent diabetes mellitus (IDDM) and moderate to severe vitamin D deficiency in adults has frequently been shown to be associated with reduced bone mineral content.[4,5] Many previous experiments have demonstrated that the rate of osseointegration around dental implants was considerably reduced.[6–8] Also, in the recent decade, dental implants have been considered as a well-accepted treatment modality to replace missing and lost teeth.[9] In this study, the rats were rendered diabetic by means of Alloxan (C4 H4 N2 O2).This drug is mainly used in treatment of pancreatic tissue cancers and also as a diabetes inducer drug in studies.
Alloxan specifically affects pancreatic B-cells.[10] Intra-peritoneal injection of Alloxan (in different dosages) results in increased blood glucose during 12-48 h in rats. Alloxan is fully dose dependent, significantly increases the number of apoptotic cells. The role of Alloxan in induction of apoptosis is unknown but it may be due to free radicals of oxygen following its injection.[11]
Vitamin D is fat-soluble, and consumed as ergocalciferol (D2) or cholecalciferol (D3) through dietary sources.
The mechanisms whereby vitamin D may impact on the development and management of diabetes are:
Insulin secretion in isolated islets of Langerhans is dependent upon vitamin D in animals.[12]
The presence of vitamin D receptors on beta cells of the islets of Langerhans, and the ability of the islets to express 1-alpha hydroxylase activates 25 hydroxy vitamin D.
An indirect effect of vitamin D on beta cell insulin secretion by means of increased parathyroid hormone (PTH)[13] which increases the intracellular calcium in the islet, resulting in inhibition of post-receptor binding action of insulin is also postulated.[14]
Vitamin D receptors have also been identified on cells of the immune system.[15]
Orwoll et al. reported that treating with 1, 25 (OH)2 D3 (1 μg/d:40 IU for 4 days) had no effect on FBS in diabetic patient.[16] Tanaka et al., reported that using 160 IU of vitamin D (SC) twice a week in rats with vitamin D deficiency did not show significant differences in perfusate insulin by 16.7 mM glucose.[17] So, we increased the dose and decided to use 160 IU (4 μg) vitamin D for 7 days to illuminate the effect of vitamin D supplement on the amount of osseointegration around tibial implants, as a marker of healing at 3 and 6 weeks.The hypothesis of this research was that the administration of vitamin D in diabetic rats could enhance the osseointegration of implants in rats’ tibia.
MATERIALS AND METHODS
Experimental animals
Forty eight healthy and vaccinated male Wistar rats (24 weeks old; weight range: 250 to 280 g) were used. The study protocol was approved by the committee on animal care of Torabinejad Dental Research Center, Isfahan University of Medical Sciences, Isfahan, Iran and executed according to national animal law.
Each 5 rats were kept in a cage in an air conditioned environment (24 ± 1°C, 50% to 60% humidity), with a circadian light rhythm of 12 h day/dark. They were fed with standard laboratory diet and allowed food and water ad libitum. Acclimatization period was 2 weeks prior to the experiment. Then the rats were rendered diabetic by IV (Tail Vein) injection of 35 mg/kg monohydrate Alloxan (St. Louis, MO, USA), freshly dissolved in physiological serum, by insulin syringe.[18] Blood glucose was measured in 24 h of alloxanisation by one-touch glucometer (BIONIME GmbH, Heinrich Wild Strasse 202, CH-9435 Heerbrugg, Switzerland) and reflected by glycosuria, hyperglycemia, polyphagia, polydipsia and body weight loss. The rats which were showing fasting blood glucose levels between 130 and 200 mg/ dl were selected for the study.
Implant insertion
After confirmation of diabetes, the screws (TRINON Titanium GbmH, Augartenstr. 1,76137 Karl sruhe, Germany), made of commercially pure titanium, 1.2 mm in diameter and 7 mm in length, were used in this study.
The rats were anesthetized by intra-abdominal injection of equal mixture of 0.1 ml/kg of ketamine hydrochloride and xylazine. The skin on tibial bone was shaved and disinfected with 70% ethanol. A 15 mm incision was made and bone was exposed. After exposing the bone, a 0.8 mm pilot hole was drilled through the medial cortex, the medulla, and the lateral cortex of the tibia. Subsequently, the hole was gradually widened by larger drills to the final diameter of the screws. Bone preparation was performed using very low rotational speeds under continuous external cooling saline.
The sterilized screws were placed in the proximal metaphyseal region of the tibia in all 48 rats. Antibiotic therapy was done with Cephalexin (15 mg/ kg) for 5 days. Flunixin Meglumine (Flunex, Razak, Iran) (2.5 mg/kg body weight) was administered to reduce pain for 3 days.
After implantation, the rats were randomly divided in two groups, and received 160 IU of vitamin D (3) or placebo orally each day for one week. BICs were measured at 3 and 6 post operative weeks.
Histologic examination
Five rats died within 3 weeks, including 2 rats of case group and 3 of control group; nine rats in each group were sacrificed in 3 weeks of implantation and the others were remained for 6 weeks investigation because of the unexpected mortality. Two more rats of control group died before 6 weeks. Then tibiae were removed with trephine bur and embedded in methyl methacrylate (Meliodent; Heraeus Kulzer, Berkshir, UK). Then, implant body was cut axially using a microtome (Denmark, Copenhagen, stuers, 50-Accutom) to prepare approximately 250 μm thick non-decalcified grinded specimens, then polished to final thickness of approximately 100 μm.The tissues were dyed with Masson's trichrome stain for microscopic observation [Figures 1 and 2].
Figure 1.

×36, stereo microscope image of case (3 weeks), BIC:91.6%
Figure 2.
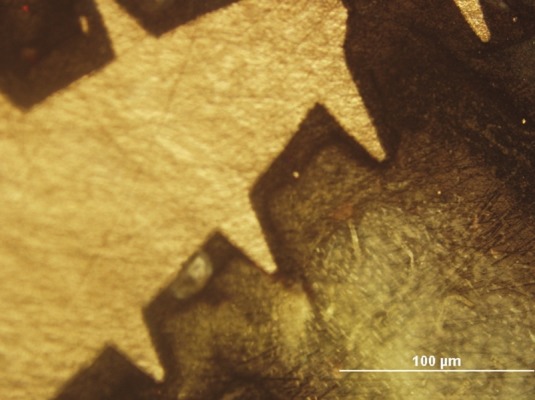
×100, polarizan microscope image of case (6 weeks), BIC: 44%
The amount of bone-implant contact and the changes in quantity of bone tissue around the implants were evaluated morphometrically by bone to implant contact (BIC) parameter and total contact length around the implants. To perform the histomorphometric analysis, a millimeter grid transparent sheet was placed on the 20 × 25-cm amplified images. By counting the filled boxes, the proportion of osseous neo-formation areas stained by the marker was quantified.[19] A total of 14 implants were dislodged during histologic processing, including 6 implants from case group and 8 from control group.
The data were analyzed with SPSS 11.5 software and ANOVA and t-test to discover the differences between the 2 groups at level of significance of P < 0.05.
RESULTS
The state of diabetes (130 mg/dl ≤ FBG levels ≤200 mg/dl) was predictably induced and monitored throughout the study. At 3 weeks, the control group (n = 5) reported a BIC level at 44 ± 19 and the vitamin D group (n = 7) at 57 ± 20. At 6 weeks, the control group (n = 5) reported BIC level at 70 ± 29 and the vitamin D group (n = 10) at 65 ± 22. The mean percentage of BIC value was 66 ± 23 [Table 1 and Figures 2 and 3].
Table 1.
Comparison of bone to implant contact between the vitamin D and control groups at 3
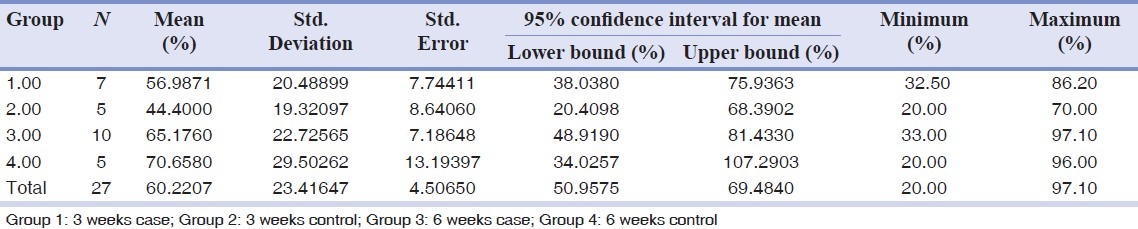
Figure 3.
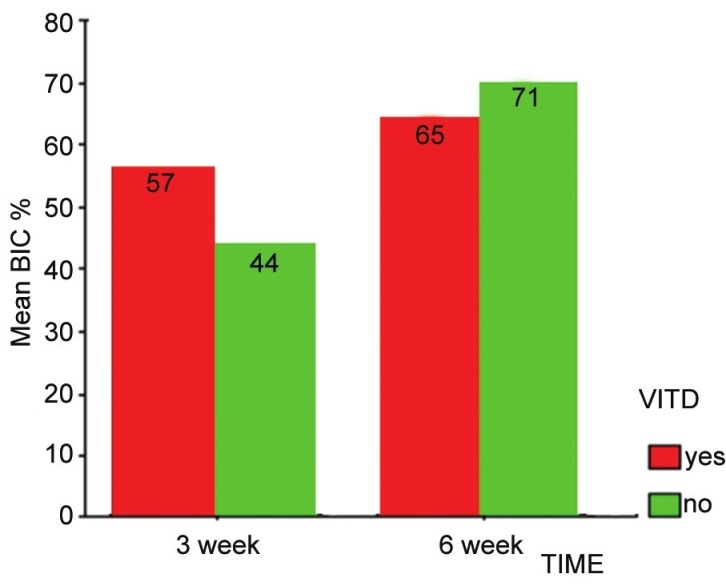
Mean percentage of BIC value changes between vitamin D and control groups at 3 and 6 week
Twenty one samples were missed because of death and incorrect histologic processes.
DISCUSSION
In this study, statistical significant difference of BIC value was not observed between case and control groups (P = 0.703) and was not time dependent as well (P = 0.074). Between group differences in BIC in short term (3 weeks) also was not statistically meaningful. (P = 0.308) After 6 weeks this difference was not noticeable (P = 0.695) [Table 1 and Figures 2 and 3].
Bone is one of the classical target tissues for vitamin D action. Vitamin D regulates calcium homeostasis by influencing on intestinal calcium absorption, renal calcium re-absorption, and bone calcium metabolism.[20] It was shown that moderate to severe vitamin D deficiency in adults was frequently associated with reduced bone mineral content,[5] and the rate of osseointegration around dental implants was considerably reduced under vitamin D insufficiency.[21] The positive effect of calcium and vitamin D supplements on improvement of bone healing around dental implants, was revealed by Park et al., in 2007 in normal rats.[22] Then in September 2011, Hong et al., approved this report by observing the healing process of surgically created alveolar sockets in dog model.[23] The last experiment was done by Dvorak,[24] in 2011 in ovariectomized rats. She reported that vitamin D deficiency was associated with a decrease in BIC value in the cortical area but no significant changes due to vitamin D depletion were noticed in the medullar compartment in comparison with control group that received 2400 IU/ kg vitamin D as a normal diet.
Vitamin D has an indirect effect on BIC through an essential role on diabetes. In vivo and in vitro studies have found that deficiency of vitamin D resulted in reduction in insulin secretion and thus in hyperglycemia, increased hemoglobin A1C and insulin resistance.[23] Rabbits and mice with vitamin D deficiency were found to have impaired insulin secretion, however vitamin D supplementation corrected this defect.[2] In the other hand, evidence indicates that persons with diabetes have lower serum concentrations of vitamin D and it plays an integral role in insulin sensitivity and secretion.[3] Our data is similar to Witham's data, because we did not see any noticeable changes in blood glucose after administration of vitamin D. In that study, vitamin D improved systolic blood pressure but not insulin resistance or glycosylated hemoglobin in patients with type 2 diabetes.[25] Insulin-dependent diabetes mellitus (IDDM) has frequently been shown to be associated with reduced bone mineral content.[5] Alterations in microvascularization and wound healing associated with diabetes lead to diminished immune responses and a reduction in bone remodelling.[26] Some animal studies have demonstrated that diabetes mellitus could negatively interfere with the process of osseointegration before 8 weeks; Ottoni et al., in 2004 reported BIC level of 39% in diabetic rats in comparison to 73% in control after 56 days.[6] Hideki et al., in 2008 demonstrated that BIC levelwas 12% indiabetic group and 61% incontrol group at 4 weeks. A 2-fold difference remained at week 8,[7] which is in agreement with a report on GK rats.[8]
This effect was also seen in our experiment, the mean percentage of BIC in all groups showed reduction (60 ± 23) but it was more than previous studies, because the lower range of blood glucose was chosen in our experiment. The consequence of diabetes on the healing process of soft tissue depends on the degree of glycemic control in the pre-operative period and the existence of chronic vascular complications. Patients with poor metabolic control have their immune defense impaired and greater predisposition to infection of the wound. The microangiopathy arising as a complication of diabetes may compromise vascularization, thus delays healing and acts as a gateway for the infection of tissue.[27] In our experiment, the duration of diabetic state was not long enough to produce chronic microangiopathies. The lower blood glucose was chosen in this study to compare with previous studies (130-200 mg/dL).[19,28,29] It may conceal the direct effect of vitamin D on diabetes. In the study of Tempe et al.,[28] 120 mg/kg of Alloxan was used to induce diabetes in rats, or Luciane et al.[29] considered the blood glucose level between 400-600 mg/dL as diabetic state.
The amount of vitamin D might not be enough in this study. The dose is important; Orwoll et al., reported that treatment with 1,25 (OH)2 D3 (1 μg/d:40 IU for 4 days) had no effect on FBS in diabetic patient.[16] Tanaka et al., reported that vitamin D (SC) supplementation for160 IU twice a week in vitamin D deficient rats did not show significant differences in perfusate insulin in response to 16.7 mM of glucose.[17] The baseline and serum concentration of vitamin D is very important, so it may suggest that prescribing the 160 IU of vitamin D for one week was insufficient and resulted in low bioavailability of vitamin D. It is necessary to determine the total dose of vitamin D3 supplement to achieve optimal serum concentration.
In human study, the relationship between diabetes and vitamin D deficiency may be affected by genetic variation in vitamin D receptors,[30] and it may be an altered factor in this study. Dose, age, body weight, race, sex and environmental factors were resembled.
In the other word, it has been reported that females with type 2 diabetes have a high prevalence of hypovitaminosis D,[31] and the review of the literature which was done in 2005, revealed that the age and gender of diabetic patients did not seem to influence implant survival,[32] so we chose male rats, but we suggest that in the future studies larger sample sizes including both sexes with higher dosage of vitamin D or longer duration of supplementation be recruited to clarify the differences between groups.
CONCLUSION
Vitamin D has no effect on osseointegration of implants in diabetic rats (130 mg/dL ≤blood sugar ≤200 mg/dL) at 3 and 6 weeks; however, for better definition of the role of vitamin D in the BIC, high-quality observational studies and RCTs that measure blood 25-hydroxyvitamin D concentration and clinically relevant glycemic outcomes are needed.
ACKNOWLEDGEMENTS
This study was done in Isfahan University of Medical Sciences, school of dentistry. The authors wish to thank Dr. Mohamad Ali Makizadeh, Dr. Shahriar Adibi and Dr. Mehdi Mehdikhani for their consultative helps.
Footnotes
Source of Support: This report is based on a research project and a thesis which was submitted to the School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran, in partial fulfillment of the requirements for the DMD degree in Dentistry .The study was approved by the Medical Ethics and Research Offi ce at the Isfahan University of Medical Sciences and financially supported by this University.
Conflict of Interest: The authors declare no conflicts of interest, real or perceived, financial or nonfinancial.
REFERENCES
- 1.Zimmet P, Shaw J, Alberti KG. Preventing Type 2 Diabetes and the dysmetabolic syndrome in the real world: A realistic view. Diabetes Metab. 2003;29:609–18. doi: 10.1046/j.1464-5491.2003.01052.x. [DOI] [PubMed] [Google Scholar]
- 2.Wollheim CB, Sharp GW. Regulation of insulin release by calcium. Physiol Rev. 1981;61:914–73. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- 3.Alfonso B, Liao E, Busta A, Poretsky L. Vitamin D in diabetes mellitus: A new field of knowledge poised for D-velopment. Diabetes Metab Res Rev. 2009;25:417–9. doi: 10.1002/dmrr.927. [DOI] [PubMed] [Google Scholar]
- 4.Selby PL. Osteopenia and diabetes. Diabet Med. 1988;5:423–8. doi: 10.1111/j.1464-5491.1988.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 6.Ottoni CE, Chopard RP. Histomorphometric evaluation of new bone formation in diabetic Rats submitted to insertion of temporary implants. Braz Dent J. 2004;15:87–92. doi: 10.1590/s0103-64402004000200001. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa H, Ozawa S, Hashimoto K, Takeichi T, Ogawa T. Type 2 diabetes impairs implant osseointegration capacity in rats. Int J Oral Maxillofac Implants. 2008;23:237–46. [PubMed] [Google Scholar]
- 8.Wang F, Song YL, Li DH, Li CX, Wang Y, Zhang N, et al. Type II diabetes mellitus impairs bone healing of dental implants in GK rats. Diabetes Res Clin Pract. 2010;88:e7–9. doi: 10.1016/j.diabres.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Pjetursson BE, Bragger U, Lang NP, Zwahlem M. Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant supported FDPs and single crowns (SCs) Clin Oral Implants Res. 2007;18:97–113. doi: 10.1111/j.1600-0501.2007.01439.x. [DOI] [PubMed] [Google Scholar]
- 10.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas,”. Physiological Research. 2001;50:537–46. [PubMed] [Google Scholar]
- 11.Behr GA, Da Silva EG, Ferreira AR, Cerski CTS, Dal-Pizzol F, Moreira JCF. Pancreas β-cells morphology, liver antioxidant enzymes and liver oxidative parameters in alloxan-resistant and alloxan-susceptible Wistar rats: A viable model system for the study of concepts into reactive oxygen species. Fundamental and Clinical Pharmacology. 2008;22:657–66. doi: 10.1111/j.1472-8206.2008.00628.x. [DOI] [PubMed] [Google Scholar]
- 12.Holick MF, Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. 10th ed. Baltimore MD: Lippincott Williams and Wilkins; 2006. Modern nutrition in health and disease. [Google Scholar]
- 13.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 14.Reusch JE, Begum N, Sussman KE, Draznin B. Regulation of GLUT-4 phosphorylation by intracellular calcium in adipocytes. Endocrinology. 1991;129:3269–73. doi: 10.1210/endo-129-6-3269. [DOI] [PubMed] [Google Scholar]
- 15.Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92:2130–5. doi: 10.1210/jc.2006-2250. [DOI] [PubMed] [Google Scholar]
- 16.Orwoll E, Riddle M, Prince M. Effects of vitamin D on insulin and glucagon secretion in non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1994;59:1083–7. doi: 10.1093/ajcn/59.5.1083. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Seino Y, Ishida M, Yamaoka K, Yabuuchi H, Ishida H, et al. Effect of vitamin D3 on the pancreatic secretion of insulin and somatostatin. Acta Endocrinol (Copenh) 1984;105:528–33. doi: 10.1530/acta.0.1050528. [DOI] [PubMed] [Google Scholar]
- 18.Tang LQ, Wei W, Chen LM, Liu S. Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J Ethnopharmacol. 2006;108:109–15. doi: 10.1016/j.jep.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Ottoni CE, Chopard RP. Histomorphometric evaluation of new bone formation in diabetic Rats submitted to insertion of temporary implants. Braz Dent J. 2004;15:87–92. doi: 10.1590/s0103-64402004000200001. [DOI] [PubMed] [Google Scholar]
- 20.Binkley N. Summary - The role of vitamin D in musculoskeletal health. J Musculoskelet Neuronal Interact. 2006;6:347–48. [PubMed] [Google Scholar]
- 21.Kelly J, Lin A, Wang CJ, Park S, Nishimura I. Vitamin D and Bone Physiology: Demonstration of Vitamin D Deficiency in an Implant Osseointegration Rat Model. J Prosthodont. 2009;18:473–8. doi: 10.1111/j.1532-849X.2009.00446.x. [DOI] [PubMed] [Google Scholar]
- 22.Park KI, Lee JY, Hwang DS, Kim YD, Kim GC, Shin SH, et al. Effect of calcium and vitamin D supplementation on bone formation around titanium implant. J Korean Assoc Oral Maxillofac Surg. 2007;33:130–7. [Google Scholar]
- 23.Hong HH, Chou TA, Yang JC, Chang CJ. The potential effects of cholecalciferol on bone regeneration in dogs. Clin Oral Implants Res. 2011 doi: 10.1111/j.1600-0501.2011.02284.x. doi: 10.1111/j.1600-0501.2011.02284.x. [DOI] [PubMed] [Google Scholar]
- 24.Dovorak G, Fugi A, Watzek G, Tanql S, Pokomy P, Gruber R. Impact of dietary vitamin D on osseointegration in the ovariectomized rat. Clin Oral Implants Res. 2012;23:1308–13. doi: 10.1111/j.1600-0501.2011.02346.x. [DOI] [PubMed] [Google Scholar]
- 25.Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D (3) on markers of vascular health in patients with type 2 diabetes: A randomised controlled trial. Diabetologia. 2010;53:2112–9. doi: 10.1007/s00125-010-1838-1. [DOI] [PubMed] [Google Scholar]
- 26.Olson JW, Shernoff AF, Tarlow JL, Colwell JA, Scheetz Jp, Bingham SF. Dental endosseous implant assessments in a type 2 diabetic population: A prospective study. Int J Oral Maxillofac Implants. 2000;15:811–8. [PubMed] [Google Scholar]
- 27.Mellado-Valero A, Ferrer Garcia JC, Herrera Ballester A, Labaiq Rueda C. Effects of diabetes on the osseointegration of dental implants. Med Oral Patol Oral Cir Bucal. 2007;12:E38–43. [PubMed] [Google Scholar]
- 28.Tempe CR, Kole PG. Comparative evaluation of antidiabetic activity of some marketed polyherbal formulations in alloxan induced diabetic rats. International Journal of Pharm Tech Research. 2009;1:43–9. [Google Scholar]
- 29.Luciane BC, Gislaine ZR, Helena MA, Karine FR, Giovanni Z, Francine FF, et al. Increased Oxidative Stress and Imbalance in Antioxidant Enzymes in the Brains of Alloxan-Induced Diabetic Rats. Experimental Diabetes Research. 2012 doi: 10.1155/2012/302682. doi:10.1155 / 2012/302682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang TJ, Lei HH, Yeh JI, Chiu KC, Lee KC, Chen MC, et al. Vitamin D receptor gene polymorphisms influence susceptibility to type 1 diabetes mellitus in the Taiwanese population. Clin Endocrinol (Oxf) 2000;52:575–80. doi: 10.1046/j.1365-2265.2000.00985.x. [DOI] [PubMed] [Google Scholar]
- 31.Isaia G, Giorqino R, Adami S. High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care. 2001;24:1496. doi: 10.2337/diacare.24.8.1496. [DOI] [PubMed] [Google Scholar]
- 32.Kotsovilis S, Karoussis IK, Fourmousis I. A comprehensive and critical review of dental implant placement in diabetic animals and patients. Clin Oral Implants Res. 2006;17:587–99. doi: 10.1111/j.1600-0501.2005.01245.x. [DOI] [PubMed] [Google Scholar]


