Abstract
Background:
Chitosan compounds have been shown to be suitable bone replacement materials. To evaluate the accelerating effects of chitosan on the bone regeneration process and assessing its histopathological adverse effects, we conducted this study on rat tibias.
Materials and Methods:
In a laboratory experimental study, micro-drilled bone defects were created in the upper tibia of each leg in 15 adult male rats. The defect in the right leg, filled by the chitosan powder, was compared with the untreated defect in the left leg in each rat at 1, 2, and 4 weeks after surgery. Bone repair and inflammation in each specimen was blindly graded by a pathologist. Reaction to the foreign body and the amount of the remaining chitosan were studied in chitosan-treated specimens at the three stages of the study.
Results:
Bone repair was significantly faster in the chitosan group, 1 week (P = 0.01) and 4 weeks (P = 0.038) after surgery, while the difference was not significant at the 2-week stage (P = 0.197) between chitosan and control groups. Chitosan-induced inflammation was not significant in any stage of the study. Reaction to the foreign body was seen in one case at 2 weeks and one case at 4 weeks postoperation.
Conclusion:
Chitosan significantly accelerated the bone regeneration process in rat tibias. Regarding its biocompatibility and osteoinductivity, it can be studied as a biomaterial in human bone healing.
Keywords: Bone regeneration, chitosan, rat
INTRODUCTION
Bone defects may develop in various clinical situations such as fractures or orthopedic surgeries. A normal bone structure should be restored in patients as soon as possible. The conventional methods of bone repair which commonly are used, including autografts and allografts, have their own shortcomings. Autografts are limited in terms of the availability of materials and may result in donor site morbidity.[1] Using allografts may be more desirable in some cases but the possible immune reaction and infection transmission limit their application. To overcome these limitations, various synthetic bone substitutes made of metal, ceramics, polymers, etc., have been introduced to accelerate and improve the process of bone regeneration, though their safety, effectiveness, and efficacy remain uncertain.[2] Recently, with the increasing number of invasive surgical procedures especially in the fields of orthopedics and dentistry, the bone repair techniques using new materials are getting more popular. The new materials which are used should help us to reduce the operation time, scar size, postoperation pain, and also improve patient recovery. One of the best materials which fulfills these requirements is chitosan.
Chitosan is a chitin-derived polymer which is produced by the deacetylation of chitin. Chitin is mainly found in exoskeletons of crustaceans and also in some fungi. These shells which were regarded as garbage in past times are a valuable source of chitin.[3] Many biomedical applications, including wound healing, bandage, skin grafting, hemostasis, hemodialysis, drug delivery, preventing dental plaque, hypertension control, calcium absorption, bilirubin absorption and cholesterol control, have been identified for improving by using chitosan.[4–8]
Several desirable properties have been described for chitosan, including high osteoinductivity, osteointegratability, easy application, and gradual biodegradability that make it a good candidate for bone regeneration. Some researchers have studied effects of chitosan compounds on animal bone repair.[9–11] Histopathological effects of chitosan on the bone regeneration process in Wistar rats are presented in this study.
MATERIALS AND METHODS
This was a laboratory experimental study on Wistar rats. In this study, a chitosan powder was obtained from capsules made by Springleaf Co. (Sydney, Australia). Each capsule contains 250 mg chitosan. The contents of 20 capsules were removed to special plastic bags and sterilized by gamma radiation of 13-15 KGy.
Animal operations
Fifteen adult male Wistar rats weighing 300-350 g were randomly assigned to one of three study groups: 1-, 2-, and 4-week groups. The rats got anesthetized by a mixture of 2% xylazine hydrochloride and 10% ketamine hydrochloride. Their legs were disinfected using the ethanol 70% spray and shaved in the areas over tibias. The tibias were exposed through skin incisions and the periosteum was removed by a fine elevator. A 3-4 mm deep microdrill hole was then created in the upper part of each tibia using a microsurgery straight hand piece driven by Surgic XT plus micromotor (NSK, Kanuma, Japan) with 3-mm round burs (Xemax Surgical Product Inc., Napa, California). Constant irrigation with cold sterile normal saline was applied to prevent thermal necrosis and wash away bone debris. The created defects were thoroughly washed and dried by sterile gauze. The defects of right leg tibias were filled in by a sterilized chitosan powder mixed with a drop of rat blood and those of the left ones were left unfilled to serve as controls. The skin was closed with 4-0 nonabsorbable sutures. The rats then were transferred to a warm room to recover from anesthesia and allowed an unrestrained activity in cages. They received 0.2 ml of ketoprofen via subdermal injection daily for 3 days as an analgesic and also 0.5 ml of floxacine for infection control.
Microscopic observation and histological study
At 1, 2, and 4 weeks postoperation, the rats in respective groups were sacrificed by putting them into a container of diethyl ether 97%. The rat legs were removed and fixed in 10% buffered formalin for 2 weeks. After fixation was completed, the legs were cut at 5 mm above and under the surgery site including the surrounding tissue and skin. Those parts containing the hard tissue (bone) need to be decalcified for block preparation and sectioning. This step was performed by placing the samples in nitric acid 10% for 24 hrs. The presence of water in the tissue prevents the penetration of the preparing material (paraffin) into the tissue. So in order to dehydrate the tissue, samples were placed in alcohol 50%, 70%, and 90% and absolute alcohol and then were placed in xylol to substitute for alcohol (dehydration process). In the infiltration step, samples were place in molten paraffin (50°C). From the samples embedded in paraffin, serial microscopic sections of 3-4 μm thick were prepared using a Leica microtome (Leica Microsystems, Wetzlar, Germany). Then the slices from the repaired bone cavity were stained with hematoxylin and eosin for microscopic examination.[12,13]
Qualitative histopathological scoring
The slices were studied using an Olympus microscope (Olympus Optical Co., Ltd, Tokyo, Japan) in a high-power field (×400). Two slices from each defect were examined and graded by an expert pathologist for bone regeneration (grades 1-3), assessing inflammation (grades 0-3), foreign body reaction (+/−), bone vitality (+/−), and remained chitosan (low, medium, high). Bone regeneration was graded 1 if woven bone was seen, 2 if both woven and lamellar bones were seen, and 3 if just lamellar bone was seen. Inflammation was graded as based on the number of infiltrative cells seen in a visual field, from 0 to 3. The decision on foreign body reaction was made based on the presence of multinuclear giant cells. Bone vitality was signed as positive if there were osteocytes inside lacunae.
Statistical analyses
To test the hypothesis that chitosan accelerates bone repair, differences in bone regeneration between the intervention and control specimens were analyzed by the Kappa test, among 1-, 2- and 4-week groups using SPSS version 11 (SPSS Inc., Chicago, IL, USA). A P value less than 0.05 was considered as significant. Fisher's exact test was used to determine if the intervention groups were significantly different from the control group in terms of inflammation and bone regeneration against inflammation.
Ethical consideration
The proposal of this study got approved by the Ethics Committee of Shahid Sadoughi University of Medical Sciences.
RESULTS
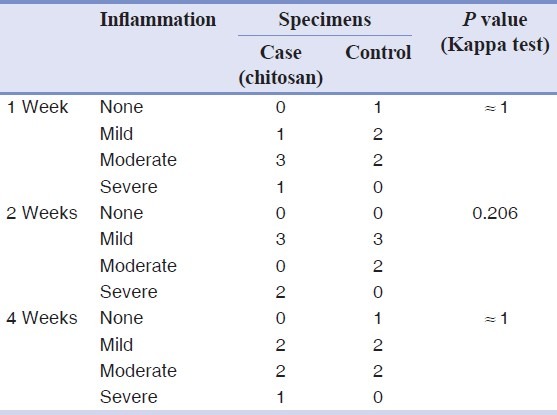
All 30 rat tibias (15 with chitosan powder and 15 controls) in three stages (1, 2, and 4 weeks after operation) were blindly studied by a pathologist. All the bones were vital and no fracture or necrosis was seen. The Kappa test on both chitosan and control samples indicated that bone defects filled with the chitosan powder showed a significantly faster healing process compared to those of control ones at 1 week (P = 0.01) and 4 weeks (P = 0.038) after operation, whereas this difference was not statistically significant between the two groups after 2 weeks (P = 0.197). One week after the surgery, no woven bone was seen in either the chitosan or control specimens [Figure 1], but 2 weeks and 4 weeks postoperation, more woven bone was observed in the chitosan specimens compared to that in the control specimens [Figures 2 and 3, respectively]. Our results indicated that there was fibrosis in 4 specimens in the chitosan group after 1 week, while this number decreased to 2 after 2 weeks, and there was no case of fibrosis in the case group after 4 weeks. There was no fibrosis after 1 week in the controls and this number increased to 4 after 2 weeks, and to 3 after 4 weeks in controls. Regarding the observation of the granulation tissue, it was seen in one of the cases in the chitosan group, and in five control cases after 1 week, while there were no cases in both groups after 2 and 4 weeks [Table 1; Figures 2 and 3]. Inflammation grading was made on the basis of the number of infiltrative cells in the high-power field of microscopic studies of the specimens. Various grades of inflammation were seen in all three stages of the study. Statistical analysis using the Kappa test interestingly showed no significant difference in inflammation between the chitosan and control specimens [Table 2]. Just 2 (out of 15) chitosan specimens showed reaction to foreign bodies at 2 and 4 weeks after surgery identified by the presence of multinucleated giant cells, but the other specimens showed no reaction. The amount of chitosan remained in the micro-drilled defects was also assessed visually by a pathologist and was graded as low, moderate, and high. Four weeks after surgery, the remained chitosan in four specimens was assessed and found to be low, and just one specimen was graded as moderate in this classification.
Figure 1.
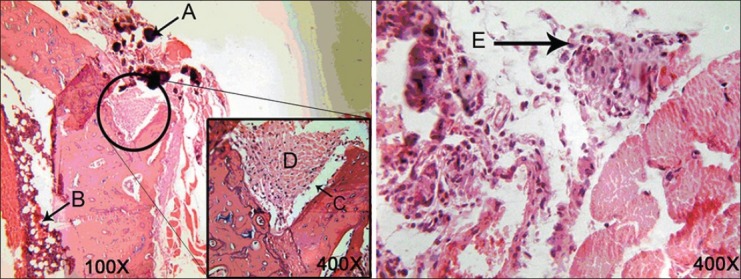
Micrographs of the tibia 1 week after surgery in a case (left) and a control (right) subject. (A) Chitosan particles, (B) bone marrow, (C) microdrilled defect, (D) fibrous tissue, (E) granulation tissue with inflammatory cells
Figure 2.
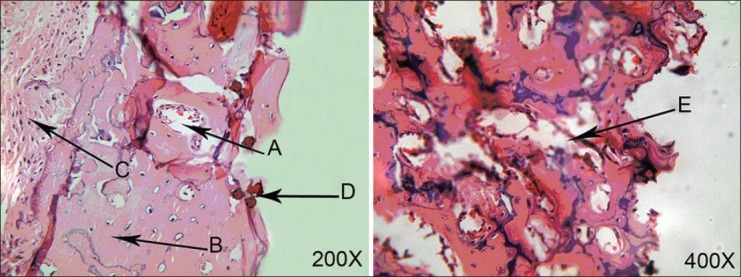
Micrograph of the tibia 2 weeks after surgery in case (left) and control (right) groups. (A and E) Woven bone, (B) tibia bone, (C) bone marrow, (D) chitosan particles
Figure 3.
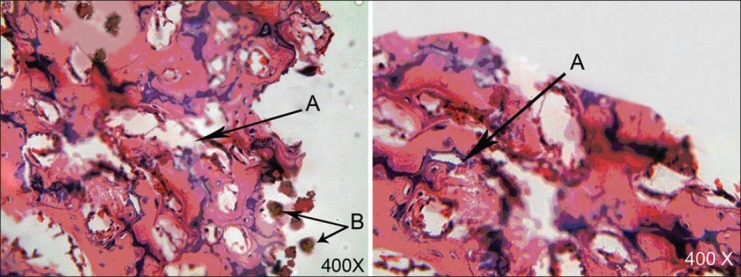
Micrograph of tibia 4 weeks after surgery in case (left) and control (right) groups. (A) woven bone and surrounding connective tissue, (B) remaining chitosan
Table 1.
Bone healing status of case (chitosan) and control specimens at three different stages
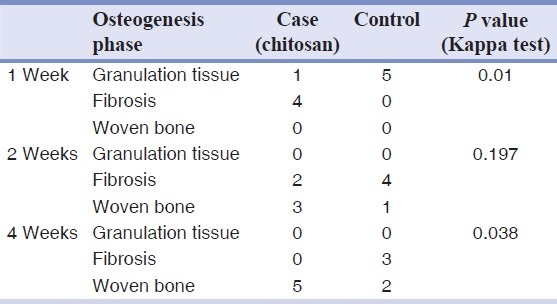
Table 2.
Comparing inflammation severity between chitosan and control specimens at three different stages
DISCUSSION
An ideal material for bone regeneration, as indicated by prior studies, should be biocompatible, biodegradable, easy to apply, and effective on bone repair.[11,14] Since chitosan has been shown by Shin et al.,[15] and other researchers to possess these properties, we studied its effectiveness on bone defect healing in micro-drilled tibias in rats.
It is important for bone replacement materials to be highly adaptable to the bone defects. We used chitosan in the form of powder to fill in the bone defects and remain there for local absorption. Also to compare the treated bone healing with those of untreated, the defects in the control bones were left unfilled. Our study is different from the others in terms of using pure chitosan instead of chitosan compounds and also applying it in a powder form to achieve good adaptation to bone defects.
Chevrier et al., showed in their study that chitosan increases vascularization and induces granulation tissue generation.[16] The achieved results at 1 week postoperation showed granulation tissue in all control specimens, while in the chitosan group fibrosis of various degrees was seen. This trend of a faster bone regeneration process could be seen in subsequent stages. At 2 weeks postoperation, a mixture of woven bone and fibrous tissue (with superiority of the woven bone) was seen in treated defects, whereas in untreated ones, the fibrous tissue was dominant. This result supports the observations of Muzarelli et al.,[17] Four weeks after surgery, woven bone was seen in all chitosan-treated specimens, whereas just two specimens in the control group developed woven bone.
It has been observed that chitosan, a natural biopolymer which is derived from chitin, and some of its derivatives, such as chitosan dihydrochloride, may act as wound healing accelerators, bone substitutes, antimicrobial agents, and may show hemostatic activities. It is documented that these substances are biodegradable and nontoxic.[16–19] Recently, chitosan composites have been widely used in bone tissue engineering due to their unique properties such as low foreign body reactions, biocompatibility, and their moldability.[20–26] Chitosan can act as a hydrating agent and can be processed into different forms such as scaffolds, nanoparticles, and gels.[26]
It is also believed that the chitosan composite improves the mechanical strength of the scaffold material.[27] Mathews et al., proposed that chitosan can increase mineralization by upregulating the associated genes as a mechanism for the osteogenesis of this substance.[25]
Boynueğri et al., in their study found that the chitosan gel combined with the demineralized bone matrix/collagenous membrane or even alone is useful for periodontal regeneration[19] although Spin-Neto et al., found that these substances were not able to promote new bone formation in critical size defects which were made in rat's calvaria.[18]
Usui et al., in their study found that chitosan tubes have a favorable compatibility with the bone tissue and promote bone regeneration and may accelerate bone formation stimulated by recombinant human bone morphogenetic protein-2.[28] In another study, Budiraharjo et al., found that HAP-coated CMCS scaffolds can act as osteogenic scaffolds for the stimulation of bone healing.[29] The study by Li et al., proved that chitosan could significantly promote osteogenesis at the graft–bone interface histologically.[30]
Geffre et al., compared the osteoinductivity of purified chitosan – CaP and the conventional form and found that the former substance had a lower osteoinductivity potential.[31]
Inflammation was associated with chitosan group in higher degrees than the control group in all three stages of the study. This finding is similar to the result reported by Chevrier et al., on subcondral bones in rats.[32] Reaction to the foreign body was seen in two cases of chitosan-treated defects, and both of them were accompanied by sever inflammation. This reaction could be due to the dislocation of the chitosan powder or some other clinical manifestation. The overall prevalence of foreign body reaction was not remarkable in this study. The remaining chitosan at 4 weeks postoperation was less than that at 1 and 2 weeks which shows a proper descending trend. This decrease could be mainly due to local absorption by the surrounding tissues, though we could not rule out the probability of physical withdrawal from the filled-in site during the time.
Our findings in some aspects are different from similar studies. These differences could be due to variation in techniques, animal subjects, or type of the bone selected. We used chitosan in a pure powder form whereas other studies used it as compounds with some other materials like hydroxyapatite and calcium sulfate. Bone mass, blood perfusion of the repairing bone, and also the species of the animal used as subjects are important parameters when considering the final results of such studies.
CONCLUSION
This study shows that chitosan is effective in bone regeneration in the micro-drilled rat tibia. Considering the osteoinductivity of chitosan, it can be used to promote bone repair in situations where rapid bone regeneration is of high importance or there is a lack of normal osteogenesis due to systemic disorders. Some more studies with different animal subjects and techniques are needed to approve safety and long-term effects of this material.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Damien CJ, Parsons JR. Bone graft and bone graft substitutes: A review of current technology and applications. J Appl Biomater. 1991;2:187–208. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 2.Langstaff S, Sayer M, Smith TJ, Pugh SM. Resorbable bioceramics based on stabilized calcium phosphates. Part II: Evaluation of biological response. Biomaterials. 2001;22:135–50. doi: 10.1016/s0142-9612(00)00139-3. [DOI] [PubMed] [Google Scholar]
- 3.Burrows F, Louime C, Abazinge M, Onokpise O. Extraction and evaluation of chitosan from crab exoskeleton as a seed fungicide and plant growth enhancer. American-Eurasian J Agric Environ Sci. 2007;2:103–11. [Google Scholar]
- 4.Vasudev SC, Chandy T, Sharma CP. Development of chitosan/polyethylene vinyl acetate co-matrix: Controlled release of aspirin-heparin for preventing cardiovascular thrombosis. Biomaterials. 1997;18:375–81. doi: 10.1016/s0142-9612(96)00131-7. [DOI] [PubMed] [Google Scholar]
- 5.Chandy T, Sharma CP. Chitosan matrix for oral sustained delivery of ampicillin. Biomaterials. 1993;14:939–44. doi: 10.1016/0142-9612(93)90136-p. [DOI] [PubMed] [Google Scholar]
- 6.Chandy T, Sharma CP. Polylysine-immobilized chitosan beads as adsorbents for bilirubin. Artif Organs. 1992;16:568–76. doi: 10.1111/j.1525-1594.1992.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 7.Chandy T, Sharma CP. Chitosan-as a biomaterial. Biomater Artif Cells Artif Organs. 1990;18:1–24. doi: 10.3109/10731199009117286. [DOI] [PubMed] [Google Scholar]
- 8.Patel VR, Amiji MM. Preparation and characterization of freeze-dried chitosan-poly (ethylene oxide) hydrogels for site-specific antibiotic delivery in the stomach. Pharm Res. 1996;13:588–93. doi: 10.1023/a:1016054306763. [DOI] [PubMed] [Google Scholar]
- 9.Hoemann CD, Sun J, McKee MD, Chevrier A, Rossomacha E, Rivard GE, et al. Chitosan-glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthritis Cartilage. 2007;15:78–89. doi: 10.1016/j.joca.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Lee JY, Nam SH, Im SY, Park YJ, Lee YM, Seol YJ, et al. Enhanced bone formation by controlled growth factor delivery from chitosan-based biomaterials. J Control Release. 2002;78:187–97. doi: 10.1016/s0168-3659(01)00498-9. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Ma J, Wang Y, He B. Bone repair in radii and tibias of rabbits with phosphorylated chitosan reinforced calcium phosphate cements. Biomaterials. 2002;23:4167–76. doi: 10.1016/s0142-9612(02)00153-9. [DOI] [PubMed] [Google Scholar]
- 12.Cho BC, Kim TG, Yog J, Chang H. Effect of calcium sulfate-chitosan composit: Pellet on bone formation in bone defect. J Craniofacial Surg. 2005;16:213–24. doi: 10.1097/00001665-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Mescher AL. Junqueira's Basic Histology: Text & Atlas. 12th ed. New York: McGraw-Hill Medical; 2010. [Google Scholar]
- 14.Ripamonti U, Duneas N. Tissue engineering of bone by osteoinductive biomaterials. MRS Bull. 1996;21:36–9. [Google Scholar]
- 15.Shin SY, Park HN, Kim KH, Lee MH, Choi YS, Park YJ, et al. Biological evaluation of chitosan nanofiber membrane for guided bone regeneration. J Periodontol. 2005;76:1778–84. doi: 10.1902/jop.2005.76.10.1778. [DOI] [PubMed] [Google Scholar]
- 16.Chevrier A, Hoemann CD, Sun J, Buschmann MD. Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthritis Cartilage. 2007;15:316–27. doi: 10.1016/j.joca.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Muzzarelli RA, Mattioli-Belmonte M, Tietz C, Biagini R, Ferioli G, Brunelli MA, et al. Stimulatory effect on bone formation exerted by a modified chitosan. Biomaterials. 1994;15:1075–81. doi: 10.1016/0142-9612(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 18.Spin-Neto R, de Freitas RM, Pavone C, Cardoso MB, Campana-Filho SP, Marcantonio RA, et al. Histological evaluation of chitosan-based biomaterials used for the correction of critical size defects in rat's calvaria. J Biomed Mater Res A. 2010;93:107–14. doi: 10.1002/jbm.a.32491. [DOI] [PubMed] [Google Scholar]
- 19.Boynueğri D, Ozcan G, Senel S, Uç D, Uraz A, Oğüş E, et al. Clinical and radiographic evaluations of chitosan gel in periodontal intraosseous defects: A pilot study. J Biomed Mater Res B Appl Biomater. 2009;90:461–6. doi: 10.1002/jbm.b.31307. [DOI] [PubMed] [Google Scholar]
- 20.Di Martino A, Sittinger M, Risbud MV. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–90. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Teng S, Lee E, Yoon B, Shin D, Kim H, Oh J. Chitosan/nanohydroxyapatite composite membranes via dynamic filtration for guided bone regeneration. J Biomed Mater Res Part A. 2009;88:569–80. doi: 10.1002/jbm.a.31897. [DOI] [PubMed] [Google Scholar]
- 22.Xianmiao C, Yubao L, Yi Z, Li Z, Jidong L, Huanan W. Properties and in vitro biological evaluation of nano-hydroxyapatite/chitosan membranes for bone guided regeneration. Mater Sci Eng C. 2009;29:29–35. [Google Scholar]
- 23.Kong L, Gao Y, Cao W, Gong Y, Zhao N, Zhang X. Preparation and characterization of nano-hydroxyapatite/chitosan composite scaffolds. J Biomed Mater Res Part A. 2005;75A:275–82. doi: 10.1002/jbm.a.30414. [DOI] [PubMed] [Google Scholar]
- 24.Thein-Han W, Misra R. Biomimetic chitosan – nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009;5:1182–97. doi: 10.1016/j.actbio.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Mathews S, Gupta PK, Bhonde R, Totey S. Chitosan enhances mineralization during osteoblast differentiation of human bone marrow-derived mesenchymal stem cells, by upregulating the associated genes. Cell Prolif. 2011;44:537–49. doi: 10.1111/j.1365-2184.2011.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu C, Lei C, Meng L, Wang C, Song Y. Chitosan as a barrier membrane material in periodontal tissue regeneration. J Biomed Mater Res B Appl Biomater. 2012;100:1435–43. doi: 10.1002/jbm.b.32662. [DOI] [PubMed] [Google Scholar]
- 27.Venkatesan J, Kim SK. Chitosan composites for bone tissue engineering-an overview. Mar Drugs. 2010;8:2252–66. doi: 10.3390/md8082252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Usui Y, Aoki K, Narita N, Murakami N, Nakamura I, Nakamura K, et al. Carbon nanotubes with high bone-tissue compatibility and bone-formation acceleration effects. Small. 2008;4:240. doi: 10.1002/smll.200700670. [DOI] [PubMed] [Google Scholar]
- 29.Budiraharjo R, Neoh KG, Kang ET. Hydroxyapatite-coated carboxymethyl chitosan scaffolds for promoting osteoblast and stem cell differentiation. J Colloid Interface Sci. 2012;366:224–32. doi: 10.1016/j.jcis.2011.09.072. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Ge Y, Zhang P, Wu L, Chen S. The effect of layer-by-layer chitosan-hyaluronic acid coating on graft-to-bone healing of a poly (ethylene terephthalate) artificial ligament. J Biomater Sci Polym Ed. 2012;23:425–38. doi: 10.1163/092050610X551989. [DOI] [PubMed] [Google Scholar]
- 31.Geffre CP, Ochoa J, Margolis DS, Szivek JA. Evaluation of the osteogenic performance of calcium phosphate-chitosan bone fillers. J Invest Surg. 2010;23:134–41. doi: 10.3109/08941930903564100. [DOI] [PubMed] [Google Scholar]
- 32.Chevrier A, Hoemann CD, Sun J, Buschmann MD. Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthritis Cartilage. 2007;15:316–27. doi: 10.1016/j.joca.2006.08.007. [DOI] [PubMed] [Google Scholar]


