Abstract
Background:
The purpose of this study was to perform a histological, histomorphometrical, and immunohistochemical evaluation of the effect of Enamel matrix derivative (EMD) on bone formation around titanium dental implant.
Materials and Methods:
In this animal study, 12 implants (10 × 3.8 mm) were inserted in the tibia bone of three dogs of Iranian breed. Two implants were placed in each tibia with EMD only on the left side. The dogs were sacrificed 2, 4, and 6 weeks after implantation. Following decalcification of the implants’ surrounding tissue and preparation of 4 μm thick sections, they were stained with hematoxylin and eosin (H and E) and immunohistochemical (IHC) stain for osteopontin (OPN) marker. Histomorphometric evaluation was performed via measurement of the percentage of the woven, lamellar, and total generated bone. Light microscopy osteoblastic intensity of OPN in osteoblasts and bone matrix was also evaluated Data were analyzed by Wilcoxon signed Ranks, and Mc Nemar tests.
Results:
In both control and EMD-applied groups, bone formation was recognized around the implants at the 4th week postimplantation. The percentage of total generated bone in the test group was higher than the control group, although being not statistically significant (P value = 0.917). Osteoclasts exhibited significantly higher proliferation activity compared the control group when stimulated by EMD (P value = 0.027). On average, the staining intensity in osteoblasts and extracellular matrix of bone, in EMD-applied subjects was higher than those of the controls (P value = 0.167 and P value = 0.414, respectively).
Conclusion:
EMD enhanced bone formation around dental implants, but this increase was not significant.
Keywords: Bone formation, dental implant, enamel matrix protein, osteopontin
INTRODUCTION
The use of dental implants has become an accepted treatment in many clinical situations these days. It has been proven by a vast amount of evidence that implant therapy is a safe and efficient option. In spite of the brilliant success of dental implant therapy, yet, one of the causes of failures is the low bone quality and unpredictability of ossteointegration.[1,2]
Various materials and techniques for bone regeneration have been suggested in the field of tissue engineering. For instance, transforming growth factor-β1 (TGF-β1), bone morphogenetic proteins (BMPs), and platelets derived growth factor (PDGF) can be counted as biomaterials in cell biology techniques.[3]
Enamel matrix derivative (EMD) has been used in regenerative periodontal treatments. There are numerous studies and reports that indicate the increase of regeneration, differentiation, and migration of osteoblasts and other periodontal cells (cementoblasts and fibroblasts) following the use of EMD.
Accordingly, based on this potentiality of tissue regeneration, EMD has been used not only to regenerate bone and periodontal membrane around teeth but recently to treat gingival recession.[3–9] Amelogenin constitutes the major portion of EMD whereas enamelin and ameloblastin are other included proteins.[7]
Amelogenin possibly acts as a growth factor through stimulating, differentiating, and regenerating the periodontal ligament cells and increasing intercellular junction. This protein enhances the activity of ameloblasts, fibroblasts, and osteoblasts, and regulates gene expression of bone multicellular units (BMUs) collagen type 1, oseteocalcin, bone sialoprotein, alkalin phosphatase, and osteopontin.[4]
It has been shown by a vast series of experiments, in vivo and in vitro, that EMD and amelogenin stimulated periodontal tissue regeneration and synthesis of Tumor necrosis factor (TNF), TGF-β, insulin-like growth factor I and II (IGF I and II), Platelet derived growth factor (PDGF)-AB-BB, and Interleukin 6 (IL6) growth factors.[4,10] After insertion of EMD in periodontal lesions, this material creates a layer on the denuded dentin and causes new acellular cementum and alveolar bone formation.[6] Since 1997, animal histological studies have reported contrary results about the effect of EMD on osteogenic cells, bone regeneration, promotion of the integration of new bone to implant and the surrounding bone.[11–16] Therefore, our purpose was to study the effect of EMD on the quality and quantity of the surrounding bone of dental implants in the dogs’ tibia.
MATERIALS AND METHODS
In this animal experimental study, three healthy male 3-year-old Iranian mixed breed dogs with identical weights (25 kg) were selected. The dogs were quarantined for 2 weeks with identical feeding diets. All surgeries were performed in animal operating room of Torabinejad Research Center, Isfahan University of Medical Sciences, Isfahan, Iran. The experimental protocol of this study was approved by the Animal Ethics Committee of the Isfahan University of Medical Sciences.
Surgical procedure
The dogs were tranquilized with intra-muscular injection of 1% Cepromazine (Woerden, Holland, Neuroran: Alfasan) with dosage of 0.02 ml/kg, and were then anesthetized with 10% Ketamine (Woerden, Holland, Ketamine: Alfasan) with dosage of 0.04 mg/kg. The anesthesia went on using oxygen and Halothone BP, Nicholas primal India (Limited India) through endotracheal intubation, Dextrose saline was infused to maintain fluid and electrolytes balance and to keep the vein open for possible medication. Atropin 1% (alfasan, Woerden Holland) was also injected to regulate heart rhythm.
Under complete anesthesia, skin of medial proximal metaphysis of both tibias were shaved, washed with betadine 7.5% and draped by sterilized cover for surgery.
Primarily, a vertical incision was made to expose the bone. Then, two 10 mm deep osteotomies with a diameter of 3.8 mm were made 20 mm apart. Normal saline was used to prevent overheating and bone necrosis during all steps of osteotomy before placing the submerged/smile master (SM) implants from DIO system (Dsi: Dong seo Inc, Korea). In the left osteotomized sites, the cavities were filled and implants were covered with EMD (Emdogain, strauman Switzerland).
In the right tibia, the implants were inserted without EMD.
Finally, Periosteum, the muscles, fascia, and skin were closed carefully with continuous suturing. At the end of surgery, intra muscular sedatives and antibiotics were injected. The samples took Penicillin-Streptomycin 40,000 IU/kg (Vasr pharmaceutical Co. Fariman, Iran) for 1 week after surgery. The dogs received intensive care during the postsurgical period.
Two weeks after surgery, one of the dogs was randomly selected and sacrificed by vital perfusion with 10% formalin under complete anesthesia. Then, tibias were amputated from under tuberosity. Caput tibiae and metaphysis including the inserted implants were soaked in 10% buffered formalin. At the fourth and sixth week, two other dogs were sacrificed and processed as above.
Histological sample preparation
After preparation of bone blocks accompanied with implants (13 × 13 × 13 mm) and formalin fixed, samples were kept in separate coded dishes containing 10% ethylenediaminetetraacetic acid (EDTA) for 8 weeks. The decalcified and softened paraffin embedded blocks were precisely cut in the middle by scalpel and the implants were removed by close care with no tissue damage. Samples were processed and framed in paraffin. Four micro meter thick sections were prepared by microtome (Accu-cut SRM 200, Sakura Fihetek, Europe BN, Holland) and coded. The final slides were stained by Hematoxylin and eosin (H and E). The immunohistochemical staining (IHC) method was done for osteopontin (OPN) marking (NCLO-Pontin Novacastra, New Castle upon tyne, UK).
Histological and histomorphometric examinations
The prepared slides were sent to the oral and maxillofacial pathology laboratories at the school of dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Under light microscopy (Olympus CX21FS, Olympus Corporation, Tokyo, Japan) with magnification of 40× and 400×, the number of osteoclasts in the bone adjacent to the implant with a radius of 1 mm was counted. The slides were evaluated for type of bone.
After photomicrography (Nikon Corporation, Tokyo, Japan) the total percentage of bone around the implant, and the woven lamellar bone in mentioned radius were calculated by Iranian HMMA ver. 1 (invented in the department of oral and maxillofacial pathology, to evaluate histomorphometry). The pathologist and the technician were not aware of samples’ contents in all steps of the process.
Immunohistochemical examination
In the stained slides, the staining intensity of OPN in osteoblasts and bone matrix was assessed under light microscopy. No staining (-), mild staining (+), moderate staining (++), and hotness staining (+++) were recorded in sample-related forms.
Statistical analysis
In the present study, the difference between the studied variables of experimental and control groups were calculated at the end of the second, fourth, and sixth weeks postsurgery. Finally, the data were analyzed by Wilcoxon signed Ranks and MC Nemar tests and significance level was predetermined as P ≤ 0.05.
RESULTS
In histomorphometric evaluations, generally, the percentage of bone increased at the end of the fourth week in both groups. In fact, it was more obvious in EMD group. Mean percentage of woven bone increased from the second week to the fourth week and decreased through the sixth week [Figures 1 and 2]. Mean percentage of the lamellar new bone decreased to the fourth and then increased through the sixth week (Table 1 shows mean percentage of total bone P = 0.917, mean percentages of the woven bone, and the lamellar bone P = 0.6). Histological examinations of the prepared specimens showed that mean number of osteoclasts in both groups had increased from the second through the fourth weeks [Figure 2], but during the third two weeks, the mean value of this variable decreased in both experimental and control groups. The number of these cells in the experimental group was more than that of the control group at all three intervals [Table 2]. On an overall basis, the mean number of osteoclasts showed a significant difference in both groups (P = 0.027).
Figure 1.
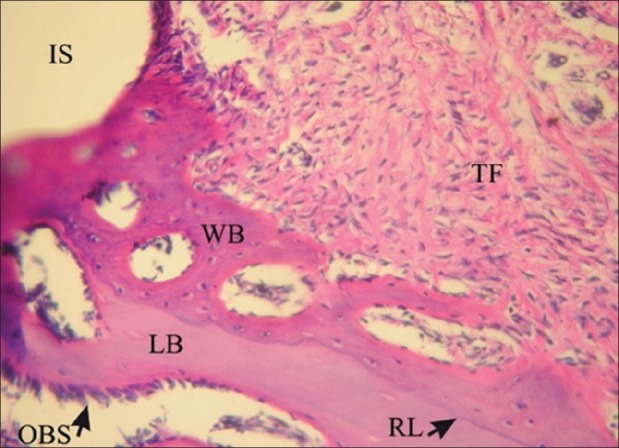
Histological image taken under a light microscope in EMD group in the fourth week. (H and E, magnification ×200). OBS: Osteoblastic rim, RL: Reversal line, FT: Fibrotic tissue. IS: Implant surface, WB: Woven bone, LB: Lamellar bone
Figure 2.
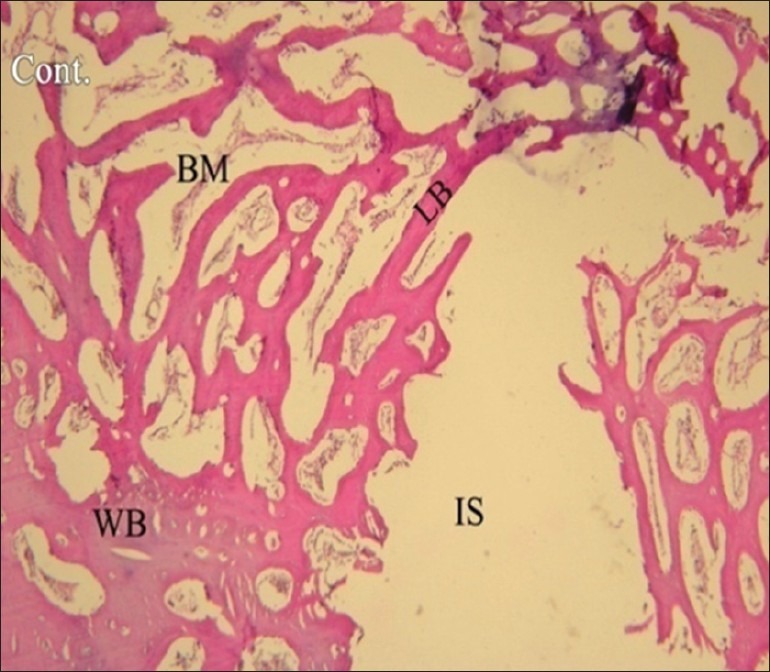
Histological image taken under a light microscope in control group in the fourth week. (H and E, magnification ×40). IS: Implant surface, WB: Woven bone, LB: Lamellar bone, BM: Bone marrow
Table 1.
Mean percentage and standard deviation (SD) of the woven, lamellar and total bone in control and EMD groups in different time intervals
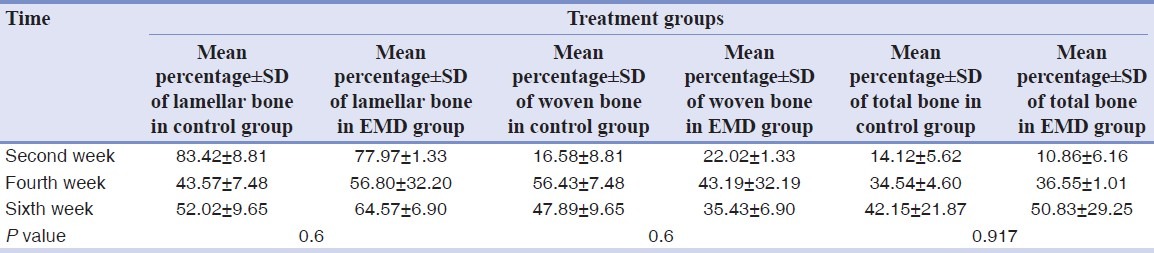
Table 2.
Mean±SD number of osteoclasts in control and EMD groups in different time intervals

Microscopic observations showed the existence of osteoblastic rim in all samples in both groups, except in one sample in control group at the end of the second week [Figure 3].
Figure 3.
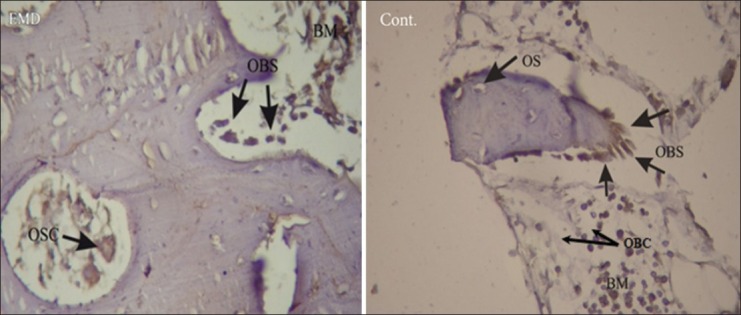
Immunohistochemical images taken under a light microscope in EMD and control groups in the sixth week (IHC, magnification ×400). OBS: Osteoblastic rim, BM: Bone marrow, OBC: Positive osteoblast cell, OSC: Osteoclast cell, OS: Osteoid
Immunohistochemical examination showed that staining intensity of osteoblasts in EMD group was high in two samples (at the end of the second and sixth weeks) [Figure 3], average in three samples (one sample in each interval), and poor in just one sample. In control group, all samples had poor staining intensity, except for one which was ranked high [Figures 1 and 2, Table 3]. The difference was not significant anyhow (P = 0.161).
Table 3.
Staining intensity of OPN in osteoblasts and extracellular matrix in control and EMD groups in different time intervals
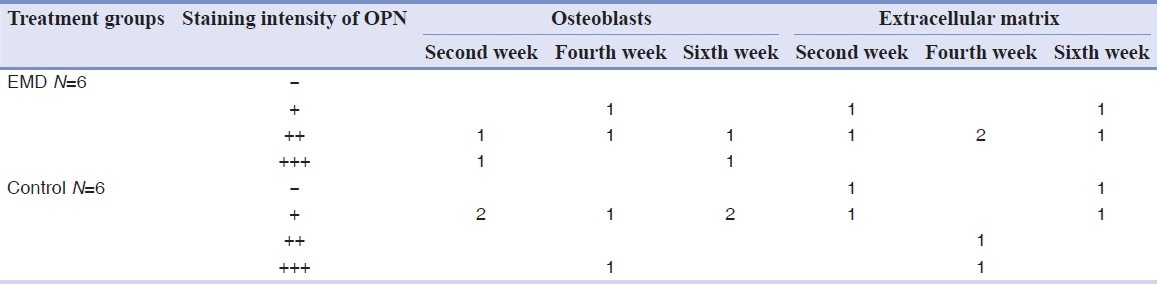
Staining intensity of osteopontin was poor in extracellular matrix for two samples of the experimental group, and its intensity was average in other specimens of this group. Staining failed in two samples and was poor in another sample in the control group while it was average in one sample by the fourth week and was high in another by the end of the fourth week (P = 0.414).
DISCUSSION
During tooth development, expression of enamel matrix protein genes takes place by ameloblasts and Hertwig's epithelial root sheet cells. These proteins play an important role in development of enamel crystals and as a mediator in formation of acellular cementum and attachment of periodontal ligament. Following complete formation of root(s), epithelial remnants of Malassez produce enamel matrix proteins, which are involved in healing and regeneration of periodontal tissues.[16–18]
In experimental animal studies, it is shown that local application of EMD in the osteotomized sites in femur of rat, and on the titanium implants increases the formation of bone trabeculae.[12,13] Yoneda et al, reported the enhancement of bone formation in osseous defects of rats’ skulls following the application of EMD.[19] There are also studies showing the inability of EMD to stimulate new bone formation around implants.[14,20]
An initial increase in bone formation around the implants placed in tibia of dogs was shown by the results of the present study. Histomorphometric evaluation of the samples showed greater percentages of total bone formation and lamellar bone enhancement around the implants in experimental group compared to control group. The reason of this increase might be found in the effect of EMD on the cells involved in healing and regeneration of the bone. Generally, the healing process of bone begins with differentiation of mesenchymal stem cells into osteoblasts and follows by formation, regeneration, and maturation of the bone.[13] Kawana et al.[12] and Shimizu et al.[13] attributed the increase in proliferation and differentiation rate of osteoblasts to the presence of EMD. Also in many in vitro studies the positive effect of EMD is reported in terms of proliferation, differentiation, and migration of osteoblasts and production of collagenase (matrixmetalloproteinase-1 (MMP-1)).[15,20–24] Yoneda et al.[20] reported that EMD increased the activity of alkaline phosphatase and promoted the formation of mineralized foci in KUSA/A1 cells, and also stimulated gene expression of collagen type I, osteopontin, osteocalcin, and TGF-β in these cells. It has been stated in other studies that EMD causes the increase of Receptor activator of nuclear factor kappa-B ligand (RANKL) in primitive osteoblasts. In addition, the activity of osteoclasts increases through RANK-RANKL interaction.[25–27]
In the present study, at all three intervals, osteoblastic rim was observed in experimental group, and the mean value of the number of osteoclasts was more than control group (P < 0.05) [Table 2].
Following new bone formation, woven bone is replaced by lamellar bone through remodeling. In this process, BMUs, which are formed by osteoclasts, blood vessels, and osteoblasts, are always involved. Osteoclasts resorb the woven bone and then lamellar bone is formed around blood vessels and secondary osteons by osteoblasts. Therefore, bone formation depends directly on the osteoclastic and osteoblastic activity.[28] In the present study, it was revealed that the increased number of osteoclasts in experimental group enhanced the bone maturation process and hence, increased the amount of lamellar bone formation in this group [Tables 1 and 2].
Immunohistochemical examination in this study showed that staining intensity of osteoblasts and intercellular matrix for osteopontin marker in experimental group was more than that of control group [Table 3]. Osteopontin is an acidic noncollagenous phosphoglycoprotein, which is found in different tissues. In the bone, this protein is produced in different phases of osteoblastic differentiation, well-differentiated osteoblasts, and osteocytes. During bone development, OPN supports the migration of osteoblasts to intercellular matrix and also stimulates osteoblastic activity during the process of bone resorbtion.[29,30] Yoneda et al.[20] reported that EMD stimulated the production of this protein in KUSA/A1 cells. It is stated in another study that EMD increases the expression of messenger-ribonucleic acid (mRNA) for osteopontin[31] and therefore increases the production of OPN and other noncollagenous proteins in bone matrix[12,13,20] Shimizu et al.[13] stated that EMD stimulated the proliferation, differentiation, and regulated the activity of osteoblastic mesenchymal cells.
Although no significant differences was observed between the two groups, based on our results it seems that EMD increased the formation of osteoblasts and production of bone matrix and subsequently enhanced bone formation in experimental group [Table 3].
Rate of increase in total bone volume was 8.5% and the quality of lamellar bone was increased with a rate of 15%. It has been reported that the increase in proliferation and activity of osteoblasts depends on the concentration of EMD.[15] Therefore, the low rate of bone increase in experimental group could be related to the low concentration or amount of applied EMD.
CONCLUSION
The findings of the present study showed that applying EMD in osteotomized sites and on titanium implant surfaces enhanced the quality and quantity of bone around implants in early steps of bone healing. Yet, this increase was not significant.
ACKNOWLEDGMENTS
We would like to express our sincere acknowledgement in the support and help of Department of Pathology, School of Dentistry, Shahid Beheshti University of Medical Sciences, Torabinejad Dental Research Center, and Dental Implants Research Center of Isfahan University of Medical Sciences, Isfahan, Iran.
Footnotes
Source of Support: This report is based on a research project which was submitted to the School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran. The study was approved by the Medical Ethics and Research Office at the Isfahan University of Medical Sciences and financially supported by this University
Conflict of Interest: The authors declare no conflicts of interest, real or perceived, financial or nonfinancial
REFERENCES
- 1.Schwartz-Arad D, Laviv A, Levin L. Failure causes, timing, and cluster behavior: An 8-year study of dental implants. Implant Dent. 2008;17:200–7. doi: 10.1097/ID.0b013e3181777906. [DOI] [PubMed] [Google Scholar]
- 2.Sakka S, Coulthard P. Implant failure: Etiology and complications. Med Oral Patol Oral Cir Bucal. 2011;16:42–4. doi: 10.4317/medoral.16.e42. [DOI] [PubMed] [Google Scholar]
- 3.Schilephake H. Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg. 2002;31:469–84. doi: 10.1054/ijom.2002.0244. [DOI] [PubMed] [Google Scholar]
- 4.Van der Pauw MT, Van den Bos T, Everts V, Beertsen W. Enamel matrix-derivedprotein stimulates attachment of periodontal ligament fibroblasts and enhances alkaline phosphatase activity and transforming growth factorbeta1 release of periodontal ligament and gingival fibroblasts. J Periodontol. 2000;71:31–43. doi: 10.1902/jop.2000.71.1.31. [DOI] [PubMed] [Google Scholar]
- 5.Heijl L. Periodontal regeneration with enamel matrix derivative in one human experimental defect. A case report. J Clin Periodontol. 1997;24:693–6. doi: 10.1034/j.1600-051x.1997.00693.x. [DOI] [PubMed] [Google Scholar]
- 6.Hammarström L. Enamel matrix, cementum development and regeneration. J Clin Periodontol. 1997;24:658–68. doi: 10.1111/j.1600-051x.1997.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 7.Simmer JP, Fincham AG. Molecular mechanisms of dental enamel formation. Crit Rev Oral Biol Med. 1995;6:84–108. doi: 10.1177/10454411950060020701. [DOI] [PubMed] [Google Scholar]
- 8.Brookes SJ, Robinson C, Kirkham J, Bonass WA. Biochemistry and molecular biology of amelogenin proteins of developing dental enamel. Arch Oral Biol. 1995;40:1–14. doi: 10.1016/0003-9969(94)00135-x. [DOI] [PubMed] [Google Scholar]
- 9.Simmer JP, Fukae M, Tanabe T, Yamakoshi Y, Uchida T, Xue J, et al. Purification, characterization, and cloning of enamel matrix serine proteinase 1. J Dent Res. 1998;77:377–86. doi: 10.1177/00220345980770020601. [DOI] [PubMed] [Google Scholar]
- 10.Lyngstadaas SP, Lundberg E, Ekdahl H, Andersson C, Gestrelius S. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J Clin Periodontol. 2001;28:181–8. doi: 10.1034/j.1600-051x.2001.028002181.x. [DOI] [PubMed] [Google Scholar]
- 11.Heijl L, Heden G, Svärdström G, Ostgren A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. J Clin Periodontol. 1997;24:705–14. doi: 10.1111/j.1600-051x.1997.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 12.Kawana F, Sawae Y, Sahara T, Tanaka S, Debari K, Shimizu M, et al. Porcine enamel matrix derivative enhances trabecular bone regeneration during wound healing of injured rat femur. Anat Rec. 2001;264:438–46. doi: 10.1002/ar.10016. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu-Ishiura M, Tanaka S, Lee WS, Debari K, Sasaki T. Effects of enamel matrix derivative to titanium implantation in rat femurs. J Biomed Mater Res. 2002;60:269–76. doi: 10.1002/jbm.10064. [DOI] [PubMed] [Google Scholar]
- 14.Franke Stenport V, Johansson CB. Enamel matrix derivative and titanium implants. J Clin Periodontol. 2003;30:359–63. doi: 10.1034/j.1600-051x.2003.00326.x. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz F, Rothamel D, Herten M, Sculean A, Scherbaum W, Becker J. Effect of enamel matrix protein derivative on the attachment, proliferation, and viability of human SaOs(2) osteoblasts on titanium implants. Clin Oral Investig. 2004;8:165–71. doi: 10.1007/s00784-004-0259-2. [DOI] [PubMed] [Google Scholar]
- 16.van den Dolder J, Vloon AP, Jansen JA. The effect of Emdogain on the growth and differentiation of rat bone marrow cells. J Periodontal Res. 2006;41:471–6. doi: 10.1111/j.1600-0765.2006.00894.x. [DOI] [PubMed] [Google Scholar]
- 17.Hammarström L. The role of enamel matrix proteins in the development of cementum and periodontal tissues. Ciba Found Symp. 1997;205:246–55. doi: 10.1002/9780470515303.ch17. [DOI] [PubMed] [Google Scholar]
- 18.Robinson C, Brookes SJ, Shore RC, Kirkham J. The developing enamel matrix: Nature and function. Eur J Oral Sci. 1998;106(Suppl 1):282–91. doi: 10.1111/j.1600-0722.1998.tb02188.x. [DOI] [PubMed] [Google Scholar]
- 19.Yoneda S, Itoh D, Kuroda S, Kondo H, Umezawa A, Ohya K, et al. The effects of enamel matrix derivative (EMD) on osteoblastic cells in culture and bone regeneration in a rat skull defect. J Periodontal Res. 2003;38:333–42. doi: 10.1034/j.1600-0765.2003.00667.x. [DOI] [PubMed] [Google Scholar]
- 20.Casati MZ, Sallum EA, Nociti FH, Jr, Caffesse RG, Sallum AW. Enamel matrix derivative and bone healing after guided bone regeneration in dehiscence-type defects around implants. A histomorphometric study in dogs. J Periodontol. 2002;73:789–96. doi: 10.1902/jop.2002.73.7.789. [DOI] [PubMed] [Google Scholar]
- 21.He J, Jiang J, Safavi KE, Spångberg LS, Zhu Q. Emdogain promotes osteoblast proliferation and differentiation and stimulates osteoprotegerin expression. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:239–45. doi: 10.1016/j.tripleo.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz Z, Carnes DL, Jr, Pulliam R, Lohmann CH, Sylvia VL, Liu Y, et al. Porcine fetal enamel matrix derivative stimulates proliferation but not differentiation of pre-osteoblastic 2T9 cells, inhibits proliferation and stimulates differentiation of osteoblast-like MG63 cells, and increases proliferation and differentiation of normal human osteoblast NHOst cells. J Periodontol. 2000;71:1287–96. doi: 10.1902/jop.2000.71.8.1287. [DOI] [PubMed] [Google Scholar]
- 23.Ohyama M, Suzuki N, Yamaguchi Y, Maeno M, Otsuka K, Ito K. Effect of enamel matrix derivative on the differentiation of C2C12 cells. J Periodontol. 2002;73:543–50. doi: 10.1902/jop.2002.73.5.543. [DOI] [PubMed] [Google Scholar]
- 24.Goda S, Inoue H, Kaneshita Y, Nagano Y, Ikeo T, Iida J, et al. Emdogain stimulates matrix degradation by osteoblasts. J Dent Res. 2008;87:782–7. doi: 10.1177/154405910808700805. [DOI] [PubMed] [Google Scholar]
- 25.Otsuka T, Kasai H, Yamaguchi K, Nishihara T. Enamel matrix derivative promotes osteoclast cell formation by RANKL production in mouse marrow cultures. J Dent. 2005;33:749–55. doi: 10.1016/j.jdent.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Itoh N, Kasai H, Ariyoshi W, Harada E, Yokota M, Nishihara T. Mechanisms involved in the enhancement of osteoclast formation by enamel matrix derivative. J Periodontal Res. 2006;41:273–9. doi: 10.1111/j.1600-0765.2005.00868.x. [DOI] [PubMed] [Google Scholar]
- 27.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 28.Buenzli PR, Pivonka P, Smith DW. Spatio-temporal structure of cell distribution in cortical bone multicellular units: A mathematical model. Bone. 2011;48:918–26. doi: 10.1016/j.bone.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 30.Daniel SP, Elizabeth CB, James A, Robert AS, Donna CM, Thomas MB, et al. mmunohistochemical Study of Osteopontin Expression During Distraction Osteogenesis in the Rat. J Histochem Cytochem. 2002;50:567–74. doi: 10.1177/002215540205000414. [DOI] [PubMed] [Google Scholar]
- 31.Tokiyasu Y, Takata T, Saaygin E, Somerman M. Enamel factors regulate expression of genes assoiated with cementoblasts. J Periodontol. 2000;71:1829–39. doi: 10.1902/jop.2000.71.12.1829. [DOI] [PubMed] [Google Scholar]


