Abstract
Objectives:
Anecdotal evidence suggests that second-generation antipsychotic agents are increasingly used to treat sleep problems. This study sought to quantify the proportion of new prescriptions for second-generation antipsychotic agents started for sleep/sedation and the correlates of such use.
Design:
A cross-sectional survey of provider decision making at the time second-generation antipsychotic agents were prescribed, documenting the reasons for the medication, patient demographics, psychiatric and medical diagnoses, patient health characteristics, and provider background.
Setting:
A single Veterans Affairs Medical Center over a 20-month period.
Participants:
Prescribers of second-generation antipsychotic agents.
Interventions:
N/A.
Results:
Seven hundred seven (32.2%) of 2,613 surveys indicated sleep/sedation was at least one reason for using a second-generation anti-psychotic agent, whereas for 266 (12.1%) it was the only reason. Quetiapine was most frequently prescribed overall as well as for sleep/sedation (47.0% and 73.6% respectively). Second-generation antipsychotic agent use for sleep/sedation was unrelated to sociodemographic characteristics, least likely in patients with schizophrenia or bipolar disorder, and most likely as a newly started second-generation antipsychotic agent.
Conclusion:
Sleep/sedation is a common reason given for new prescriptions of second-generation antipsychotic agents. Quetiapine is most frequently used for this purpose. A greater understanding of why providers use second-generation antipsychotic agents rather than safer and less costly alternatives for sleep problems may advance the development of interventions to reduce adverse effects.
Citation:
Hermes EDA; Sernyak M; Rosenheck R. Use of second-generation antipsychotic agents for sleep and sedation: a provider survey. SLEEP 2013;36(4):597-600.
Keywords: Atypical antipsychotics, decision-making, hypnotic, off-label, pharmacoepidemiology
INTRODUCTION
More than one-third of the US population is affected by insomnia, and in 10% the disorder is chronic.1 Insomnia also plays a prominent role in the psychopathology of serious mental illnesses such as schizophrenia, mood disorders, and posttraumatic stress disorder (PTSD). Although more than 10 medications have approval from the US Food and Drug Administration (FDA) for the treatment of insomnia, many others are commonly used off-label for sleep problems. A 2002 study found that trazodone was the most commonly prescribed medication to promote sleep, accounting for 2.7 million prescription occurrences.2 Among medications used for sleep, those with the greatest recent growth in use and risk of adverse effects are second-generation antipsychotic agents. In 2002, the second-generation antipsychotic agent quetiapine was the most common antipsychotic prescribed to promote sleep, accounting for 500,000 occurences.2
Research on the pharmacoepidemiology of second-generation antipsychotic agents using encounter codes has found that only 5-9% of patients prescribed these drugs are given a diagnosis of insomnia, likely an underestimation of the true prevalence of use for this purpose.3,4 To address this deficiency, this study used data from surveys of prescribers on their reasons for using second-generation antipsychotic agents to identify the proportion of new second-generation antipsychotic agent starts that were for sleep or sedation. It further sought to identify correlates of second-generation antipsychotic agent use for sleep/ sedation that might expand the understanding of this practice.
METHODS
Data were generated as part of a minimally restrictive intervention consisting of academic detailing and a required provider decision-making survey used in an effort to reduce the rate of new prescriptions for on-patent second-generation antipsychotic agents at a single Veterans Health Administration (VHA) medical center. A recent study found that this intervention did not reduce the number of second-generation antipsychotic agent prescriptions over the study period.5 The survey involved all healthcare workers who were prescribers of second-generation antipsychotic agents (psychiatric physicians, psychiatric advance practice registered nurses [APRNs], psychiatric physician assistants [PAs], and other clinicians with permission from the pharmacy) and was completed at the time any second-generation antipsychotic agent on formulary (olanzapine, long-acting injectable risperidone, oral risperidone, aripiprazole, ziprazidone, or quetiapine) was ordered as a new, non-refill prescription for an outpatient between October 2007 and May 2009. Other sedative-hypnotic medications on formulary included most generic benzodiazepines, generic tricyclic antidepressants, generic first-generation antipsychotic agents, generic antihistamines, zolpidem, mirtazapine, and trazodone. Newer, on-patent sedatives-hypnotic agents could be ordered after pharmacy review. The survey was electronically delivered as part of the medication ordering system at the time any new prescription for a second-generation antipsychotic agent was placed, and completion was required before the prescription could be electronically sent to the pharmacy. Therefore, 100% of providers writing prescriptions for second-generation antipsychotic agents were assumed to have been surveyed regarding 100% of patients receiving second-generation antipsychotic agents over this period. This study was approved by the Institutional Review Boards of the VA Connecticut Healthcare System and Yale University.
The survey consisted of 20 questions that documented patient demographics, the proposed medication, the primary psychiatric diagnosis under treatment, important comorbid psychiatric and medical diagnoses, and the professional background of the provider as well as other patient health characteristics such as the patient's current Global Assessment of Functioning (GAF) and body mass index. A list of forced choice selections and write-in answers were given for each question. Providers made multiple selections from 10 reasons for using the second-generation antipsychotic agent: intolerance to current drug, efficacy, less extrapyramidal symptoms, decreased risk for tardive dyskinesia (TD), less akathisia, less sedation, increased sleep/sedation, treatment of TD, patient preference, or other.
The sample draws from a total of 2,643 completed surveys. Surveys in which information on the prescribed second-generation antipsychotic agent was missing (n = 30) and no response was given to the following question: reason for proposed medication (Check all that apply), were excluded (n = 415). Chi-square tests and analysis of variance compared three types of prescriptions based on the clinician's reason for using the second-generation antipsychotic agent: exclusively for sleep/ sedation, for sleep/sedation as one of several reasons, and for reasons other than sleep/sedation. In a secondary analysis, the use of quetiapine compared with other second-generation antipsychotic agents was also analyzed for only those surveys where sleep or sedation was given as a reason.
RESULTS
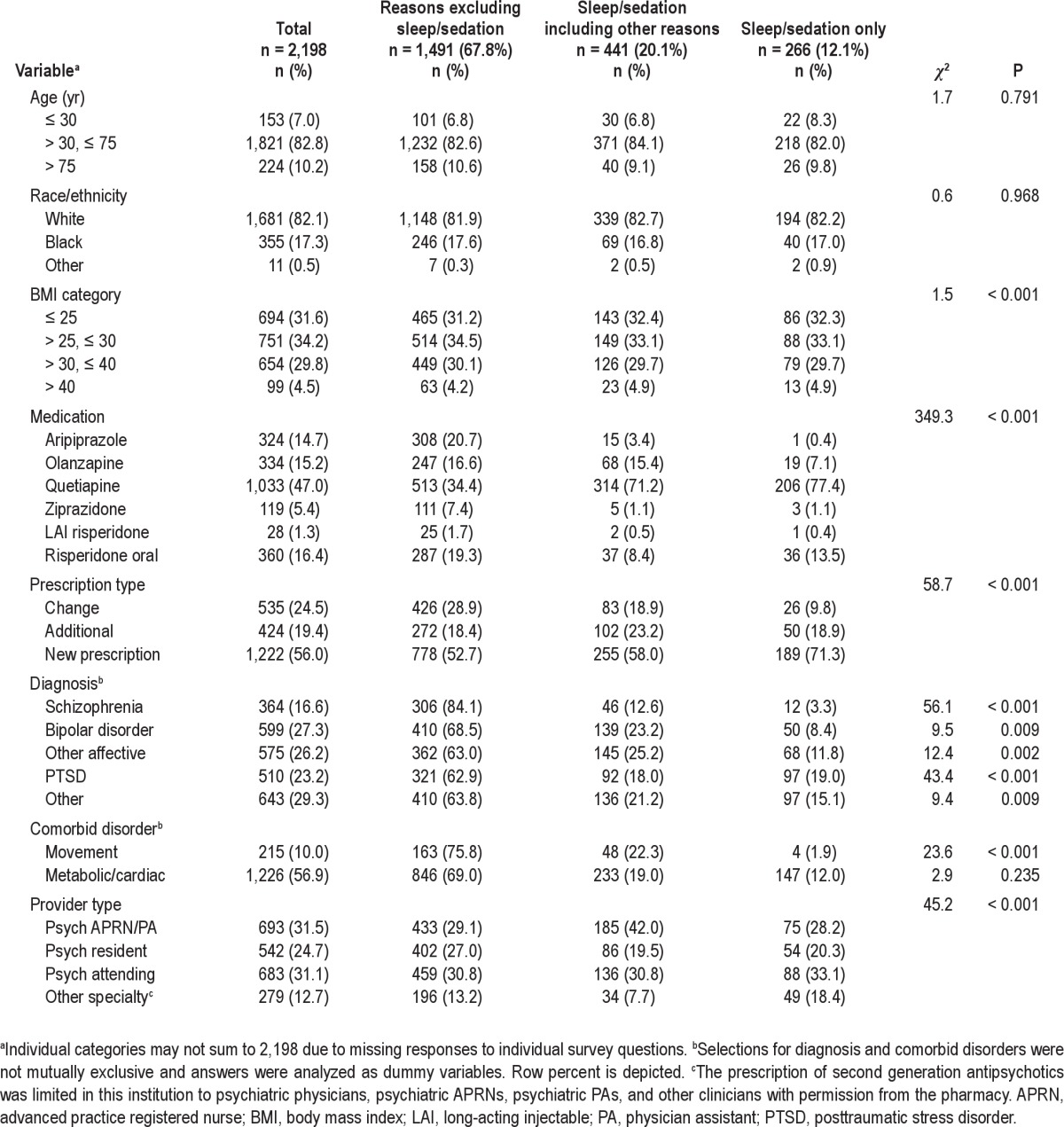
Of the 2,198 surveys included in the analysis, sleep/sedation was given as one reason among others in 441 surveys (20.1%), and the sole reason in 266 surveys (12.1%) (Table 1). Sleep/ sedation was the third most common reason selected, after efficacy (51.0%) and patient preference (32.4%). There was a statistically significant association between the second-generation antipsychotic agent prescribed and sleep/sedation categories (χ2 = 349.3, P < 0.001). Quetiapine was the most frequently used second-generation antipsychotic agent both in the entire sample and for sleep/sedation, in 71.2% of surveys when sleep/sedation was among other reasons, and 77.4% of surveys in which it was the sole reason. Most prescriptions for sleep/sedation (63%) represented a new second-generation antipsychotic agent prescription rather than a change in or additional prescription (χ2 = 58.7, P < 0.001). The use of a second-generation antipsychotic agent for sleep/sedation was least frequent in schizophrenia (15.9%), whereas it was most frequent as the sole reason in PTSD (19.0%). There was also a statistically significant association between the provider's professional background and sleep categories (χ2 = 45.2, P < 0.001). APRNs and PAs were more frequent prescribers of second-generation antipsychotic agents for sleep/sedation in combination with other reasons, whereas physicians from other specialties were more frequent prescribers solely for sleep/sedation. Patients for whom providers selected sleep/sedation as a reason had a significant but only slightly higher mean GAF, 46.2 compared with 44.3 (F = 4.9, P = 0.008). When occurrences where second-generation anti-psychotic agents were used for sleep/sedation were specifically analyzed, an increased proportion of individuals with affective disorders other than bipolar disorder and a decreased proportion of those with schizophrenia and PTSD were treated with quetiapine compared with other second-generation antipsychotic agents (χ2 = 9.13, P = 0.003; χ2 = 4.44, P = 0.035; χ2 = 10.74, P = 0.001; respectively), whereas other factors listed in Table 1 did not display a significant association in this subanalysis.
Table 1.
Bivariate analysis by usage for sleep/sedation of responses to a provider survey completed at the time second-generation antipsychotics were prescribed
DISCUSSION
This analysis of provider survey data found that sleep/sedation was a factor in the decision to use a particular second-generation antipsychotic agent in almost one-third of prescriptions, whereas in 12% it was the only reason. Results indicate that prescriptions for second-generation antipsychotic agents for sleep/sedation were most commonly for quetiapine and were new to the patient, probably at low dose.
Previous studies of antipsychotic use for these reasons have evaluated diagnostic codes for insomnia and other related diagnoses, which are employed infrequently. Consequently, the estimation of second-generation antipsychotic agent use for this purpose may not be accurate. Our findings are substantially higher than the 5.1% noted by Linden and Thiels.3 In addition, our finding that 50.3% of all new quetiapine prescriptions were given for sleep is much higher than that found in a study of inpatients for whom 9% of quetiapine prescriptions were for insomnia.4 Unfortunately, the only other similar survey study queried patients and not their providers about why antipsychotic agents were prescribed. In this study, 14.5% of prescriptions for antipsychotic agents were written as hypnotics.6 Findings from the current study emulate those from a study of Oregon Medicaid patients, where the most frequent second-generation antipsychotic agent prescribed was quetiapine, accounting for 40% of prescriptions, 86% of which were for doses considered subtherapeutic for psychoses and thus presumed to be for sleep/ sedation.7 However, some of these prescriptions may have been for refractory major affective disorder.
The current findings are especially important because second-generation antipsychotic agents have potentially serious side effects. Although widely believed to have fewer neurologic side effects than older antipsychotic agents, some of these medications, especially quetiapine, can cause important medium-and long-term problems such as weight gain, disturbances in glucose homeostasis, and hyperlipidemia.8 In one evaluation, quetiapine at a low dose was associated with a 1 lb per mo weight gain.9 Second-generation antipsychotic agents have also been shown to increase the risk of death in patients with dementia.10 There are also several reports suggesting some abuse potential for quetiapine. Additional drawbacks of second-generation antipsychotic agents include expense, as most are still on patent and can cost up to $10 a day.
Empirical studies of quetiapine as a sedative-hypnotic agent in either general or psychiatric populations with sleep disorders have been limited, and there have been no comparative effectiveness trials of second-generation antipsychotic agents with other sleep medications. There are a plethora of other medications that have an FDA indication for sleep or a more proven track record. Providers should consider the risk-benefit profile of second-generation antipsychotic agents, especially quetiapine, prior to off-label use for this indication.
This analysis has several limitations that deserve mention. The original study's objectives did not involve the analysis of decision making with regard to sleep treatments. Providers completed surveys only related to prescriptions for second-generation antipsychotic agents and not other sedative hypnotic agents. It is unclear how responses were affected by the institution's generic-only sedative-hypnotic agent formulary policy and to what extent providers were limited by the survey's forced choice format. In addition, neither the prescription duration nor dose was recorded, which may be important in determining side-effect burden. The survey neither contained a diagnostic category nor a reason addressing substance abuse, which is unfortunate because quetiapine has been investigated as a potential treatment for alcohol abuse.11 Given the high co-occurrence of sleep, alcohol use, and other psychiatric disorders, it may be that providers are using quetiapine to target multiple problems related to addiction. Also, it must be understood that this study uses data from a single institution within the VHA system and represents the use of only one medication type. Therefore, the generalizability of these findings may be limited.
Why providers choose second-generation antipsychotic agents over other medications with FDA-approved indications for sleep or less serious side effects remains unclear. Providers may discount the relatively long-term side effects of drugs such as quetiapine, and be more concerned about the relatively short-term abuse potential of hypnotic agents such as benzodiazepines. Substantial legal settlements suggest that off-label marketing of some second-generation antipsychotic agents for sleep problems may have also promoted their use for this purpose.
Sleep and sedation emerge in this study as a common reason for prescribing second-generation antipsychotic agents, most often quetiapine, where there are no FDA-approved indications, sparse comparative effectiveness data, and important long-term side effects. Results justify academic detailing interventions to reduce the use of second-generation antipsychotic agents for sleep, emphasizing side-effect risks and potentially safer alternatives.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Sernyak has received honoraria from Pfizer. Dr. Rosenheck has received research support from Janssen Pharmaceutical Products, Wyeth Pharmaceuticals, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, and Eli Lilly and Co. He has also received consulting fees from Bristol-Myers Squibb, Eli Lilly and Co., Roche Pharmaceuticals and Janssen Pharmaceutical Products. He also testified as an expert witness on behalf of Janssen Pharmaceutical Products. Dr. Hermes has indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Elina Stefanovics, PhD, VA Connecticut Healthcare System, for her help in data management and analysis.
REFERENCES
- 1.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. Sleep. 1999;22:S347–53. [PubMed] [Google Scholar]
- 2.Walsh JK. Drugs used to treat insomnia in 2002: regulatory-based rather than evidence-based medicine. Sleep. 2004;27:1441. doi: 10.1093/sleep/27.8.1441. [DOI] [PubMed] [Google Scholar]
- 3.Linden M, Thiels C. Epidemiology of prescriptions for neuroleptic drugs: tranquilizers rather than antipsychotics. Pharmacopsychiatry. 2001;34:150–4. doi: 10.1055/s-2001-15880. [DOI] [PubMed] [Google Scholar]
- 4.Philip NS, Mello K, Carpenter LL, Tyrka AR, Price LH. Patterns of quetiapine use in psychiatric inpatients: An examination of off-label use. Ann Clin Psychiatry. 2008;20:15–20. doi: 10.1080/10401230701866870. [DOI] [PubMed] [Google Scholar]
- 5.Hermes EDA, Sernyak M, Rosenheck R. Impact of a program encouraging the use of generic antipsychotics. Am J Manag Care. 2012;18:e307–314. [PubMed] [Google Scholar]
- 6.Weiss E, Hummer M, Koller D, Ulmer H, Fleischhacker WW. Off-label use of antipsychotic drugs. J Clin Psychopharmacol. 2000;20:695–8. doi: 10.1097/00004714-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Hartung DM, Wisdom JP, Pollack DA, et al. Patterns of atypical anti-psychotic subtherapeutic dosing among Oregon Medicaid patients. J Clin Psychiatry. 2008;69:1540–7. doi: 10.4088/jcp.v69n1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–96. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- 9.Williams SG, Alinejad NA, Williams JA, Cruess DF. Statistically signifi-cant increase in weight caused by low-dose quetiapine. Pharmacotherapy. 2010;30:1011–5. doi: 10.1592/phco.30.10.1011. [DOI] [PubMed] [Google Scholar]
- 10.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia. JAMA. 2005;294:1934–43. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 11.Guardia J, Roncero C, Galan J, Gonzalvo B, Burguete T, Casas M. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36:265–9. doi: 10.1016/j.addbeh.2010.11.006. [DOI] [PubMed] [Google Scholar]


