Abstract
Background:
Enterococcus faecalis has been widely used as a valuable microbiological pathogen for in vitro studies due to its ability to successfully colonize the root canal in a biofilm-like style, invade dentinal tubules, and resist endodontic treatment procedures.The aim of this study was to compare the bactericidal efficacy of photodynamic therapy (PDT), 2% chlorhexidine, 2.5% NaOCl, and combination of PDT and 2.5% NaOCl against E. faecalis.
Materials and Methods:
Sixty single-rooted teeth had their canals contaminated with E. faecalis in brain heart infusion broth and were incubated for 48 hours.The canals were then subjected to 2% chlorhexidine, 2.5% NaOCl, PDT (red light emitting diode 625 nm+ Toludine Blue) and PDT + 2.5% NaOCl. Controls consisted of no treatment (positive control) and without inoculation of bacterium (negative control). Following treatment, the canal contents were sampled with sterile paper points.The samples were dispersed in transport medium, serially diluted, and cultured on blood agar to determine the number of colony forming units (CFU). Data were analyzed by Mann-Whitney U test at 5% significance level. The significance level for all analyses was set at P <.05.
Results:
Combination of PDT and 2.5% NaOCl achieved maximum reduction in recovered viable bacteria, no viable bacteria was observed after treatment of PDT + 2.5% NaOCl.
Conclusion:
Combination of PDT and 2.5% NaOCl simultaneously is effective in the elimination of E. faecalis from dentinal tubules under the conditions of this study.
Keywords: Enterococcus faecalis, sodium hypochlorite, photosensitization, root canals, toluidine blue
INTRODUCTION
One possible reason for persistent endodontic infection may be retention of microorganisms in the dentine tissue of the root canal walls. Dentinal tubules of root canal walls have been shown to harbor microorganisms.[1] An infection of the pulp can result in microbial colonization of the entire root canal system, together with the dentinal tubules adjacent to the canal.[2] Selective pressures related to oxidation–reduction potential, nutrient supply, and microbial interactions are related to the maintenance of endodontic infections. Enterococcus faecalis has been identified frequently in cases with refractory endodontic infections.[3] It is the dominant microorganism in root-filled teeth presenting post-treatment apical periodontitis and can be isolated from the root canal in pure culture. E. faecalis has been the focus of attention as a recognized pathogen, isolated both in mixed microbiota and in monocultures. Several virulence factors (aggregation substance, enterococcal surface proteins (Esp), gelatinase, cytolysin toxin, extracellular superoxide production, capsular polysaccharides, antibiotic resistance determinant) can facilitate tissue invasion, immunomodulation, and cause toxin-mediated damage.[3,4] Sodium hypochlorite (NaOCl) and chlorhexidine are common irrigants used in endodontic therapy.[3] Sodium hypochlorite presents antimicrobial activity with action on bacterial essential enzymatic sites promoting irreversible inactivation originated by hydroxyl ions and chloramination action. Dissolution of organic tissue can be verified in the saponification reaction when sodium hypochlorite destroys fatty acids and lipids resulting in soap and glycerol.[5] Chlorhexidine gluconate solutions of varying concentrations have recently been recommended as endodontic irrigants[6,7] and dressings.[8–10] Chlorhexidine is a cationic agent (biguanide group; 4-chlorophenyl radical), which exhibits antibacterial activity. The cationic nature of the compound promotes connection with anionic compound at the bacterial surface (phosphate groups from teicoic acid at gram-positive and lipopolysaccharide at gram-negative bacteria) capable of altering its integrity. The potassium ion, being a small entity, is the first substance to appear when the cytoplasmic membrane is damaged.[11] Although NaOCl and Chlorhexidine present antibacterial activity, both substances have distinct characteristics. Various research results have been inconclusive when comparing the antimicrobial effect of these solutions.[11–13] Disadvantages of conventional root canal treatments include their skill-dependent nature, long treatment time, possible weakening of teeth due to widening of the root canal, and use of medicaments such as sodium hypochlorite.[14] Furthermore, the field of antibacterial chemotherapy is a constant challenge. The current problem of bacterial drug resistance perhaps best illustrates the continuing requirement both for new agents and new approaches to eliminate infection from root canal system. The commercial phenothiazine dye, toluidine blue (TB), is an effective photosensitizing agent for the inactivation of pathogenic organisms, including viruses, bacteria, and yeast.[15,16] In recent years, TB has been reported as an antifungal and antibacterial drug for inactivation of yeast and some gram-positive and gram-negative bacteria.[15] Photodynamic therapy (PDT) matured as feasible medical technology in 1980s at several institutions throughout the world to eradicate premalignant and early-stage cancer and reduce the tumor size in end-stage cancers involving 3 components: 1. A photosensitizer 2. Light source, and 3. Tissue oxygen. The word photodynamics means the study of activating effects of light on living organisms. Employing the same principle, PDT can be described as a medical treatment that utilizes light to activate a photosensitizing agent in presence of oxygen. PDT or photoactivated disinfection uses light of a specific wavelength to activate a nontoxic photoactive dye (photosensitizer) in the presence of oxygen.[17] The photoactivated sensitizer can interact with the biological substrate, leading to the production of highly reactive oxygen species, such as singlet oxygen and free radicals, which can kill microorganisms by damaging essential cellular molecules, including proteins, membrane lipids, and nucleic acid.[5,18,19] Gram-negative bacteria are less susceptible to photoinactivation than gram-positive species.[20]
The aim of this study was to compare the effectiveness of 2% chlorhexidine, 2.5% NaOCl, PDT, and combination of two factors (PDT plus 2.5% NaOCl) as intracanal disinfectants in extracted teeth against E. faecalis.
MATERIALS AND METHODS
Preparation of specimens
Ninety freshly extracted, intact, adult, human, single-rooted, mature teeth with a single canal were collected and stored in sterile saline. Calculus and stains were removed from the root surface using an ultrasonic scaler (Cavitron, Dentsply Ltd, Weybridge, UK).[11] After preparation of coronal two-thirds of all canals using Gates Glidden files number 4, 3 and 2, they were sequentially prepared within 1 mm apical end of the canal, using Hedström files (Antaeos, Munich, Germany) up to size 40. The canal was irrigated with physiologic saline after the use of each size file. To remove the smear layer that had developed on the canal wall as a result of the instrumentation, each canal was rinsed with 10% citric acid. Only teeth with closed apices tight for solutions were used in this study. All teeth were dried with paper points and weighed. The teeth were then stored in demethylated ethanol 70%.[2] The prepared tooth was mounted in the lid of a bijou bottle. The assembled tooth, lid, and bottle were covered with aluminium foil and autoclaved at 121°C, for 15 min. The bottle was then aseptically filled with sterile brain heart infusion broth (BHI) (Merk, Poole, UK) so that the root was covered.[14]
A clinical strain of E. faecalis strain ATCC 29212, isolated from a treated root canal at the Department of Endodontics of Shahid Beheshti university of Medical Sciences was used in this experiment. Bacterial suspension was prepared by a pure culture of this E. faecalis strain, grown in 1 mL of sterile BHI broth to obtain an optical turbidity of 0.5 McFarland standard, and incubated for 1 hour at 37°C in the incubator. The inoculums were injected into the prepared root canal using a sterile syringe and incubated for 48 h at 37°C.[14]
Testing procedures
Teeth were randomly divided into five experimental groups (each composed of 15 teeth) according to the post-instrumentation procedures. Groups were as follow:
Sodium hypochlorite (NaOCl) irrigation
The canals were filled with NaOCl (2.5% v/v) for 5 min, removed with sterile paper points, and irrigated with normal saline solution (.85% v/v).[14]
Diode laser plus 2.5% NaOCl
The canals were filled with NaOCl (2.5% v/v) for 5 min, removed with sterile paper points and then was irradiated with diode laser.[15] The irradiation source was a diode laser (fotoson CMS Dental, Denmark) with a total power of 200 mw/cm2 and 625 nm of wavelength. Total irradiation time was 60 s.
Photodynamic therapy
Root canal infected with E. faecalis was subjected to lethal photosensitization with toluidine blue with concentration 15 μg/mL[19] and diode laser for 60 s.[15] The irradiation source was a diode laser (fotoson CMS Dental, Denmark) with a total power of 200 mw/cm2 and 625 nm of wavelength.
2.5% NaOCl plus PDT
The canals were filled with NaOCl (2.5% v/v) for 5 min, removed with sterile paper points, and then subjected to PDT as described above.
Chlorhexidine irrigation
Root canals were injected with Chlorhexidine (2% v/v) (Altrincham, Cheshire, WA14 5DH, UK) for 5 min, removed with sterile paper points, and irrigated with normal saline solution (.85% v/v).[2,14]
Control groups
Controls consisted of no treatment (positive control) and without inoculation of bacterium (negative control) for five experimental groups.
Sampling procedures
Following all treatments, the liquid content of the root canal was carefully absorbed with paper points. Canals were filled with sterile 0.85% normal saline solution, and sample was taken by the sequential use of three paper points placed to the working length. Paper points were transferred to tubes containing 1 mL of 0.85% normal saline solution and agitated for 1 minute. After 10-fold serial dilutions in saline, aliquots of 0.1 mL were plated onto blood agar plates and incubated at 37°C for 48 hours. The colony forming units (CFUs) grown were counted and then transformed into actual counts based on the known dilution factors.[14,19]
Data were analyzed by Mann-Whitney U test at 5% significance level. The significance level for all analyses was set at P <.05.[18,20]
RESULTS
Table 1 depicts the mean, median, range, and percent reduction of CFUs observed for all groups. The individual and combined effects of antibacterial agents are presented in Table 1. Counting of all CFU of E. faecalis remaining in the control (no treatment) and groups was done to determine effectiveness of treatments. There was a significant reduction in the CFU counts (P < 0.05) compared with the initial numbers recorded in positive control, photodynamic therapy alone, and chlorohexidine therapy; both had mild antibacterial effects. A maximum of viable bacterial reduction was observed with a combination of PDT plus 2.5% NaOCl that resulted in 100% bacterial kill [Figure 1]. The group analyses were performed to investigate the ability of each procedure for reducing the bacterial counts when compared with the previous conditions. A reduction in the number of CFUs was statistically significant for all groups (P < 0.05 for all groups). The reduction factors were significantly higher in group 2 (NaOCl plus diode laser) compared to group 3 (PDT) and group 5 (chlrohexidine) (P <.05). The Mann-Whitney U test did not show significant differences when comparing samples from group 2 and group 1 (P = 0.09). The group comparisons failed to disclose significant differences between group 1 and group 2. However, group 4 (PDT plus NaOCl) was significantly more effective in eliminating bacteria from the root canals when compared to all [Figure 1] (P < 0.05). Highest CFU of viable bacteria was from positive control group, while the negative control group was free of microorganisms under the experimental conditions [Table 1].
Table 1.
Counts of Enterococcus faecalis colony-forming units after effect of antibacterial agents
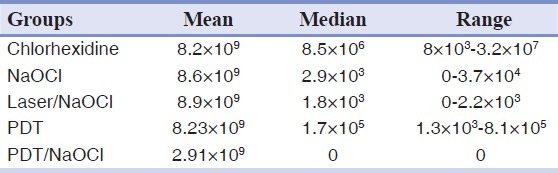
Figure 1.
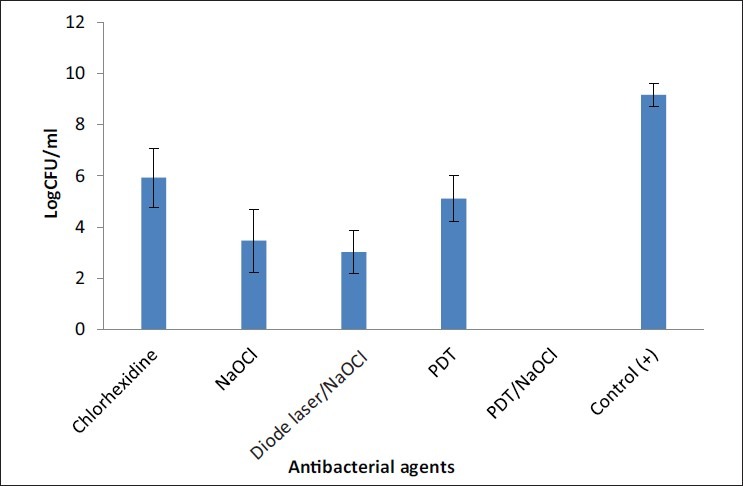
Effect of antibacterial agents against E. faecalis
DISCUSSION
E. faecalis has been the most frequent species with post-treatment apical periodontitis.[21] Although this species has been recently questioned as to its importance in the etiology of treatment failures, such a role cannot be surely disregarded in the light of current evidence. Moreover E. faecalis has been widely used as a valuable microbiological marker for in vitro studies because it has been shown to successfully colonize the root canal in a biofilm-like style, invade dentinal tubules, and resist to some endodontic treatment procedures.[19] In present study, E. faecalis was chosen as the test microorganism because it is one of the most resistant microorganism found in infected root canals,[22,23] and it has been reported that cases with refractory endodontic treatment were associated with this bacterial strain.[24–26] From the results obtained with the control teeth, it is clear that E. faecalis was capable of migrating up to 400 μm and deeper into the dentinal tubules.
In the present study, 2% chlorhexidine solution was used because previous results have shown that 2% solution was more efficient in the shortest period of time than all other concentrations.[2] Previous reports have shown that chlorhexidine has a marked effect against E. faecalis[27–30] and could be effective even at low concentrations against the microorganisms most frequently present in infected root canals,[29] anaerobic bacteria,[22] and Candida albicans.[8,31] E. faecalis CFU were significantly decreased following NaOCl irrigation. The 2.5% NaOCl (5 min) alone exhibited reduction of E. faecalis in the root canal system of extracted human teeth. However when combined with diode laser, 2.5% NaOCl was more effective on E. faecalis killing. The effect of NaOCl plus PDT was the most effective therapy and achieved 100% kill of E. faecalis after 2-min contact time.[31] In this study 2.5% NaOCl was used because it was shown to denature bacterial toxins and dissolve organic tissues.[14,32,33] Studies have shown that the magnitude of the antimicrobial efficacy of a 2.5% NaOCl and chlorhexidine can be influenced by the methodology, microbial characteristics in the biofilm, exposure time, and concentration of the substance tested.[11,34] The antimicrobial effect of NaOCl by direct contact on E. faecalis occurred after 2 min.[11,31] Our results demonstrate that 2% chlorhexidine and 2.5% NaOCl had a significant effect on the viability of E. faecalis. Thus, our result confirms those of previous studies on oral microorganisms.[2,8,9,19] In this study, PDT exhibited effect of E. faecalis killing. In previous studies of bactericidal activity against E. faecalis biofilm extracted from human teeth, it was reported that the total energy output of a diode laser was 36 J[35] and total energy level was 76 J and combined with a photosensitizer was adequate for a high level of sterilization.[36] Nagayoshi et al., found that irradiation at 5 W for 60 seconds in the presence of a photosensitizer decreased lesion defect area.[36] In the present study, irradiation with laser, TBO and irrigation with NaOCl 2.5% (5 min) with an absorption maxima (625 nm) had an excellent efficacy against E. faecalis, because in the total of 15 canals incubated with this bacterial strain and then treated with PDT/laser/NaOCl, no bacteria could be detected in 100% cases. Seal and coworkers (2002) reported effect of 100% killing teeth infected with S. intermedius biofilm treated with 3% NaOCl for 10 min.[14] Previous results have shown that singlet oxygen production was not the most relevant parameter to consider when evaluating the antimicrobial activity of PDT against S. mutans.[37] A major advantage of PDT in treatment of root canal infections is the absence of thermal side-effects in the tissues surrounding the roots, as associated with the use of high power lasers. The anticipated benefits of access of laser light and photosensitizer were more limited than hypothesized. Refinement of the laser delivery system by introduction of the laser beam into the root canal and/or increased energy delivery may be needed to achieve a better antimicrobial effect.[14] Overall PDT of single species grown in a tooth model was interestingly effective considering the light dose at the access cavity. But ultimately, combination of it with 2.5% NaOCl and PDT is the best option to maximize root canal disinfection and can predictably disinfect root canals in clinical settings. This protocol is a viable option to be used in future. There are many variables to be taken into account when developing a PDT protocol, including light parameters, photosensitizers, and light delivery techniques.[19] The peak of absorption of a photosensitizer should match the wavelength of the light used for irradiation in order to promote formation of singlet oxygen, a highly reactive oxygen molecule responsible for PDT-mediated bacterial killing.[19,38,39] Both methylene blue and toluidine blue, are phenothiazine dyes that have a maximum wavelength absorption of 656 nm and 625 nm, respectively.[40,41] Antibacterial effectiveness of PDT may also depend on the photosensitizer concentration.[38] For endodontic disinfection, photosensitizers have been tested at concentrations ranging from 6.25 mg/mL to 25 mg/ mL for methylene blue and from 10 mg/mL to 100 mg/ mL for toluidine blue. Souza and workers used TBO with concentration of 15 μg/ml against E. faecalis.[19] In this study, we used concentration of 15 μg/ml. Souza reported that PDT with either methylene blue or toluidine blue did not have a significant additional effect to the chemo-mechanical preparation using 2.5% NaOCl as an irrigant in the reduction of E. faecalis populations. The additional antibacterial effect of PDT with either photosensitizer (methylene blue and toluidine blue) was reported, but it was statistically significant only for toluidine blue.[19] The results of our study showed that reduction in the number of CFUs was statistically significant for all groups: chlorhexidine, NaOCl, laser plus TBO (PDT), laser/NaOCl, and PDT/NaOCl against E. faecalis. However, no viable cells were detected with combination PDT/NaOCl. Further research is essential to offer new guidelines for treatment protocols of endodontic infections.
CONCLUSION
One of the aims of root canal treatment is to eliminate the bacteria, their products, and the substrate from the root canal system. The result of this research suggests that effect of PDT plus 2.5% NaOCl simultaneously is effective in the elimination of E. faecalis from dentinal tubules under the conditions of this study.
AKNOWLEDGEMENT
This article was based on postgraduate thesis by Amin Nazari Nasab which was successfully completed under the supervision of Dr. Ali Kangarlou by Dental school of Shahid Beheshti University of Medical Sciences.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Safavi KE, Spangberg LS, Langeland K. Root Canal Dentinal Tubule Disinfection. J Endod. 1990;16:207–10. doi: 10.1016/s0099-2399(06)81670-5. [DOI] [PubMed] [Google Scholar]
- 2.Schafer E, Boosmann K. Antimicrobial efficacy of chlorhexidine and two calcium hydroxide formulation against Enterococcus faecalis. J Endod. 2005;31:53–6. doi: 10.1097/01.don.0000134209.28874.1c. [DOI] [PubMed] [Google Scholar]
- 3.Estrela C, Estrela CR, Decurcio DA, Hollanda AC, Silva JA. Antimicrobial efficacy of ozonated water, gaseous ozone, sodium hypochlorite and chlorhexidine in infected human root canals. Int Endod J. 2007;40:85–93. doi: 10.1111/j.1365-2591.2006.01185.x. [DOI] [PubMed] [Google Scholar]
- 4.Portenier I, Waltimo TMT, Haapasalo M. Enterococcus faecalis – the root canal survivor and ‘star’ in post-treatment disease. Endodontic Top. 2003;6:135–59. [Google Scholar]
- 5.Vela SF, Junqueira JC, Barbosa JO, Majewski M, Munin E, Jorge AO. Photodynamic inactivation of Staphylococcus aureus and Escherichia coli biofilms by malachite green and phenothiazine dyes: An in vitro study. Arch Oral Biol. 2011;2730:704–10. doi: 10.1016/j.archoralbio.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Jeansonne MJ, White RR. A comparison of 2.0% chlorhexidinegluconate and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J Endod. 1994;20:276–78. doi: 10.1016/s0099-2399(06)80815-0. [DOI] [PubMed] [Google Scholar]
- 7.Lin S, Zuckerman O, Weiss EI, Mazor Y, Fuss Z. Antibacterial efficacy of a new chlorhexidine slow release device to disinfect dentinal tubules. J Endod. 2003;29:416–8. doi: 10.1097/00004770-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Schäfer E, Bössmann K. Antimicrobial efficacy of chloroxylenol and chlorhexidine in the treatment of infected root canals. Am J Dent. 2001;14:233–7. [PubMed] [Google Scholar]
- 9.Delany GM, Patterson SS, Miller CH, Newton CW. The effect of chlorhexidine gluconate irrigation on the root canal flora of freshly extracted necrotic teeth. Oral Surg Oral Med Oral Pathol. 1982;53:518–23. doi: 10.1016/0030-4220(82)90469-8. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa CA, Gonçalves RB, Siqueira JF, Jr, De-Uzeda M. Evaluation of the antibacterial activities of calcium hydroxide, chlorhexidine, and camphorated paramonochlorphenol as intracanal medicament: A clinical and laboratory study. J Endod. 1997;23:297–300. doi: 10.1016/S0099-2399(97)80409-8. [DOI] [PubMed] [Google Scholar]
- 11.Esterla C, Ribeiro RG, Estrela CR, Pecora JD, Sousa-Neto MD. Antimicrobial Effect of 2% Sodium Hypochlorite and 2% Chlorhexidine Tested by Different Methods. Braz Dent J. 2003;14:58–62. doi: 10.1590/s0103-64402003000100011. [DOI] [PubMed] [Google Scholar]
- 12.Heling I, Chandler NP. Antimicrobial effect of irrigant combinations within dentinal tubules. Int Endod J. 1998;31:8–14. [PubMed] [Google Scholar]
- 13.Samadi N, Zaree R, Bakhtiar H, Salehnia A, Azimi S. Comparative antibacterial efficacy of endemic Satureja Khuzistanica Jamzad essential oil, Sodium Hypochlorite and Chlorhexidine Gluconate solutions as root canal irrigations. Dent Res J (Isfahan) 2011;8:28–32. [PMC free article] [PubMed] [Google Scholar]
- 14.Seal Gj, Ng Yl, Spratt D, Bhatti M, Gulabivala K. An in vitro comparison of the bacterial efficacy of lethal photosensitization or sodium hypochlorite irrigation on Streptococcus intermedius biofilms in root canals. Int Endod J. 2002;35:268–74. doi: 10.1046/j.1365-2591.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- 15.Usacheva MN, Teichert MC, Biel MA. Comparison of the Methylene Blue and Toluidine Blue Photobactericidal Efficacy Against Gram-Positive and Gram-Negative Microorganisms. Lasers Surg Med. 2001;29:165–73. doi: 10.1002/lsm.1105. [DOI] [PubMed] [Google Scholar]
- 16.Wainwright M. Non-porphyrin photosensitizers in biomedicine. Chem Soc Rev. 1997;25:351–259. [Google Scholar]
- 17.Veerenda NR, Rekha RK, Ghandana G, Sehrawat S. Photodynamic therapy: Review. Indian J Dent Adv. 2009;1:46–50. [Google Scholar]
- 18.Bertoloni G, Salvato B, Dall’Acqua M, Vazzoler M, Jori G. Hematoporphyrin-sensitized photoinactivation of Streptococcus faecalis. Photochem Photobiol. 1984;39:811–6. doi: 10.1111/j.1751-1097.1984.tb08864.x. [DOI] [PubMed] [Google Scholar]
- 19.Souza LC, Brito PR, de Oliveira JC, Alves FR, Moreira EJ, Sampaio-Filho HR, et al. Photodynamic therapy with Two different photosensitizers as a supplement to instrumentation/irrigation procedures in promoting intracanal reduction of Enterococcus faecalis. J Endod. 2010;36:292–6. doi: 10.1016/j.joen.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 20.Soukos NS, Chen PS, Morris JT, Ruggiero K, Abernethy AD, Som S, et al. Photodynamic therapy for endodontic disinfection. J Endod. 2006;32:979–84. doi: 10.1016/j.joen.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–8. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 22.Ørstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod Dent Traumatol. 1990;6:142–9. doi: 10.1111/j.1600-9657.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 23.Love RM. Enterococcus faecalis: A mechanism for its role in endodontic failure. Int Endod J. 2001;34:399–405. doi: 10.1046/j.1365-2591.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 24.Siren EK, Haapasalo PP, Ranta K, Salmi P, Kerosuo EN. Microbiological findings and clinical treatment procedures in endodontic cases selected for microbiological investigation. Int Endod J. 1997;30:91–5. [PubMed] [Google Scholar]
- 25.Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative retreatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:86–93. doi: 10.1016/s1079-2104(98)90404-8. [DOI] [PubMed] [Google Scholar]
- 26.Nair PN, Sjøgren U, Krey G, Kahnberg KE, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy resistant periapical lesion: A long-term light and electron-microscopic follow- up study. J Endod. 1990;16:580–8. doi: 10.1016/S0099-2399(07)80201-9. [DOI] [PubMed] [Google Scholar]
- 27.Basrani B, Tjäderhane L, Santos JM, Pascon E, Grad H, Lawrence HP, et al. Efficacy of chlorhexidine- and calcium hydroxide-containing medicaments against Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Radiol Endod. 2003;96:618–24. doi: 10.1016/s1079-2104(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 28.Gomes BP, Souza SF, Ferraz CC, Teixeira FB, Zala AA, Valdrighi L, Souza-Filho FJ. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J. 2003;36:267–75. doi: 10.1046/j.1365-2591.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 29.D’Arcangelo C, Varvara G, De-Fazio P. An evaluation of the action of different root canal irrigants on facultative aerobic-anaerobic, obligate anaerobic, and microaerophilic bacteria. J Endod. 1999;25:351–3. doi: 10.1016/S0099-2399(06)81170-2. [DOI] [PubMed] [Google Scholar]
- 30.Lin YH, Mickel AK, Chogle S. Effectiveness of selected materials against Enterococcus faecalis: Part 3.The antibacterial effect of calciumhydroxide and chlorhexidine on Enterococcus faecalis. J Endod. 2003;29:565–6. doi: 10.1097/00004770-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Abdullah M, Ng YL, Gulabivala K, Moles DR, Spratt DA. Susceptibilities of two Enterococcus faecalis phenotypes to root canal medications. J Endod. 2005;31:30–6. doi: 10.1097/01.don.0000136205.80807.5a. [DOI] [PubMed] [Google Scholar]
- 32.Safavi KE, Nichols FC. Alteration of biological properties of bacterial lipopolysaccharide by calcium hydroxide treatment. J Endod. 1994;20:76–8. doi: 10.1016/S0099-2399(06)80057-9. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner JC, Falkler WA., Jr Bacteria in the apical 5 mm of infected root canals. J Endod. 1991;17:380–3. doi: 10.1016/s0099-2399(06)81989-8. [DOI] [PubMed] [Google Scholar]
- 34.Spratt DA, Pratten J, Wilson M, Gulabivala K. An in vitro evaluation of the antimicrobial efficacy of irrigant on biofilms of root canal isolates. Int Endod J. 2001;34:300–7. doi: 10.1046/j.1365-2591.2001.00392.x. [DOI] [PubMed] [Google Scholar]
- 35.Lim Z, Cheng JL, Lim TW, Teo EG, Wong J, George S, et al. Light activated disinfection: An alternative endodontic disinfection strategy. Aust Dent J. 2009;54:108–14. doi: 10.1111/j.1834-7819.2009.01102.x. [DOI] [PubMed] [Google Scholar]
- 36.Nagayoshi M, Nishihara T, Nakoshima K, lwaki S, Chen KK, Terashita M, et al. Bactericidal effect of diode laser irridation on Enterococcus faecalisusing periapical lesion defect model. ISRN Dent. doi: 10.5402/2011/870364. Published online 2011 July 14. doi: 10.5402 / 2011/870364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolim JP, De-Melo MA, Guedes SF, Albuquerque-Filho FB, De-Souza JR, Nogueira NA, et al. The antimicrobial activity of photodynamic therapy against Streptococcus mutansusing different photosensitizers. J Photochem Photobiol B. 2012;106:40–6. doi: 10.1016/j.jphotobiol.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Konopka K, Goslinski T. Photodynamic therapy in dentistry. J Dent Res. 2007;86:694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- 39.Bonsor SJ, Nichol R, Reid TM, Pearson GJ. Microbiological evaluation of photo-activated disinfection in endodontics (an in vivo study) Br Dent J. 2006;200:337–41. doi: 10.1038/sj.bdj.4813371. [DOI] [PubMed] [Google Scholar]
- 40.Wainwright M. The development of phenothiazinium photosensitisers. Photodiagnosis Photodyn Ther. 2005;2:263–72. doi: 10.1016/S1572-1000(05)00110-9. [DOI] [PubMed] [Google Scholar]
- 41.Mattiello FD, Coelho AA, Martines OP, Mattiello RD, Ferrão Júnior JP. In vitro effect of photodynamic therapy on Aggregatibacteractinomycetemcomitans and Streptococcus sanguinis. Braz Dent J. 2011;22:398–403. doi: 10.1590/s0103-64402011000500009. [DOI] [PubMed] [Google Scholar]


