Abstract
Background:
The aim of this study was to compare push-out bond strength of a new bioceramic endodontic sealer, EndoSequence BC sealer (Brasseler USA, Savannah, GA), used with gutta-percha in the presence or absence of phosphate-buffered saline solution (PBS) within the root canals.
Materials and Methods:
Forty single-rooted human teeth were prepared and randomly divided into four groups. Samples in groups 1 and 2 were dried, but those in groups 3 and 4 were moistened with PBS before obturation. All root canals were obturated with gutta-percha/EndoSequence BC sealer. The specimens were stored in PBS for 7 days in groups 1 and 3 and for 2 months in groups 2 and 4. Push-out bond strength values and failure modes were evaluated. The data on push-out bond strength were analyzed using two-way ANOVA.
Results:
The mean value for the bond strength of the obturation material in moistened canals was significantly higher than that in dried ones at 1 week (P = 0.00). Contrarily, there was no significant difference between dried and moistened root canals at 2 months (P = 0.61). In dried canals, bond strength increased significantly with time but in moistened ones, the difference was not significant. Inspection of the specimens revealed the bond failure to be mainly cohesive for all groups.
Conclusion:
The presence of PBS within the root canals increased the bond strength of EndoSequence BC sealer/gutta-percha at 1 week. However, no difference was found between the bond strength of EndoSequence BC sealer/gutta-percha in the presence or absence of PBS in the root canals at 2 months.
Keywords: Bioceramic, bond strength, phosphate-buffered saline, root canal sealer
INTRODUCTION
The use of gutta-percha combined with an endodontic sealer is the most widely accepted technique for root canal obturation; however, it does not provide a complete bacteria-tight seal for the root canal system.[1,2] New root canal sealers are constantly being developed in an attempt to provide all of the favorable properties. Recently, a new bioceramic root canal sealer, EndoSequence BC sealer (Brasseler USA, Savannah, Georgia; also known as iRoot SP, Innovative Bioceramix, Vancouver, Canada), has been introduced to the market.[3] It is described by the manufacturer to be an injectable, premixed, hydrophilic, radiopaque, insoluble, and aluminum-free material based on a calcium silicate composition. The BC sealer is composed of calcium silicates, calcium phosphate monobasic, calcium hydroxide, and zirconium oxide. Zhang et al.[4] showed that iRoot SP is equivalent to AH Plus sealer in apical sealing ability. Furthermore, it has been demonstrated that iRoot SP and Endosequence BC sealer are significantly less toxic than AH Plus.[5,6] iRoot SP has also been shown to be effective against Enterococcus faecalis.[3]
The ability of an endodontic sealer to bond to intraradicular dentin is advantageous in maintaining the integrity of the sealer–dentin interface during mechanical stresses caused by tooth flexure, preparation of post space, or operative procedures.[7–9] It has been shown that calcium and hydroxyl ions released from the calcium silicate-containing material interact with phosphate-containing fluids to form an apatite layer.[10–13] Formation of this interfacial layer develops a chemical bond between calcium silicate-based materials and dentinal walls.[10] Huffman et al.[8] showed that ProRoot Endo Sealer which is based on calcium silicate exhibited higher push-out strength after immersing in a phosphate-containing fluid for 4 weeks. Calcium silicate-based root canal sealers have been found to show bioactivity while being in contact with phosphate ions.[8–14] It has been shown that the use of a phosphate-buffered saline (PBS) as an intracanal dressing promotes the biomineralization process at the inner side of MTA apical plugs.[15]
According to the manufacturer, the EndoSequence BC sealer utilizes the moisture that remains within the dentinal tubules to initiate and complete its setting reaction. Its setting time is 4 h and may be extended in overly dry canals.
It has been suggested to take advantage of the bioactivity of MTA by leaving the root canals slightly moist with PBS or using PBS as the final rinse to encourage the deposition of carbonated apatite as a protocol to reduce leakage in root canals.[16] Since calcium silicate-based biomaterials interact with phosphate-containing fluids, it may be advantageous to wet dentinal walls with these fluids prior to obturation of the root canals using these materials. Therefore, the purpose of this study was to determine the bond strength of root canal fillings with the EndoSequence BC sealer in combination with gutta-percha in the presence or absence of PBS within the root canals after storage for 1 week or 2 months.
MATERIALS AND METHODS
Forty extracted, single-rooted human teeth, stored in 0.5% chloramine T, were selected. The teeth were decoronated to standardized root lengths of 13 mm. Working length was determined for each sample at approximately 1 mm short of the apex. The root canals were prepared using Mtwo rotary instruments (VDW, Munich, Germany) to apical size 35/0.04. Throughout preparation, the canals were irrigated with 5.25% NaOCl. After canal preparation, the root canals were irrigated with 3 mL of 17% EDTA for 1 min followed by 3 mL of 5.25% NaOCl. Then, the specimens were randomly divided into four groups (n = 10) as follows.
Groups 1 and 2: In these groups, at the completion of instrumentation, the root canals were dried with paper points until the last point appeared dry after removal.
Groups 3 and 4: After instrumentation, the root canals were flushed with 3 mL of distilled water. The root canals were dried with paper points and then filled with PBS solution (pH = 7.2). Then, for each canal a #35 paper point was used to absorb the PBS from inside the root canal for a period of 10 s.
Obturation was performed similarly in all groups with a size 35/0.02 gutta-percha as a master cone in each canal in combination with the EndoSequence BC sealer according to the manufacturer's instructions. Additional size 20/0.02 gutta-percha points were placed into the canals using cold lateral compaction technique.
The root canal entrances were sealed with a temporary filling material. In groups 1 and 3, the specimens were stored in PBS at 37°C and incubated for 7 days, and in groups 2 and 4, they were incubated for 2 months (60 days).
Push-out test
The middle portion of each root was sectioned perpendicular to the long axis into 1.00 ± 0.05 mmthick serial slices by using a water-cooled diamond blade on a precision cut-off machine (Mecatome, Presi, France). Twenty slices were achieved per group.
The filling material was loaded with a 0.7 mm diameter cylindrical stainless steel plunger. Loading was performed on a universal testing machine (Z050, Zwick/Roell, Ulm, Germany) at a speed of 0.5 mm/min in an apical-coronal direction. The maximum load applied to the filling material before debonding was recorded in Newtons. To express the bond strength in megapascals (MPa), the load at failure recorded in Newtons (N) was divided by the interfacial area.[17]
The slices were then examined under a stereomicroscope at ×40 magnification to determine the failure mode. Modes of failure were considered as follows: (1) adhesive: at the filling material/dentin interface, (2) cohesive: within filling material, and (3) mixed failure.
The data were analyzed using two-way ANOVA. The significance level was set at P = 0.05.
RESULTS
The mean values of bond strength recorded for test groups are presented in Table 1. The bond strength of root canal fillings in wet canals (group 3) was significantly higher than that in dry canals (group 1) at 1 week (P = 0.00). There was no significant difference between groups of dry and moist root canals at 2 months (groups 2 and 4) (P = 0.61). The results showed that in dry canals, higher bond strength of filling materials was found at 2 months compared with 1 week (P = 0.00). However, in moist canals, there was no significant difference between bond strength at 1 week and 2 months (P = 0.62).
Table 1.
Mean push-out bond strengths (±SD) and failure modes for the experimental groups
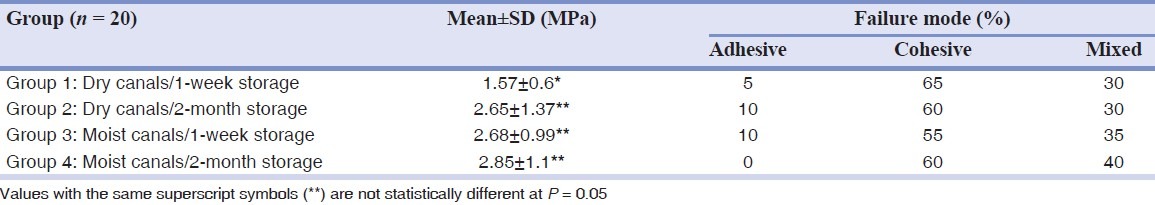
Inspection of the specimens revealed the bond failure to be mainly cohesive for all groups.
DISCUSSION
Recently, a variety of root canal sealers based on bioceramic or calcium silicate have been developed.[8,14] Bioceramic materials are ceramic products or components employed in medical and dental applications, mainly as implants and replacements, which have osteoinductive properties.[18] Many materials used today in dentistry are considered to be bioceramics, such as alumina, zirconia, bioactive glass, and calcium silicate-based materials such as MTA.[19]
Since PBS is considered as a synthetic tissue fluid (10), the specimens were stored in PBS before testing the bond strength at 37°C to partially simulate the in vivo conditions.
In the present study, the bond strength of BC sealer in dry canals was significantly lower compared with that in the presence of PBS within the root canals at 1 week. It has been demonstrated that release of calcium and hydroxyl ions from the calcium silicate-containing materials will result in apatite formation in the presence of phosphate-containing fluids.[10–13] The higher bond strength in the presence of PBS within the root canals may be attributed to the bioactivity of the BC sealer. The EndoSequence BC sealer is mainly composed of calcium and silicate. Formation of apatite is a common characteristic of calcium silicate-containing biomaterials.[20,21]
It has been shown that the formation of apatite crystals develops a chemical bond between mineral trioxide aggregated (MTA), a calcium silicate-containing biomaterial, and the dentinal walls.[10] Furthermore, Reyes-Carmona et al.[12] showed that a white precipitate formed over MTA in the first hour after immersion in PBS; and after 5 days, the surface of the material was entirely covered. Gandolfi et al.[13] analyzed MTA and calcium silicate cement surface stored 24 h in Dulbecco PBS and found apatite deposition over the materials. Therefore, it is anticipated that the presence of PBS in the root canal will result in the higher bond strength even after 1-week storage period. In this study, the presence of PBS within the root canals resulted in the bond strength of the EndoSequence BC sealer at 1 week as high as that observed at 2 month.
According to the manufacturer, the EndoSequence BC sealer needs water for setting and utilizes the moisture within the dentinal tubules to initiate and complete its setting reaction. The moisture in the dentinal tubules might not be enough for setting this material which might be a reason for explaining the lower bond strength of the EndoSequence BC sealer in root canals dried before obturation. Accordingly, Gancedo-Caravia and Garcia-Barbero[22] showed that the curing conditions do play an important role on the retention characteristics of MTA, since in their study the push-out strength of dry-cured MTA was clearly lower than that of wet-cured MTA.
In this study, the bond strength of the EndoSequence BC sealer in dry canals was significantly higher at 2 months compared with that observed at 1 week. This might be attributed to diffusion of phosphate ions from phosphate-containing storage media into the microporous sealer via the apical foramen.[14] It has been shown that during a 7-day storage period in a phosphate-containing fluid, roots filled with a calcium silicate-based sealer and gutta-percha will develop amorphous calcium phosphate precursors along the apical third of the root canal walls. With increased storage period, apatite-like crystalline clusters will be seen up to the middle third of the canal walls. With more extended storage in phosphate-containing fluid, there would be more phosphate ions available for the interaction with the calcium and hydroxyl ions released by the calcium silicate-based sealer,[14] which could result in higher bond strength of calcium silicate-containing sealers in the upper levels of the roots. This probably explains why the bond strength of the EndoSequence BC sealer was higher in the slices prepared from the middle thirds of dried root canals after 2-month storage in PBS compared with 1-week storage in this study. However, comparing the influence of humidity on the bond strength of MTA, Gancedo-Caravia and Garcia-Barbero[22] found that the bond strength of MTA in dry environment did not increase at 21 days compared with that observed at 3 days. In the present study, the roots were stored in PBS to partially simulate in vivo conditions which might explain why the bond strength of BC sealer increased by lengthening of time in dry canals.
The results of failure analysis demonstrated that the mode of bond failure was mainly cohesive for all groups. This finding is in accordance with Huffman et al.,[8] who showed that the failure mode for a calcium silicate-based sealer was predominantly cohesive. Furthermore, Ersahan and Aydin[23] revealed the predominant failure mode to be cohesive for iRoot SP.
Considering the effect of moisture content on the hardness of the EndoSequence BC sealer, Loushine et al.[24] showed that its microhardeness significantly declined when additional water was included in the sealer. In the study by Loushine et al.,[24] the sealer was mixed with water to simulate different amounts of water that may be incorporated into the sealer as it contacts root canal walls with different amounts of wetness. In the present study, the wetness was produced by leaving the PBS on dentinal walls. As calcium silicate-based materials show bioactivity in the presence of phosphate-containing fluids,[10–13] the effect of water within the root canals might be different from that of PBS on physical properties of calcium silicate-based sealers and their bond strength. This needs to be investigated in future studies.
CONCLUSION
Under the conditions of this in vitro study, the presence of PBS within the root canals increased the bond strength of gutta-percha in combination with the EndoSequence BC sealer at 1 week. However, no difference was found between the bond strength of this obturation material in the presence or absence of PBS in the root canals at 2 months. Further studies should be conducted to assess the effect of PBS within the root canals and its amount on chemomechanical properties of endodontic bioceramic calcium silicate-based sealers.
ACKNOWLEDGMENT
This research has been supported by Tehran University of Medical Sciences and Health Services grant (No. 11764). The authors wish to thank Dr. Farzaneh Aghajani and Ms. Golriz Farmani for their assistance in data collection.
Footnotes
Source of Support: This study was supported by a grant (No. 11764) from Tehran University of Medical Sciences and Health Services.
Conflict of Interest: None declared.
REFERENCES
- 1.Magura ME, Kafrawy AH, Brown CE, Jr, Newton CW. Human saliva coronal microleakage in obturated root canals: an in vitro study. J Endod. 1991;17:324–31. doi: 10.1016/S0099-2399(06)81700-0. [DOI] [PubMed] [Google Scholar]
- 2.Shipper G, Teixeira FB, Arnold RR, Trope M. Periapical inflammation after coronal microbial inoculation of dog roots filled with gutta-percha or resilon. J Endod. 2005;31:91–6. doi: 10.1097/01.don.0000140569.33867.bf. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Shen Y, Ruse ND, Haapasalo M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J Endod. 2009;35:1051–5. doi: 10.1016/j.joen.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Li Z, Peng B. Assessment of a new root canal sealer's apical sealing ability. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:e79–82. doi: 10.1016/j.tripleo.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Li Z, Peng B. Ex vivo cytotoxicity of a new calcium silicate-based canal filling material. Int Endod J. 2010;43:769–74. doi: 10.1111/j.1365-2591.2010.01733.x. [DOI] [PubMed] [Google Scholar]
- 6.Zoufan K, Jiang J, Komabayashi T, Wang YH, Safavi KE, Zhu Q. Cytotoxicity evaluation of Gutta Flow and Endo Sequence BC sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:657–61. doi: 10.1016/j.tripleo.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 7.Saleh IM, Ruyter IE, Haapasalo MP, Orstavik D. Adhesion of endodontic sealers: scanning electron microscopy and energy dispersive spectroscopy. J Endod. 2003;29:1595–601. doi: 10.1097/00004770-200309000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Huffman BP, Mai S, Pinna L, Weller RN, Primus CM, Gutmann JL, et al. Dislocation resistance of ProRoot Endo Sealer, a calcium silicate-based root canal sealer, from radicular dentine. Int Endod J. 2009;42:34–46. doi: 10.1111/j.1365-2591.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 9.Rached-Junior FJ, Souza-Gabriel AE, Alfredo E, Miranda CE, Silva-Sousa YT, Sousa-Neto MD. Bond strength of Epiphany sealer prepared with resinous solvent. J Endod. 2009;35:251–5. doi: 10.1016/j.joen.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31:97–100. doi: 10.1097/01.don.0000133155.04468.41. [DOI] [PubMed] [Google Scholar]
- 11.Tay FR, Pashley DH, Rueggeberg FA, Loushine RJ, Weller RN. Calcium phosphate phase transformation produced by the interaction of the Portland cement component of white mineral trioxide aggregate with a phosphate-containing fluid. J Endod. 2007;33:1347–51. doi: 10.1016/j.joen.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Reyes-Carmona JF, Felippe MCS, Felippe WT. Biomineralization ability and interaction of Mineral Trioxide Aggregate and Portland cement with dentin in a phosphatecontaining fluid. J Endod. 2009;35:731–6. doi: 10.1016/j.joen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Gandolfi MG, Van Landuyt K, Taddei P, Modena E, van Meerbeek B, Prati C. Environmental scanning electron microscopy connected with energy dispersive x-ray analysis and Raman techniques to study ProRoot mineral trioxide aggregate and calcium silicate cements in wet conditions and in real time. J Endod. 2010;36:851–7. doi: 10.1016/j.joen.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Weller RN, Tay KC, Garrett LV, Mai S, Primus CM, Gutmann JL, et al. Microscopic appearance and apical seal of root canals filled with gutta-percha and ProRoot Endo Sealer after immersion in a phosphate-containing fluid. Int Endod J. 2008;41:977–86. doi: 10.1111/j.1365-2591.2008.01462.x. [DOI] [PubMed] [Google Scholar]
- 15.Reyes-Carmona JF, Felippe MS, Felippe WT. A phosphate-buffered saline intracanal dressing improves the biomineralization ability of mineral trioxide aggregate apical plugs. J Endod. 2010;36:1648–52. doi: 10.1016/j.joen.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Martin RL, Monticelli F, Brackett WW, Loushine RJ, Rockman RA, Ferrari M, et al. Sealing properties of mineral trioxide aggregate orthograde apical plugs and root fillings in an in vitro apexification model. J Endod. 2007;33:272–5. doi: 10.1016/j.joen.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Gesi A, Raffaelli O, Goracci C, Pashley DH, Tay FR, Ferrari M. Interfacial strength of Resilon and gutta-percha to intraradicular dentin. J Endod. 2005;31:809–13. doi: 10.1097/01.don.0000158230.15853.b7. [DOI] [PubMed] [Google Scholar]
- 18.Bioceramics. Encyclopædia Britannica. 2010. [Last accessed on 2011 Nov 18]. Available from: http://www.britannica.com/EBchecked/topic/65775/bioceramics .
- 19.Koch K, Brave D. The increased use of bioceramics in endodontics. [Last accessed on 2011 Dec 01]. Available from: http://www.chalcogen.infim.ro/267_Bayazit.pdf .
- 20.Gou Z, Chang J, Zhai W, Wang J. Study on the self-setting property and the in vitro bioactivity of beta-Ca2SiO4. J Biomed Mater Res B Appl Biomater. 2005;73:244–51. doi: 10.1002/jbm.b.30203. [DOI] [PubMed] [Google Scholar]
- 21.Zhao W, Wang J, Zhai W, Wang Z, Chang J. The self-setting properties and in vitro bioactivity of tricalcium silicate. Biomaterials. 2005;26:6113–21. doi: 10.1016/j.biomaterials.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 22.Gancedo-Caravia L, Garcia-Barbero E. Influence of humidity and setting time on the push-out strength of mineral trioxide aggregate obturations. J Endod. 2006;32:894–6. doi: 10.1016/j.joen.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Ersahan S, Aydin C. Dislocation resistance of iRoot SP, a calcium silicate-based sealer, from radicular dentine. J Endod. 2010;36:2000–2. doi: 10.1016/j.joen.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 24.Loushine BA, Bryan TE, Looney SW, Gillen BM, Loushine RJ, Weller RN. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J Endod. 2011;37:673–7. [Google Scholar]


