Abstract
Study Objectives:
To investigate sleep quality in adolescents with juvenile primary fibromyalgia syndrome (JPFS) and determine whether sleep abnormalities, including alpha-delta sleep (ADS), correlate with pain intensity. We hypothesized that successful treatment for pain with exercise therapy would reduce ADS and improve sleep quality.
Design:
Single-center preintervention and postintervention (mean = 5.7 ± 1.0 weeks; range = 4.0-7.3 weeks) observational study.
Patients:
Ten female adolescents (mean age = 16.2 ± 0.65 SD yr) who met criteria for JPFS and completed treatment.
Interventions:
Multidisciplinary pain treatment, including intensive exercise therapy.
Measurements and Results:
Pain and disability were measured by a pain visual analog scale (VAS) and the Functional Disability Inventory. Subjective sleep measures included a sleep VAS, an energy VAS, and the School Sleep Habits Survey. Objective sleep measures included actigraphy, polysomnography (PSG), and the Multiple Sleep Latency Test. Baseline PSG was compared with that of healthy age- and sex-matched control patients. At baseline, patients had poorer sleep efficiency, more arousals/awakenings, and more ADS (70.3% of total slow wave sleep [SWS] versus 21.9% SWS, P = 0.002) than controls. ADS was unrelated to pain, disability, or subjective sleep difficulty. After treatment, pain decreased (P = 0.000) and subjective sleep quality improved (P = 0.008). Objective sleep quality, including the amount of ADS, did not change.
Conclusions:
Although perceived sleep quality improved in adolescents with JPFS after treatment, objective measures did not. Our findings do not suggest exercise therapy for pain improves sleep by reducing ADS, nor do they support causal relationships between ADS and chronic pain or subjective sleep quality.
Citation:
Olsen MN; Sherry DD; Boyne K; McCue R; Gallagher PR; Brooks LJ. Relationship between sleep and pain in adolescents with juvenile primary fibromyalgia syndrome. SLEEP 2013;36(4):509-516.
Keywords: Adolescents, alpha-delta sleep, juvenile primary fibromyalgia syndrome, pain, polysomnography, sleep
INTRODUCTION
Chronic pain is associated with significant morbidity in children and adolescents, including functional disability1,2 and symptoms of depression and anxiety.3,4 One of the most disabling co-morbidities is poor sleep quality, endorsed by more than 50% of children and adolescents with chronic pain5 and nearly all children with juvenile primary fibromyalgia syndrome (JPFS).6 Subjective sleep problems include difficulty falling asleep, frequent awakenings, waking unrefreshed, and daytime somnolence.7,8 These subjective complaints are supported by objective sleep measures, including polysomnography (PSG) and actigraphy, which demonstrate shorter sleep duration, poorer sleep efficiency, more arousals/awakenings and limb movements, and longer sleep onset latency among children and adolescents with chronic pain compared with healthy controls.9–13
Patients with fibromyalgia also exhibit electroencephalographic (EEG) abnormalities, particularly alpha intrusions into slow wave sleep (SWS).14–16 Alpha-delta sleep (ADS) has been associated with pain, decreased energy, mood disturbances, and unrefreshing sleep in adults with fibromyalgia,14,17,18 although it is not specific to the disorder.19 The direction of causality between ADS and pain remains unclear. Some suggest that chronic pain leads to poor sleep quality,20,21 whereas others propose that improving sleep quality will decrease pain.16,17 The relationship between chronic pain and disrupted sleep is widely considered to be bidirectional.22 However, the strength of association between ADS and unrefreshing sleep is uncertain.23
The relationship of ADS to pain and subjective sleep quality has been less well studied in children. Children with juvenile rheumatoid arthritis24 and JPFS11 have demonstrated significantly more ADS and subjective sleep difficulties than healthy controls. Mothers with fibromyalgia have more ADS, nonrestorative sleep, and morning fatigue than children with JPFS,11 suggesting these symptoms may worsen over time. Elucidation of relationships among pain, ADS, and subjective sleep quality in JPFS might lead to earlier and more effective interventions.
Exercise therapy is an established treatment for fibromyalgia, with efficacy studies in adults documenting significant improvements in physical fitness, pain threshold and intensity, and sleep.25–28 However, whether and how exercise therapy affects sleep architecture has not been well studied. Exercise and physical fitness have been associated with greater SWS in both children and adults29–32 and it has been suggested that exercise therapy may improve sleep in fibromyalgia specifically by reducing ADS.16,33 To test this hypothesis, we investigated whether successful treatment of pain via exercise therapy was associated with improved subjective and objective sleep quality, including ADS, in adolescents with JPFS. We had three main hypotheses: (1) at baseline, adolescents with JPFS will have poor objective sleep quality compared with healthy controls, including more ADS; (2) ADS will be associated with greater pain, disability, and subjective sleep difficulties; and (3) after treatment, pain intensity, subjective sleep quality, and ADS will improve.
METHODS
Participants
Patients between the ages of 13 and 17 years were recruited from a multidisciplinary pain treatment program that includes intensive exercise therapy. Patients were eligible for participation if they met diagnostic criteria for JPFS34 and reported pain intensity ≥ 50 mm on a 100-mm pain visual analog scale (VAS). Patients were excluded if they could not complete the exercises, had a previous sleep study, had used pain or sleep medications within one week of the study, or had co-morbid illnesses that could affect sleep or pain. Ten girls of mean age 16.2 ± 0.65 SD years (range = 15.1-17.4 years) completed the study. Ageand sex-matched controls were selected from healthy asymptomatic controls who had participated in previous studies of adolescent sleep.
This study was approved by the Institutional Review Board for human subjects research. All participants and legal guardians provided appropriate informed consent/assent.
Study Design
This was a single-center, preintervention, and postintervention observational study. Self-report measures were used to assess pain intensity, functional disability, and subjective sleep quality. Actigraphy, PSG, and the Multiple Sleep Latency Test were used to evaluate objective sleep quality. Each subject was compared to herself before and after treatment (Figure 1). Baseline PSG data were also compared to age- and sex-matched controls.
Figure 1.

Timeline for data collection pretreatment and post-treatment (mean ± standard deviation)
Intervention
Treatment was similar to that previously described35 and included six hours of one-to-one physical and occupational therapy daily, emphasizing intense aerobic training and desensitization. Treatment can be outpatient or residential, depending on the family's travel needs and insurance dictates, but the intervention is identical for both groups. Patients saw a psychologist for cognitive behavioral therapy, received sleep hygiene counseling, and participated in art and music therapy. Treatment duration depends on individual needs, but typically lasts four weeks. Similar programs have been shown to improve pain and functioning in children with chronic pain.35–37 Our own preliminary outcomes data for patients with total body pain or JPFS show significant improvement: pain was resolved in 58%, and 75% report no or minimal disability within one year of treatment.
Subjective Measures
Pain VAS
A 100-mm VAS was used to assess the intensity of patients' usual pain in the previous two weeks. The Pain VAS38 has established reliability and validity.39,40 Healthy adolescents report pain scores < 3 on the VAS,9 significantly less than adolescents with chronic pain.9,10,41
Functional Disability Inventory
The Functional Disability Inventory (FDI)2 measures the effect of pain on daily activities. FDI scores range from 0-60, with higher scores indicating greater impairment. Healthy children generally score ≤ 5,41 whereas average scores for pediatric pain patients range from 17.2 to 26.3.2,42,43 In patients with recurrent abdominal pain, coefficient alpha was 0.89 and 3-month test-retest reliability was 0.60.2
Sleep and Energy Visual Analog Scales
Patients used a 100-mm Sleep VAS and a 100-mm Energy VAS to rate the severity of subjective sleep and energy difficul-ties, respectively, in the previous two weeks.
School Sleep Habits Survey
The School Sleep Habits Survey is a reliable and validated scale that assesses sleep/wake patterns and daytime functioning.44–46 It includes four scales: a sleepiness scale measuring daytime somnolence; a sleep/wake behavior problems scale assessing the frequency of erratic sleep behaviors (i.e., trouble falling asleep); a depressive mood scale47 measuring the frequency of depressive symptoms; and a morningness/eveningness questionnaire48 assessing circadian preference.
Objective Measures
Actigraphy
Actigraphy captures sleep patterns over longer periods of time and is well validated in measuring treatment outcomes.49–51 Subjects wore the Actiwatch 16/64 (Respironics, Inc., Bend, OR) on their nondominant wrist for 7-14 days before and during each overnight PSG, removing it only when bathing. This Actiwatch has been validated against PSG.52 Using a 10-min immobility time and low wake threshold as previously described,52 activity counts from each subject for each night at home were analyzed and compared. Patients also kept sleep diaries, which have been recommended for use with actigraphy to improve accuracy in total sleep time (TST) and sleep efficiency,53 for one week before and after treatment. Actigraphy variables included TST, sleep efficiency, sleep latency, and wake after sleep onset.
Polysomnography
Subjects reported to the sleep laboratory at approximately 7 PM Lights out occurred at the subject's usual week-day bedtime. PSG recordings began between 9 PM and 11 PM and were completed by 7 AM The following parameters were recorded (Rembrandt, Embla, Broomfield, CO): EEG (C3/A2, C4/A1, O1/A2, O2/A1), electrooculogram (left and right), submental electromyogram (EMG), tibial EMG, modified lead two electrocardiogram, chest and abdominal wall motion by respiratory inductance plethysmography (SensorMedics, Yorba Linda, CA), airflow by nasal pressure (Pro-Tech Services, Inc, Mukilteo, WA) and three-pronged thermistor (Pro-Tech Services, Inc, Mukilteo, WA); end-tidal partial pressure of carbon dioxide by capnography (Novametrix 7000; Novametrix, Wallingford, CT), arterial oxygen saturation (Novametrix 7000 or Masimo, Irvine, CA), oximeter pulse waveform, and digital video. Data were collected in 30-sec epochs. PSG variables included TST, sleep efficiency, sleep latency, arousal/awakening index, sleep staging, and ADS.
Each study was deidentified and scored, according to American Academy of Sleep Medicine (AASM) guidelines,54 by a board-certified sleep physician (LJB) in a blinded fashion. Time in each sleep stage was expressed as a percentage of TST. ADS was expressed both in minutes and as a percentage of total SWS (Stage N3). The same observer scored each study to eliminate inter-observer variability. Intraobserver reliability was assessed by assigning each patient and control a new identification number and rescoring all studies in a blinded fashion. Original and rescored studies were compared by calculating the Cronbach alpha coefficient of reliability and intraclass correlation.
Multiple Sleep Latency Test
The Multiple Sleep Latency Test55 evaluates daytime somnolence by providing opportunities for sleep at 2-h intervals throughout the day and measuring the time to sleep onset. Sleep onset in less than 5 minutes reflects pathological sleepiness, whereas 10-20 minutes is normal.55
Statistical Analysis
Primary outcomes (pain, disability, and sleep quality) were analyzed by paired t-test. The mean change in each variable pretreatment and posttreatment and its standard deviation were computed. One-sample Kolmogorov-Smirnov tests of normal distribution found no evidence that our variables were not normally distributed; therefore, Pearson correlations were calculated to assess the relationship between ADS and pain intensity/ duration, subjective sleep difficulty, and functional disability. One-sample t-tests were used to compare our sample to literature norms for all measures except PSG. PSG for subjects and controls were compared using parametric t-tests. SPSS version 17.0 (SPSS Inc., Chicago, IL, release date: August 23, 2008) was used for all data analyses.
RESULTS
A total of 14 patients consented to participate. Two participants withdrew consent before completing any studies, and one could not sleep in the laboratory. The fourth was withdrawn due to behavioral issues that prevented completion of the program. A final total of 10 Caucasian females with a mean age of 16.2 years and average symptom duration of 48.6 ± 50.7 SD months completed this study. Seven of the patients completed treatment as outpatients, and the remaining three were residential.
Pain and Disability
Pain and disability pretreatment and posttreatment are presented in Table 1. At baseline, patients reported high usual pain intensity and severe functional disability.1 After treatment, pain (P = 0.000) and disability (P = 0.004) improved significantly. Half of the subjects' pain scores decreased to ≤ 4, including three subjects with pain scores of 0 after treatment, and 8 of 10 patients had FDI scores within the normal range (≤ 5).
Table 1.
Pain, disability, and subjective sleep measures pretreatment versus posttreatment

Subjective Sleep Difficulties
Sleep VAS and Energy VAS
At baseline, patients endorsed considerable sleep and energy difficulties. Both sleep (P = 0.008) and energy (P = 0.001) improved significantly after treatment (Table 1).
School Sleep Habits Survey
School Sleep Habits Survey scores pretreatment and post-treatment are presented in Table 1. At baseline, circadian preference was significantly shifted to eveningness compared with healthy female adolescents (P = 0.018).48 Patients had significantly higher scores on the Sleepiness Scale (P = 0.003)46 but significantly lower scores on the Sleep-Wake Behavior Problems Scale (P = 0.001)46 and depressed mood scale (P = 0.000)47 compared with healthy adolescents.
After treatment, circadian preference shifted significantly toward morningness (P = 0.014). No significant changes were observed in other School Sleep Habits Survey subscales, although differences in Sleep-Wake Behavior Problems Scale scores approached significance (P = 0.056) (Table 1).
Actigraphy
Complete preactigraphy and postactigraphy data were available for only 6 subjects due to technical difficulties. Individual nights that appeared anomalous were eliminated from analysis. For example, one subject displayed significantly different sleep patterns on December 24th (Christmas Eve): therefore, this night was excluded.
At baseline, compared with normative data,10 patients had significantly poorer sleep efficiency (P = 0.003) and more wake after sleep onset (P = 0.025), but similar TST (P = 0.131) and sleep latency (P = 0.148). None of these variables changed significantly after treatment (Table 2).
Table 2.
Objective sleep measures pretreatment versus posttreatment

Polysomnography
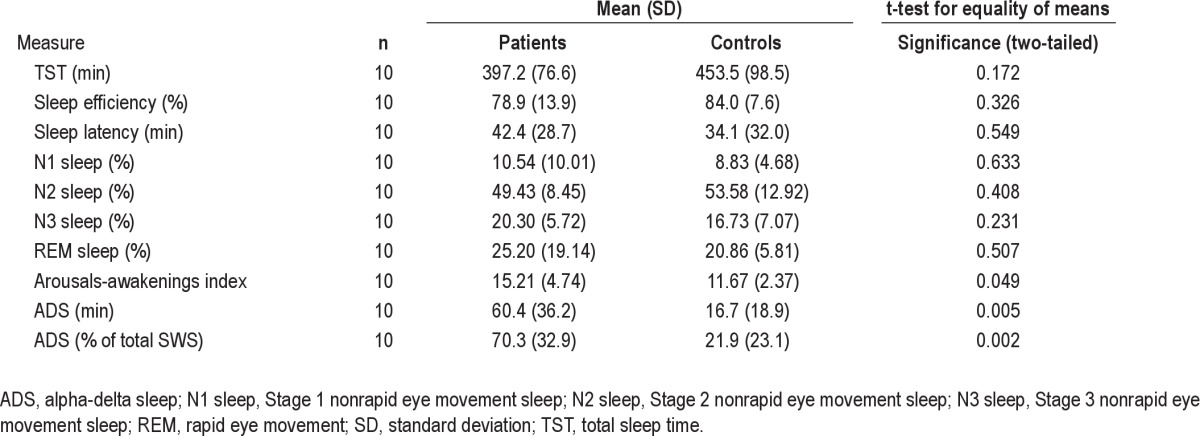
At baseline, patients had significantly more arousals and awakenings than controls (P = 0.049; Table 3), suggesting sleep fragmentation. However, there were no significant differences between patients and controls for TST, sleep efficiency, sleep latency, or sleep staging (Table 3). None of these variables changed significantly after treatment (Table 2).
Table 3.
PSG data for patients at baseline versus healthy control patients
Alpha-Delta Sleep
All patients displayed ADS at baseline, averaging 60.6 ± 35.8 SD minutes and encompassing 70.7 ± 32.0 SD% of total SWS, significantly more than controls both in terms of both minutes (P = 0.005) and percentage of total SWS (P = 0.002) (Table 3). There was no significant correlation between ADS and pain intensity or duration, subjective sleep difficulty, or functional disability. Neither the amount nor the percentage of ADS changed after treatment (Table 2). Intraobserver reliability for scoring ADS was excellent, with a reliability coefficient of 0.98 and intraclass correlation of 0.97 (95% confidence interval: 0.87, 0.99).
Multiple Sleep Latency Test
At baseline, mean sleep onset latency was normal55 (14.47 ± 3.58 SD minutes, range = 8.9-20.0 minutes). There was no significant change after treatment (Table 3).
DISCUSSION
Pain, disability, and subjective sleep quality improved significantly in our adolescents with JPFS following completion of a multidisciplinary pain treatment program that included intensive exercise therapy. As expected, patients with pain had significantly more ADS than controls at baseline; however, neither ADS nor other objective measurements of sleep quality changed after treatment. In other words, patients' perceptions of sleep improved even though objective sleep quality did not. We were unable to demonstrate any relationship between ADS and pain intensity or subjective sleep quality, at least in the short term.
ADS was first described in 197356 and has since been established as common in individuals with fibromyalgia. An early study16 found considerable ADS in adults with fibromyalgia, and these findings have been replicated by other studies.57,58 Similar to our results, several other studies have found significantly more ADS and subjective sleep difficulties in adults with fibromyalgia compared with healthy controls.11,14,15,59 Although Horne and Shackell60 did not find a significant difference in ADS between adults with fibromyalgia and healthy controls, one of their inclusion criteria was that subjects consider themselves to be good sleepers. Because poor sleep is described by 90% of adults61 and 96% of children and adolescents with fibromyalgia,6 the sample in this particular study may not be representative of the disease population.
Although ADS has been less well studied in children, it also appears to be associated with JPFS. Roizenblatt and colleagues11 found significantly more ADS in both children and mothers with fibromyalgia than in healthy controls, similar to our results. Also consistent with our findings, they observed significantly poorer sleep efficiency and more arousals in children with JPFS than in controls. ADS was significantly associated with the number of tender points and inversely related to pain threshold in both children and mothers with fibromyalgia, a correlation we expected to find but did not observe. In our study, pain improved significantly after treatment but the amount of ADS did not change, suggesting these constructs may not be causally related. Though further study is clearly indicated, our results suggest that the etiologies of pain and ADS may be independent in JPFS.
In addition to its prevalence in fibromyalgia, ADS has been associated with pain, mood disturbances, and subjective reports of unrefreshing sleep.14,16,17,57,62 However, the direction of causality between pain and ADS remains unclear. Some studies suggest that pain is caused by ADS, whereas others seem to indicate the reverse. Depriving healthy adults of SWS via auditory stimulation induced musculoskeletal symptoms of fibromyalgia, including muscle aching, stiffness, and tenderness.63 These results were replicated in a later study64 and are further supported by findings of increased morning pain intensity in patients with fibromyalgia who have significant ADS overnight.57 Conversely, Drewes and colleagues65 induced ADS in healthy adults by applying painful stimuli to muscles during SWS. Collectively, these findings suggest a bidirectional interaction between pain and ADS22; however, this relationship is not supported by our finding that ADS persisted after pain diminished, nor by a previous study of adults with fibromyalgia that found no correlation between ADS and symptom severity.66 Additionally, another recent study of adults with fibromyalgia found that neither sleep duration nor time awake after sleep onset significantly predicted pain intensity.67
The efficacy of exercise therapy in treating fibromyalgia is well documented. It has been shown to improve sleep in adult patients,25 although whether it affects sleep architecture is unknown. In healthy children and adolescents, exercise and physical fitness are associated with increased SWS and better sleep quality.29,30 It has been suggested that poor physical fitness in fibromyalgia68 contributes to both pain and SWS disturbances,69 and that ADS may interfere with the restorative role of sleep.14,16 Evidence that exercise promotes and preserves SWS32,70 has led to the suggestion that physical fitness may protect against ADS.16 In support of this hypothesis, Moldofsky and colleagues16 found that sedentary but otherwise healthy adults developed musculoskeletal and mood symptoms of fibromyalgia when deprived of SWS, whereas those who exercised regularly did not. Our findings do not support the idea that exercise therapy for pain improves sleep quality by reducing ADS. ADS was not correlated with subjective sleep difficulty and persisted in the absence of reports of unrefreshing sleep. The prevalence of ADS in our sample was high at baseline and, despite significant improvements in subjective sleep quality and physical fitness, did not change after treatment.
It is worth emphasizing that ADS is not specific to fibromyalgia. ADS has been observed in patients with chronic fatigue syndrome71,72 as well as in healthy individuals.73 It is possible that, rather than pain causing ADS (or vice versa), some other factor, such as genetics, predisposes some individuals to chronic pain, ADS, or both. The existence of a genetic component to fibromyalgia has been suggested,74–77 but susceptibility to ADS might be separately heritable. In healthy individuals, Scheuler and colleagues73 found some evidence for a familial component to ADS, but further research is needed.
The statistical power of this study is limited by its small sample size. Actigraphy data from several subjects were excluded from analysis due to technical difficulties, further decreasing the sample size for actigraphy variables. Additionally, only PSG data were collected from controls. Although we compared our sample to healthy literature controls for other variables, an optimal study design would include collecting control subject data for all measures. It is also important to acknowledge that the intervention described consists of multiple components, including sleep hygiene counseling and cognitive behavioral therapy, in addition to intensive exercise. Although intensive aerobic training constitutes most daily treatment-related activity, it is likely that other aspects of treatment contributed to our patients' recovery. Therefore, we cannot say with certainty that exercise alone is responsible for the improvements we observed in pain, disability, and subjective sleep quality. Finally, our sample consisted of a very specific population, Caucasian female adolescents, which may limit the generalizability of our findings. Although the overrepresentation of girls is consistent with the 4:1 female-to-male ratio among adolescents with amplified musculoskeletal pain78 and JPFS is most often diagnosed in white adolescent girls,6,79 it is important to determine whether sex and/or race influence outcomes.
This study provides an important foundation for understanding the relationship between pain and sleep in adolescents with JPFS. We have demonstrated that sleep difficulties, including ADS, are common in these patients, and that intensive treatment, including exercise therapy, results in markedly improved pain and self-reported sleep difficulties. However, there were no objective improvements in sleep, particularly sleep fragmentation and ADS. Our results do not suggest causal relationships between ADS and pain or subjective sleep quality, although further study is clearly indicated. Future research should include a larger, more diverse sample and incorporate additional longitudinal points to address the possibility that ADS is slower to resolve than pain symptoms. It may be that EEG abnormalities, including ADS, require more time to resolve than the one to two months between measurements in this study. Future studies might also consider other factors, such as genetics, that may predispose patients to both chronic pain and ADS.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Sohee Kim for help in gathering and organizing the data; the sleep technologists at the Children's Hospital of Philadelphia; the contributions of Karen and Christine Lubert; and the patients and their families for their cooperation and participation. This work was performed at The Children's Hospital of Philadelphia and was supported by hospital research funds from the Reflex Neurovascular Dystrophy (RND) Program, by NIH T35 HD 007446, and by UL1RR024134 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. This work did not involve any off-label or investigational use.
ABBREVIATIONS
- ADS
alpha-delta sleep
- DMS
Depressive Mood Scale
- FDI
Functional Disability Inventory
- JPFS
juvenile primary fibromyalgia syndrome
- MEQ
Morningness/Eveningness Questionnaire
- MSLT
Multiple Sleep Latency Test
- PSG
polysomnography
- SLS
Sleepiness Scale
- SSHS
School Sleep Habits Survey
- SWBPS
Sleep-Wake Behavior Problems Scale
- SWS
slow wave sleep
- TST
total sleep time
- VAS
Visual Analog Scale
- WASO
wake after sleep onset
REFERENCES
- 1.Kashikar-Zuck S, Flowers SR, Claar RL, et al. Clinical utility and validity of the Functional Disability Inventory among a multicenter sample of youth with chronic pain. Pain. 2011;152:1600–7. doi: 10.1016/j.pain.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 3.Eccleston C, Crombez G, Scotford A, Clinch J, Connell H. Adolescent chronic pain: patterns and predictors of emotional distress in adolescents with chronic pain and their parents. Pain. 2004;108:221–9. doi: 10.1016/j.pain.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Smith M, Martin-Herz S, Womack W, Marsigan J. Comparative study of anxiety, depression, somatization, functional disability, and illness attribution in adolescents with chronic fatigue or migraine. Pediatrics. 2003;111:e376–81. doi: 10.1542/peds.111.4.e376. [DOI] [PubMed] [Google Scholar]
- 5.Roth-Isigkeit A, Thyen U, Stöven H, Schwarzenberger J, Schmucker P. Pain among children and adolescents: restrictions in daily living and triggering factors. Pediatrics. 2005;115:e152–62. doi: 10.1542/peds.2004-0682. [DOI] [PubMed] [Google Scholar]
- 6.Siegel DM, Janeway D, Baum J. Fibromyalgia syndrome in children and adolescents: clinical features at presentation and status at follow-up. Pediatrics. 1998;101:377–82. doi: 10.1542/peds.101.3.377. [DOI] [PubMed] [Google Scholar]
- 7.Meltzer LJ, Logan DE, Mindell JA. Sleep patterns in female adolescents with chronic musculoskeletal pain. Behav Sleep Med. 2005;3:193–208. doi: 10.1207/s15402010bsm0304_2. [DOI] [PubMed] [Google Scholar]
- 8.Palermo TM, Kiska R. Subjective sleep disturbances in adolescents with chronic pain: relationship to daily functioning and quality of life. J Pain. 2005;6:201–7. doi: 10.1016/j.jpain.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Palermo T, Fonareva I, Janosy N. Sleep quality and efficiency in adolescents with chronic pain: Relationship with activity limitations and health-related quality of life. Behav Sleep Med. 2008;6:234–50. doi: 10.1080/15402000802371353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palermo TM, Toliver-Sokol M, Fonareva I, Koh JL. Objective and subjective assessment of sleep in adolescents with chronic pain compared to healthy adolescents. Clin J Pain. 2007;23:812–20. doi: 10.1097/AJP.0b013e318156ca63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roizenblatt S, Tufik S, Goldenberg J, Pinto LR, Hilario MO, Feldman D. Juvenile fibromyalgia: clinical and polysomnographic aspects. J Rheumatol. 1997;24:579–85. [PubMed] [Google Scholar]
- 12.Tayag-Kier CE, Keenan GF, Scalzi LV, et al. Sleep and periodic limb movement in sleep in juvenile fibromyalgia. Pediatrics. 2000;106:E70. doi: 10.1542/peds.106.5.e70. [DOI] [PubMed] [Google Scholar]
- 13.Tsai S, Labyak S, Richardson L, et al. Brief report: Actigraphic sleep and daytime naps in adolescent girls with chronic musculoskeletal pain. J Pediatr Psychol. 2008;33:307–11. doi: 10.1093/jpepsy/jsm117. [DOI] [PubMed] [Google Scholar]
- 14.Anch AM, Lue FA, MacLean AW, Moldofsky H. Sleep physiology and psychological aspects of the fibrositis (fibromyalgia) syndrome. Can J Psychol. 1991;45:179–84. doi: 10.1037/h0084280. [DOI] [PubMed] [Google Scholar]
- 15.Branco J, Atalaia A, Paiva T. Sleep cycles and alpha-delta sleep in fibromyalgia syndrome. J Rheumatol. 1994;21:1113–7. [PubMed] [Google Scholar]
- 16.Moldofsky H, Scarisbrick P, England R, Smythe H. Musculosketal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med. 1975;37:341–51. doi: 10.1097/00006842-197507000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Moldofsky H, Lue FA. The relationship of alpha and delta EEG frequencies to pain and mood in “fibrositis” patients treated with chlorpromazine and L-tryptophan. Electroencephalogr Clin Neurophysiol. 1980;50:71–80. doi: 10.1016/0013-4694(80)90324-7. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro CM, Devins GM, Hussain MR. ABC of sleep disorders. Sleep problems in patients with medical illness. BMJ. 1993;306:1532–5. doi: 10.1136/bmj.306.6891.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahowald ML, Mahowald MW. Nighttime sleep and daytime functioning (sleepiness and fatigue) in less well-defined chronic rheumatic diseases with particular reference to the “alpha-delta NREM sleep anomaly.”. Sleep Med. 2000;1:195–207. doi: 10.1016/s1389-9457(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 20.Ohayon MM. Relationship between chronic painful physical condition and insomnia. J Psychiatr Res. 2005;39:151–9. doi: 10.1016/j.jpsychires.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Ulus Y, Akyol Y, Tander B, Durmus D, Bilgici A, Kuru O. Sleep quality in fibromyalgia and rheumatoid arthritis: associations with pain, fatigue, depression, and disease activity. Clin Exp Rheumatol. 2011;29:S92–6. [PubMed] [Google Scholar]
- 22.Ohayon M. Chronic pain and sleep. Int J Sleep Disorders. 2006;1:16–21. [Google Scholar]
- 23.Stone KC, Taylor DJ, McCrae CS, Kalsekar A, Lichstein KL. Nonrestorative sleep. Sleep Med Rev. 2008;12:275–88. doi: 10.1016/j.smrv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Passarelli CM, Roizenblatt S, Len CA, et al. A case-control sleep study in children with polyarticular juvenile rheumatoid arthritis. J Rheumatol. 2006;33:796–802. [PubMed] [Google Scholar]
- 25.Gowans SE, deHueck A, Voss S, Richardson M. A randomized, controlled trial of exercise and education for individuals with fibromyalgia. Arthritis Care Res. 1999;12:120–8. doi: 10.1002/1529-0131(199904)12:2<120::aid-art7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Gowans S, deHueck A, Voss S, Silaj A, Abbey S, Reynolds W. Effect of a randomized, controlled trial of exercise on mood and physical function in individuals with fibromyalgia. Arthritis Rheum. 2001;45:519–29. doi: 10.1002/1529-0131(200112)45:6<519::aid-art377>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.McCain GA. Role of physical fitness training in the fibrositis/fibromyalgia syndrome. Am J Med. 1986;81:73–7. doi: 10.1016/0002-9343(86)90881-8. [DOI] [PubMed] [Google Scholar]
- 28.McCain GA, Bell DA, Mai FM, Halliday PD. A controlled study of the effects of a supervised cardiovascular fitness training program on the manifestations of primary fibromyalgia. Arthritis Rheum. 1988;31:1135–41. doi: 10.1002/art.1780310908. [DOI] [PubMed] [Google Scholar]
- 29.Brand S, Gerber M, Beck J, Hatzinger M, Puhse U, Holsboer-Trachsler E. Exercising, sleep-EEG patterns, and psychological functioning are related among adolescents. World J Biol Psychiatry. 2010;11:129–40. doi: 10.3109/15622970903522501. [DOI] [PubMed] [Google Scholar]
- 30.Dworak M, Wiater A, Alfer D, Stephan E, Hollmann W, Strüder HK. Increased slow wave sleep and reduced stage 2 sleep in children depending on exercise intensity. Sleep Med. 2008;9:266–72. doi: 10.1016/j.sleep.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 31.Griffin S, Trinder J. Physical fitness, exercise, and human sleep. Psycho-physiology. 1978;15:447–50. doi: 10.1111/j.1469-8986.1978.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 32.Hobson JA. Sleep after exercise. Science. 1968;162:1503–5. doi: 10.1126/science.162.3861.1503. [DOI] [PubMed] [Google Scholar]
- 33.Kashikar-Zuck S, Graham T, Huenefeld M, Powers S. A review of biobehavioral research in juvenile primary fibromyalgia syndrome. Arthritis Care Res. 2000;13:388–97. doi: 10.1002/1529-0131(200012)13:6<388::aid-art9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 34.Wolfe F, Smythe H, Yunus M, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 35.Sherry D, Wallace C, Kelley C, Kidder M, Sapp L. Short-and long-term outcomes of children with complex regional pain syndrome type I treated with exercise therapy. Clin J Pain. 1999;15:218–23. doi: 10.1097/00002508-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 36.de Blécourt AC, Schiphorst Preuper HR, Van Der Schans CP, Groothoff JW, Reneman MF. Preliminary evaluation of a multidisciplinary pain management program for children and adolescents with chronic musculo-skeletal pain. Disabil Rehabil. 2008;30:13–20. doi: 10.1080/09638280601178816. [DOI] [PubMed] [Google Scholar]
- 37.Hechler T, Blankenburg M, Dobe M, Kosfelder J, Hübner B, Zernikow B. Effectiveness of a multimodal inpatient treatment for pediatric chronic pain: a comparison between children and adolescents. Eur J Pain. 2010;14:97.e1–9. doi: 10.1016/j.ejpain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Varni JW, Thompson KL, Hanson V. The Varni/Thompson Pediatric Pain Questionnaire. I. Chronic musculoskeletal pain in juvenile rheumatoid arthritis. Pain. 1987;28:27–38. doi: 10.1016/0304-3959(87)91056-6. [DOI] [PubMed] [Google Scholar]
- 39.McGrath P. Pain in children: Nature, assessment, and treatment. New York: Guilford Press; 1990. [Google Scholar]
- 40.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 41.Kashikar-Zuck S, Vaught M, Goldschneider K, Graham T, Miller J. Depression, coping, and functional disability in juvenile primary fibromyalgia syndrome. J Pain. 2002;3:412–9. doi: 10.1054/jpai.2002.126786. [DOI] [PubMed] [Google Scholar]
- 42.Gauntlett-Gilbert J, Eccleston C. Disability in adolescents with chronic pain: Patterns and predictors across different domains of functioning. Pain. 2007;131:132–41. doi: 10.1016/j.pain.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Kashikar-Zuck S, Swain NF, Jones BA, Graham TB. Efficacy of cognitive-behavioral intervention for juvenile primary fibromyalgia syndrome. J Rheumatol. 2005;32:1594–602. [PubMed] [Google Scholar]
- 44.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–87. [PubMed] [Google Scholar]
- 45.Carskadon M, Seifer R, Acebo C. Reliability of six scales in a sleep questionnaire for adolescents. Sleep Res. 1991:421. [Google Scholar]
- 46.Bruni O, Russo PM, Ferri R, Novelli L, Galli F, Guidetti V. Relationships between headache and sleep in a non-clinical population of children and adolescents. Sleep Med. 2008;9:542–8. doi: 10.1016/j.sleep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Kandel DB, Davies M. Epidemiology of depressive mood in adolescents: an empirical study. Arch Gen Psychiatry. 1982;39:1205–12. doi: 10.1001/archpsyc.1982.04290100065011. [DOI] [PubMed] [Google Scholar]
- 48.Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–62. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- 49.Acebo C, LeBourgeois MK. Actigraphy. Respir Care Clin N Am. 2006;12:23–30. viii. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–41. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 51.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6:113–24. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- 52.Boyne K, Sherry DD, Gallagher PR, Olsen M, Brooks LJ. Accuracy of computer algorithms and the human eye in scoring actigraphy. Sleep Breath. 2012 May 13; doi: 10.1007/s11325-012-0709-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 54.Iber C, Ancoli-Israel S, Chesson A, Quan S. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 55.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 56.Hauri P, Hawkins DR. Alpha-delta sleep. Electroencephalogr Clin Neurophysiol. 1973;34:233–7. doi: 10.1016/0013-4694(73)90250-2. [DOI] [PubMed] [Google Scholar]
- 57.Roizenblatt S, Moldofsky H, Benedito-Silva AA, Tufik S. Alpha sleep characteristics in fibromyalgia. Arthritis Rheum. 2001;44:222–30. doi: 10.1002/1529-0131(200101)44:1<222::AID-ANR29>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 58.Saskin P, Moldofsky H, Lue FA. Sleep and posttraumatic rheumatic pain modulation disorder (fibrositis syndrome) Psychosom Med. 1986;48:319–23. doi: 10.1097/00006842-198605000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Touchon J, Besset A, Billiard M, Simon L, Herrisson C, Cadilhac J. Fibrositis syndrome: polysomnographic and psychological aspects. In: Koella W, Obal F, Schulz H, Visser P, editors. Sleep ‘86. Stuttgart: Gustav Fischer Verlag; 1988. pp. 445–7. [Google Scholar]
- 60.Horne JA, Shackell BS. Alpha-like EEG activity in non-REM sleep and the fibromyalgia (fibrositis) syndrome. Electroencephalogr Clin Neurophysiol. 1991;79:271–6. doi: 10.1016/0013-4694(91)90122-k. [DOI] [PubMed] [Google Scholar]
- 61.Moldofsky H. The significance, assessment, and management of nonrestorative sleep in fibromyalgia syndrome. CNS Spectr. 2008;13:22–6. doi: 10.1017/s1092852900026808. [DOI] [PubMed] [Google Scholar]
- 62.Drewes AM, Nielsen KD, Taagholt SJ, Bjerregård K, Svendsen L, Gade J. Sleep intensity in fibromyalgia: focus on the microstructure of the sleep process. Br J Rheumatol. 1995;34:629–35. doi: 10.1093/rheumatology/34.7.629. [DOI] [PubMed] [Google Scholar]
- 63.Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35–44. doi: 10.1097/00006842-197601000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999;26:1586–92. [PubMed] [Google Scholar]
- 65.Drewes AM, Nielsen KD, Arendt-Nielsen L, Birket-Smith L, Hansen LM. The effect of cutaneous and deep pain on the electroencephalogram during sleep: an experimental study. Sleep. 1997;20:632–40. doi: 10.1093/sleep/20.8.632. [DOI] [PubMed] [Google Scholar]
- 66.Carette S, Oakson G, Guimont C, Steriade M. Sleep electroencephalography and the clinical response to amitriptyline in patients with fibromyalgia. Arthritis Rheum. 1995;38:1211–7. doi: 10.1002/art.1780380906. [DOI] [PubMed] [Google Scholar]
- 67.Anderson RJ, McCrae CS, Staud R, Berry RB, Robinson ME. Predictors of clinical pain in fibromyalgia: examining the role of sleep. J Pain. 2012;13:350–8. doi: 10.1016/j.jpain.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bennett RM. Physical fitness and muscle metabolism in the fibromyalgia syndrome: an overview. J Rheumatol Suppl. 1989;19:28–9. [PubMed] [Google Scholar]
- 69.Bennett RM. Beyond fibromyalgia: ideas on etiology and treatment. J Rheumatol Suppl. 1989;19:185–91. [PubMed] [Google Scholar]
- 70.Zloty RB, Burdick JA, Adamson JD. Sleep of distance runners. Act Nerv Super (Praha) 1973;15:217–21. [PubMed] [Google Scholar]
- 71.Manu P, Lane TJ, Matthews DA, Castriotta RJ, Watson RK, Abeles M. Alpha-delta sleep in patients with a chief complaint of chronic fatigue. South Med J. 1994;87:465–70. doi: 10.1097/00007611-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Whelton CL, Salit I, Moldofsky H. Sleep, Epstein-Barr virus infection, musculoskeletal pain, and depressive symptoms in chronic fatigue syndrome. J Rheumatol. 1992;19:939–43. [PubMed] [Google Scholar]
- 73.Scheuler W, Kubicki S, Marquardt J, Scholz G. Koella W, Obal F, Schultz H, Visser P, editors. The alpha sleep pattern: quantitative analysis and functional aspects. Sleep ‘86. Gustav Fisher Verlag. 1988:284–6. [Google Scholar]
- 74.Buskila D, Neumann L. Fibromyalgia syndrome (FM) and nonarticular tenderness in relatives of patients with FM. J Rheumatol. 1997;24:941–4. [PubMed] [Google Scholar]
- 75.Buskila D, Neumann L, Hazanov I, Carmi R. Familial aggregation in the fibromyalgia syndrome. Semin Arthritis Rheum. 1996;26:605–11. doi: 10.1016/s0049-0172(96)80011-4. [DOI] [PubMed] [Google Scholar]
- 76.Pellegrino MJ, Waylonis GW, Sommer A. Familial occurrence of primary fibromyalgia. Arch Phys Med Rehabil. 1989;70:61–3. [PubMed] [Google Scholar]
- 77.Yunus MB, Khan MA, Rawlings KK, Green JR, Olson JM, Shah S. Genetic linkage analysis of multicase families with fibromyalgia syndrome. J Rheumatol. 1999;26:408–12. [PubMed] [Google Scholar]
- 78.Sherry DD, Malleson PN. The idiopathic musculoskeletal pain syndromes in childhood. Rheum Dis Clin North Am. 2002;28:669–85. doi: 10.1016/s0889-857x(02)00007-8. [DOI] [PubMed] [Google Scholar]
- 79.Yunus MB, Masi AT. Juvenile primary fibromyalgia syndrome. A clinical study of thirty-three patients and matched normal controls. Arthritis Rheum. 1985;28:138–45. doi: 10.1002/art.1780280205. [DOI] [PubMed] [Google Scholar]


