Abstract
Study Objectives:
Primary insomnia (PI) is a sleep disorder characterized by difficulty with sleep initiation, maintenance, and/or the experience of nonrestorative sleep combined with a subsequent impairment of daytime functioning. The hyperarousal hypothesis has emerged as the leading candidate to explain insomnia symptoms in the absence of specific mental, physical, or substance-related causes. We hypothesized that the cellular energetic metabolites, including beta nucleoside triphosphate, which in magnetic resonance spectroscopy approximates adenosine triphosphate (ATP), and phosphocreatine (PCr), would show changes in PI reflecting increased energy demand.
Design and Setting:
Matched-groups, cross-sectional study performed at two university-based hospitals.
Patients:
Sixteen medication-free individuals (eight males, eight females; mean ± standard deviation (SD) age = 37.2 ± 8.4 y) with PI and 16 good sleepers (nine males, seven females; mean ± SD age = 37.6 ± 4.7 y).
Measurements:
Diagnosis was established for all individuals by unstructured clinical interview, Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (SCID), sleep diary, and actigraphy. Polysomnography was collected in individuals with PI. Phosphorous magnetic resonance spectroscopy (31P MRS) data were collected on all individuals at 4 Tesla. We assessed cell membrane (anabolic precursors and catabolic metabolites) and bioenergetic (ATP, phosphocreatine) metabolites in gray matter and white matter to determine their relationship to the presence and severity of PI.
Results:
Individuals with PI showed lower phosphocreatine in gray matter and an unexpected decrease of phosphocholine, a precursor of the cell membrane compound phosphatidylcholine, in white matter. In addition, there was a trend toward a negative association between polysomnographically determined wake after sleep onset and gray matter beta-nucleoside triphosphate and white matter phosphocholine in the primary insomnia group.
Conclusions:
These results support the hyperarousal hypothesis in PI based on lower phosphocreatine in gray matter in the PI group.
Citation:
Harper DG; Plante DT; Jensen JE; Ravichandran C; Buxton OM; Benson KL; O'Connor SP; Renshaw PF; Winkelman JW. Energetic and cell membrane metabolic products in patients with primary insomnia: a 31-phosphorus magnetic resonance spectroscopy study at 4 tesla. SLEEP 2013;36(4):493-500.
Keywords: Adenosine triphosphate (ATP), long-term potentiation, phosphocholine, phosphocreatine, sleep, spectroscopy
INTRODUCTION
Insomnia is characterized by difficulty with sleep initiation, maintenance, and/or the experience of nonrestorative sleep combined with a subsequent impairment of daytime functioning. Insomnia is a significant public health problem associated with functional impairment and a decreased quality of life, as well as increased disability and health care utilization.1 Insomnia may be divided into primary and comorbid forms, with comorbid insomnia constituting most instances of chronic insomnia, with symptoms related to concomitant medical, psychiatric, and/or pharmacologic considerations. Primary insomnia (PI) is defined by insomnia symptoms unrelated to any other medical or psychiatric disorder or to a substance or medication, accounts for approximately 25% of chronic insomnia, and has a prevalence between 2% and 4% in the general population.2 The etiology of PI is unclear given the limited knowledge of pathophysiologic mechanisms and uncertainty about whether PI represents one homogeneous disorder or a single symptomatic entity with heterogeneous mechanisms.
The most prominent proposition regarding the etiology of insomnia is the hyperarousal hypothesis. This model posits a combination of neurobiologic and behavioral processes that lead to a state of conditioned autonomic arousal and perception of sleeplessness.3,4 Several lines of evidence, including increased high frequency (beta/gamma) activity during sleep electroencephalogram, elevated whole body metabolic rate, and alterations in endocrine and immunologic factors, support the hyperarousal model of insomnia.4
Neuroimaging evidence for hyperarousal in PI includes data from 18fluorodeoxyglucose positron emission tomography (18FDG PET) and proton magnetic resonance spectroscopy (1H MRS) studies. Glucose metabolism is increased in PI across the whole brain during wakefulness and sleep, with smaller decreases between wake and sleep in brain regions associated with arousal (ascending reticular formation and hypothalamus), emotion regulation (hippocampus, amygdala, and anterior cingulate cortex), and cognition (median prefrontal cortex),5 and increased wake after sleep onset (WASO) in individuals with insomnia with higher glucose metabolism.6 A 1H MRS study, using the same study participants as the current study, revealed that the global level of the primary inhibitory neurotransmitter gamma-aminobutyric acid was decreased in patients with PI compared with control patients, and was inversely correlated with WASO, suggesting an imbalance of inhibitory and excitatory neurotransmitters was associated with heightened arousal in these patients.7
Phosphorus-31 magnetic resonance spectroscopy (31PMRS) has the ability to quantify some of the important energetic compounds in the brain, including nucleoside-triphosphate compounds (adenosine triphosphate (ATP), guanosine triphosphate), phosphocreatine (PCr; an important high-energy phosphate buffer of ATP), and inorganic phosphate (Pi: a metabolite of ATP) yielding an impression of energetic activity. In addition, precursors (phosphocholine [PCho] and phosphoethanolamine [PEtn]) and metabolites (glycerophosphocholine [GPCho] and glycerophosphoethanolamine [GPEtn]) of the two most prevalent membrane phospholipids (phosphatidylcholine and phosphatidylethanolamine) can be quantified, yielding an overall impression of cell membrane health. 31P MRS has been used to provide cellular energetic and phospholipid information in studies of depression,8,9 Huntington disease,10 Alzheimer disease,11 bipolar disorder,12 and schizophrenia.13
Here we report the first phosphorous (31P) MRS study in PI, which examines the cellular energetic and membrane metabolites in the brains of individuals with PI compared with those of normal control individuals. Based on the hyperarousal hypothesis of insomnia we hypothesized that the cellular energetic metabolite, beta nucleoside triphosphate (bNTP), which in MRS approximates ATP, and PCr, would show changes in PI reflecting increased energy demand (lowered PCr and bNTP in gray matter where energy utilization and demand are highest) and that these changes would be associated with increased WASO. In addition, we hypothesized that changes in cell membrane metabolites, reflecting increased membrane turnover, would be present in PI and that these changes would be associated with increased WASO.
METHODS AND MATERIALS
Study Participants
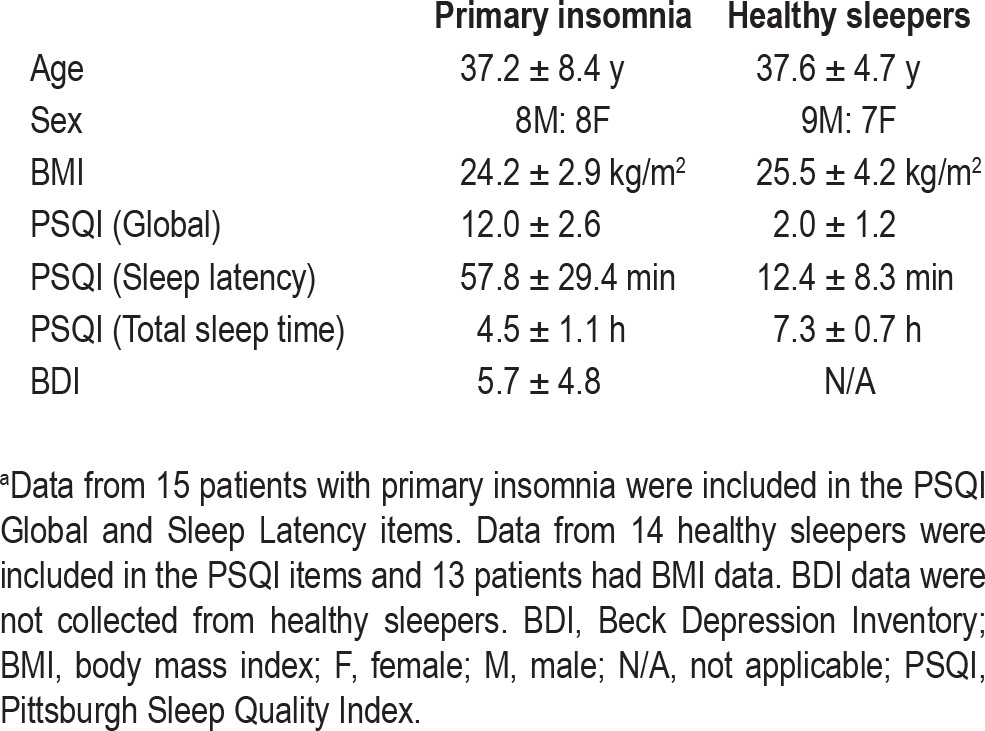
Study participant recruitment, inclusion, and exclusion criteria are detailed previously as 1H MRS results from this cohort have been previously reported.7 Sixteen patients with PI (eight males, eight females; mean ± standard deviation [SD] age = 37.2 ± 8.4 y) were recruited via advertisements for a larger study of glucose metabolism and neuroimaging in insomnia at Brigham and Women's Hospital and McLean Hospital from January 2007 to May 2008. Sixteen control patients (nine males, seven females; mean ± SD age = 37.6 ± 4.7 y) without sleep complaints were also recruited via advertising (Table 1).
Table 1.
Demographic and questionnaire information from patients with primary insomnia and normal healthy sleepersa
All patients were assessed using an unstructured clinical interview for history of medical and sleep disorders, Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (SCID), physical examination, laboratory assessment, and routine scales to assess mood and sleep as described elsewhere.7 Additionally, sleep diaries collected over 2 to 4 wk and supplemented by wrist actigraphy were used to verify sleep-wake patterns. Patients with PI met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for the disorder and additionally met specific severity criteria: duration > 6 mo, total sleep time ≤ 6.5 h and either sleep onset latency (SOL) > 45 min or WASO > 45 min or SOL + WASO > 60 min.
Exclusion criteria for all patients were: current or recent (within the preceding year) diagnosis of a DSM-IV Axis I disorder (including alcohol or drug dependence/abuse) other than PI; symptoms, diagnosis, or history of any sleep disorder other than PI; body mass index > 32 or < 19.8; regular treatment with central nervous system active agents within 3 mo of the first visit; current nicotine use (> 10 cigarettes per day), consumption of more than two caffeinated beverages per day, or more than two standard alcoholic drinks per day for a period > 1 mo in the preceding year; and history of swing shift, night shift, or rotating shift-work within the preceding year.
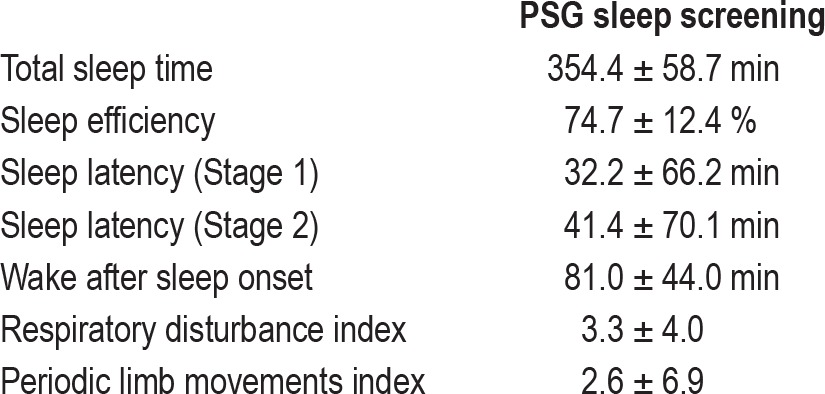
Patients with PI who met initial screening criteria underwent routine attended in-laboratory polysomnography (PSG) within 2 wk of MRS acquisition (Table 2). A cutoff of 15 apnea + hypopneas or 20 periodic limb movements per hour of sleep led to exclusion from the study. Additionally, evidence of paradoxical insomnia (sleep efficiency greater than 90% and subjective report of sleep similar to usual) was exclusionary. One patient with PI did not have a PSG for analysis.
Table 2.
Aggregate polysomnography data from patients with primary insomnia (n = 15)
This study was approved by the Institutional Review Boards of Partners Healthcare, the parent organization of Brigham and Women's Hospital, and McLean Hospital. Informed consent was obtained from all patients prior to the performance of any experimental procedures. All patients were compensated for their participation in this study.
4 T Magnetic Resonance Imaging/Magnetic Resonance Spectroscopy
A set of T1-weighted magnetization-prepared Fast, Low-Angle Shot, three-dimensional (mp-FLASH3D) images were acquired in sagittal to expose the relevant anatomy used to guide the slab placement (echo time/repetition time (TE/TR) = 6.2/11.4 ms, field-of-view (FOV) = 24 cm × 24 cm, readout-duration = 4 ms, receive bandwidth = ± 32 kHz, in-plane matrix size = 128 × 256, in-plane resolution = 0.94 × 0.94 mm, readout points = 512, axial-plane matrix size = 16, axial-plane resolution = 2.5 mm sagittal, scan time = 1 min, 15 sec). Once complete, an axial set of 32 images using the same mpFLASH3D sequence acquired a set of T1-weighted axial images of high resolution. (TE/TR = 6.2/11.4 ms, FOV = 24 cm × 24 cm, readout-duration = 4 ms, receiver bandwidth = ± 32 kHz, in-plane matrix size = 256 × 256, in-plane resolution = 0.94 × 0.94 mm, readout points = 512, axial-plane matrix size = 32, axial-plane resolution = 2.5 mm sagittal, scan time = 2 min, 30 sec).
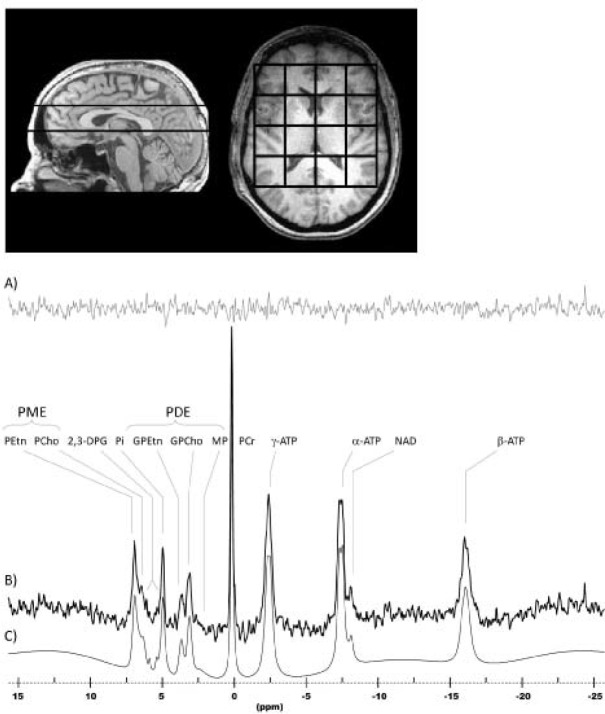
The 31P-chemical shift imaging (CSI) acquisition consisted of a two-dimensional CSI (2D-CSI) sequence that used weighted k-space sampling for optimal sensitivity and optimal time-efficiency.14,15 The parameters of this 31P-CSI acquisition were: TR = 3 sec; tip angle = 90 degrees; Rx bandwidth = ± 2 kHz; complex points = 1,024; readout duration = 256 ms; prepulses = 5; preacquisition delay = 1.75 ms; FOV = 24 × 24 cm; nominal volume = 27 cc (approximately 55 cc effective volume); sampled matrix = 8 × 8. Voxels were isotropic in two dimensions (axial: anterior-posterior and left-right) with the full width half maximum of the two-dimensional point-spread function, including the effects of filtering, being approximately 5.8 cm in diameter. Because we used a 2D-CSI approach, the slice-selective (S-I)) voxel profile is a 3-cm rectangular slab-profile. The duration of the 31P-CSI scan was 23 min (Figure 1).
Figure 1.
Sagittal and axial T1-weighted images depicting 3-cm-thick CSI slab placement and positioning of the 4 × 4 submatrix voxel-grid. A representative in vivo human brain spectrum acquired with this protocol is shown with the residual (A), original data (B) and the fitted model (C). ATP, adenosine triphosphate; DPG, diphosphoglycerides; GPCho, glycerophosphocholine; GPEtn, glycerophosphoethanolamine; MP, membrane phospholipid; PCho, phosphocholine; PEtn, phosphoethanolamine; Pi, inorganic phosphate.
Data Processing/Analysis
The 31P-CSI data were first read into a zero-padded 8 × 8 matrix and corrected by a set of scalar correction factors that correct each k-space sample for the discrepancy of defining an optimal, theoretical k-space filter with an integer number of averages for each phase-encode step. The corrected 31P-CSI data were then Fourier-transformed to spatially resolve each voxel throughout the brain. Each spatially-resolved spectrum was stored as a time-domain free-induction decay for each voxel.
Spectral Fitting
All 31P-CSI spectra were fitted with a nonlinear, iterative routine developed onsite, based on the Marquardt-Levenberg algorithim for nonlinear, least-squares fitting of complex waveforms.14 The routine incorporates the use of prior spectral knowledge such as J-coupling constants, chemical shifts, and linewidths and applies preoptimized constraints to converge on an optimal fit. With this methodology, we are able to determine peak areas in an automated fashion, thus eliminating any user interactive bias.14 The template that we used for 31P fitting at 4 T models each metabolite as a series of lorentzian and gaussian lineshapes. In our template we have modeled PCr, nucleo-side triphosphate, diphosphoglycerides (DPG), and inorganic phosphate (Pi) as lorenztians and PEtn, PCho, phosphoserine (PSer), GPEtn, GPCho, and membrane phospholipid (MP) as gaussians.
Tissue Segmentation and Image Postprocessing
Brain images were segmented into different classes, white matter, gray matter, and cerebrospinal fluid, using FMRIB's Automated Segmentation Tool (FAST).16 Results were used to determine the contributions of tissue type (gray or white matter) and cerebrospinal fluid in each MRS voxel. In this process, we convolved the mathematically-modeled, three-dimensional point-spread function (3D-PSF) from the sparse k-space sampling scheme, digitally sampled in a 256 × 256 × 64 matrix, with the co-registered binary images to obtain pixel counts of the contribution of each tissue type to each voxel based on the 3D-PSF weighted distribution.14
Statistics
The two demographic variables considered in this study were tested for significant differences between the control and PI groups by t-test (age) or χ2 statistic (sex). Linear mixed-effects models were constructed for each of the 31P metabolites measured in this study.17,18 A minimal model identified peaks that were questionable due to contamination by muscle or other artifact. Voxels were rejected if the studentized residual from the minimal model for each voxel was > 3 or < -3.
The full model used for data analysis included a random effect of subject and a fixed effect of total phosphorous signal-metabolite of interest, as in the minimal model, with additional fixed effects of diagnosis, partial volume (gray matter + white matter) and tissue type (gray matter – white matter).19–21 Interaction terms were added for partial volume by diagnosis and tissue type by diagnosis because we hypothesized metabolite differences between tissue types (gray matter and white matter) in patients with insomnia and normal control patients. Age and sex were added to the model as covariates if they were predictors of metabolite levels at the alpha = 0.05 significance level.
Our measures of interest included metabolite differences in total tissue between patients with insomnia and control patients, which we defined as the mean difference in the metabolite of interest in a voxel composed of equal amounts of gray matter and white matter, and metabolite differences in gray matter and white matter between patients with insomnia and control patients. For each metabolite, we tested for a difference in mean level between patients with PI and control patients (a main effect of PI) and a difference in association with PI between gray matter and white matter (a tissue type by PI interaction). To aid in interpretation, we further estimated mean metabolite concentrations in the gray matter component and the white matter component of voxels of mixed composition from the model.18
Bonferroni corrections were applied separately for phospholipid metabolites (PCho, PEtn, GPCho), and GPEtn) and energetic metabolites (bNTP, PCr). Phospholipid P values were multiplied by four to account for our choice of four outcomes in the phospholipid category, whereas bNTP and PCr P values were multiplied by two to account for two outcomes in the energetic category. A resulting corrected P value less than 0.05 was required for significance. Trends toward statistical significance were noted for a corrected P value less than 0.10. All metabolite levels are reported as least squares mean ± corrected 95% confidence interval. Linear mixed effects models were fitted with the restricted maximum likelihood method implemented by JMP release 7 (SAS Institute, Cary, NC).
Additional linear mixed effects models were constructed within the PI group to test the hypothesis that metabolite concentration varied as a function of WASO as determined by PSG. Models were constructed as previously discussed with the exception that the fixed effect of WASO within the PI group was substituted for the fixed effect of PI. Multiple comparison corrections were applied as noted previously.
RESULTS
Control patients and patients with PI were not significantly different in age (t23 = 0.18; P = 0.86) or sex (χ12 = 0.126; P = 0.72). We observed significant fits for our statistical models for all of the metabolites examined in this study (Table 3). Age and sex were both found to be significant predictors of PCho, whereas sex alone was found to be a significant predictor of GPCho (P < 0.05). Age and sex were not found to be signifi-cant predictors of any of the other metabolites examined in this study (P > 0.20).
Table 3.
Model goodness-of-fit measures and results from comparison of total tissue (50% gray matter, 50% white matter)a
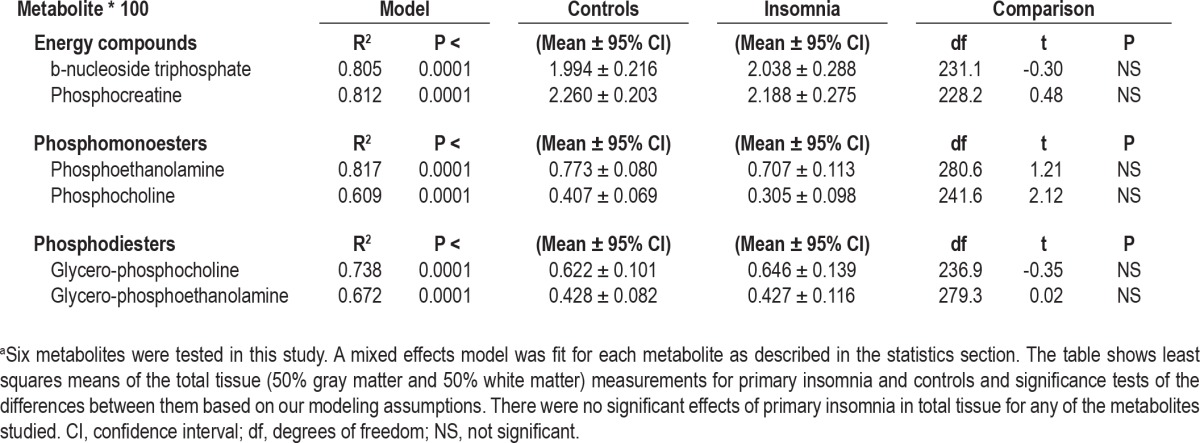
None of the metabolites examined in this study showed a significant difference between normal control patients and patients with PI in partial volume corrected total tissue (Table 3). However, when we assessed energy metabolism and membrane precursors (PCho; PEtn) and metabolites (GPCho; GPEtn) separately in gray and white matter, significant differences emerged in these two tissue compartments. PCr showed a significant difference in its association with PI between gray matter and white matter (tissue type by diagnosis interaction: t462 = 3.46; P = 0.0006). Examination of the least squares means of gray matter voxels revealed, in accordance with our hypothesis, a mean reduction of gray matter PCr in PI (Figure 2) equal to 29% of the control mean. We also saw an unexpected mean elevation of white matter PCr in patients with PI equal to 26% of the control mean (t267 = -3.72; P < 0.001). There was no observed difference in the association with PI between gray matter and white matter for bNTP (t464 = 1.23; not significant).
Figure 2.
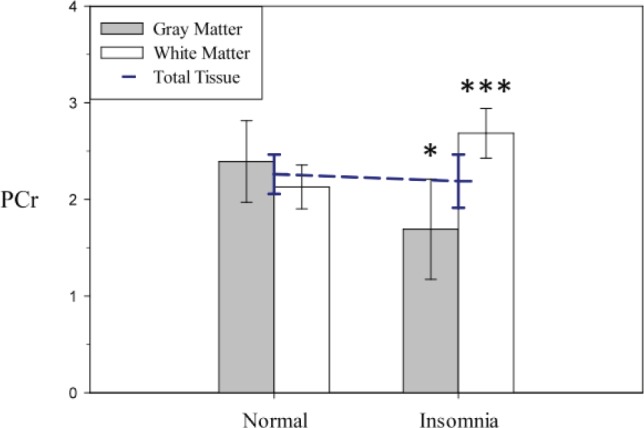
Model derived least squares means of phosphocreatine (PCr) area under the curve (error bars represent 95% confidence interval adjusted for multiple comparisons) extracted from theoretical voxels composed of 100% gray matter and 100% white matter. PCr showed a significant difference in association with insomnia between gray matter and white matter, in accordance with our hypothesis, when compared with change in white matter in patients with primary insomnia versus control patients (t462 = 3.46; P = 0.0006). Post hoc analysis revealed significantly lower PCr in patients with primary insomnia compared with control patients in gray matter (t463 = 2.35; P = 0.04) and significantly higher PCr in patients with primary insomnia when compared with control patients in white matter (t267 = -3.72; P = 0.0005). The dashed line in blue is the total tissue effect in the model, and is depicted for comparison purposes.
Cell membrane precursors and metabolites also showed significant differences in the association with PI between gray matter and white matter. PCho tissue type analysis revealed a difference (tissue type by diagnosis interaction: t457 = -2.58; P = 0.04) largely accounted for by a reduction in white matter PCho in the PI group. The mean white matter PCho in the PI group was reduced by 65% of the control mean, whereas mean gray matter PCho was 20% higher in the PI group compared with the control mean, a difference that did not reach statistical significance (Figure 3). PEtn showed no significant differences between the participant groups (t169 = 1.20; not significant) in a tissue type by diagnosis interaction.
Figure 3.
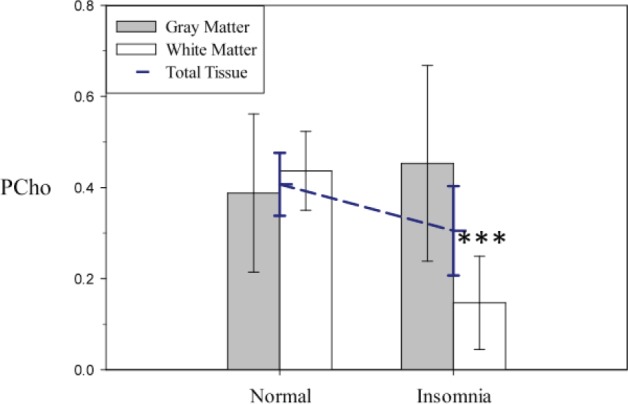
Model-derived least squares means of phosphocholine (PCho) area under the curve adjusted for age and sex (error bars represent 95% confidence intervals adjusted for multiple comparisons) extracted from theoretical voxels composed of 100% gray matter and 100% white matter. Analysis of the PCho total tissue reduction in patients with primary insomnia revealed a significant difference in association with primary insomnia between gray matter and white matter (t457 = -2.58; P = 0.04). Post hoc testing indicates that the observed reduction in PCho is occurring primarily in white matter (t437 = 5.66; P < 0.0001) as opposed to gray matter (t403 = -0.59; not significant). The dashed blue line is the total tissue main effect of the model (Table 1), which is depicted for comparison purposes.
GPCho showed a statistical trend toward differential alteration in gray matter versus white matter between patients with PI and control patients (tissue type by diagnosis interaction: t471 = -2.32; P = 0.08). This difference was largely accounted for by a mean reduction in white matter GPCho in the PI group equivalent to 23% of the normal control mean (Figure 4). GPEtn did not show any significant differences in the association with insomnia between gray matter and white matter (t467.0 = -1.54; not significant).
Figure 4.
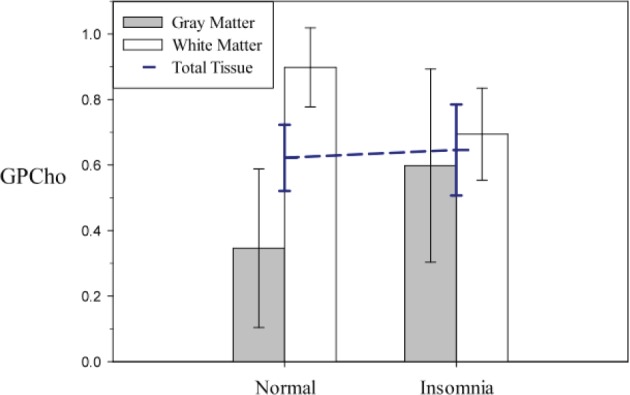
Model-derived least squares means of glycerophosphocholine (GPCho) area under the curve adjusted for sex (error bars represent 95% confidence interval adjusted for multiple comparisons) extracted from theoretical voxels composed of 100% gray matter and 100% white matter. GPCho showed a statistical trend toward a different association with insomnia between gray matter and white matter (t471 = -2.32; P = 0.08). Patients with insomnia had lower GPCho in white matter and higher GPCho in gray matter, though only the white matter change was significant in post hoc testing (t454 = 2.83; P = 0.02, white matter: t414 = -1.65; not significant, gray matter). The dashed blue line is the total tissue main effect of the model (Table 1), which is depicted for comparison purposes.
In patients with PI, WASO was not associated with PCr levels either as a main effect or when compared between tissue types. However, WASO showed a trend toward an association with bNTP, especially in gray matter where increased WASO was associated with decreased bNTP (t215 = -2.09; P = 0.07). Sex-adjusted WASO was also significantly associated with PCho reduction in total tissue (t136 = -2.72; P = 0.014). This reduction was significantly more prominent in white matter (t216 = -3.94; P < 0.001) than in gray matter (t216 = -0.82; not significant). Sex- and age-adjusted WASO did not significantly associate with GPCho either in total tissue (t92 = 0.92; not significant) or differentially based on tissue type (t196 = 0.30; not significant).
DISCUSSION
This study provides evidence of abnormalities in both bioenergetics and membrane dynamics in patients with PI compared with healthy-sleeping control patients. Our finding of reduced PCr in gray matter suggests that there is greater energy demand in PI. The decrease of PCho and GPCho in white matter in PI suggests that membrane homeostasis in PI could also be affected. Further supporting the validity of these two findings is the trend for an association between both reduced bNTP in gray matter and white matter PCho with greater WASO in the PI group, suggesting a severity-dependent effect of PI on these metabolites.
The interpretation of these differences in relative concentrations of metabolites between PI and healthy sleepers requires an understanding of the features of ATP production and regulation as well as cell membrane synthesis and degradation in the brain. In the brain, most ATP is generated in the mitochondria via oxidative phosphorylation, with a much smaller amount generated by glycolysis.22 The brain has evolved an intricate system to keep energy supplies, in the form of ATP and other nucleoside triphosphates, constant under different energy requirements or loads, especially at sites distal from the mitochondria. The most important of these mechanisms is the creatine kinase equilibrium, which transfers the high-energy phosphate of PCr to adenosine diphosphate (ADP), yielding ATP. Therefore PCr acts as a high energy phosphate reservoir for ATP and the equilibrium of this reaction is such that, under increasing load, PCr is depleted to a greater extent than ATP.23,24 PCr also shuttles high-energy phosphate from the mitochondria to ADP in the cytosol, suggesting that the main mode of energy distribution from mitochondria in the cell is through PCr.25
Reduced gray matter PCr, as observed in this study, can be interpreted to indicate an increased use or demand for cellular energy, consistent with the hyperarousal model of insomnia. Similar decrements in PCr have been observed in experiments using evoked seizures where the PCr level decreases in response to increased demand on ATP.23,24 This interpretation is further bolstered by the trend toward a reduction in the bNTP resonance, as a function of increased WASO in the gray matter of patients with PI. Notably, we also found a significant increase (26%) in PCr in white matter in patients with PI compared with that in control patients. From experiments on glucose uptake, white matter is known to have a much lower energy demand than gray matter.26 Therefore, the increase in PCr in white matter may represent an adaptation to increased energy demand in gray matter.
However, reduced gray matter PCr could also be the result of impaired oxidative phosphorylation due to mitochondrial dysfunction, a change in creatine kinase equilibrium dynamics or the effect of some third, unspecified effector that is preventing the formation of PCr. These explanations would suggest that neurodegenerative phenomena are occurring in PI, and that idea is supported by the observation that gray matter is reduced in patients with chronic insomnia.27 However, these are less likely explanations for several reasons. Under fully aerobic conditions, there is a direct relationship between energy consumption in the brain, measured by glucose or oxygen utilization and ATP production through oxidative phosphorylation.22 Given that patients with PI have severity-dependent increases in cerebral glucose metabolism,6 this finding would suggest that oxidative phosphorylation does not decrease in PI as it would if mitochondrial dysfunction were the cause of the gray matter PCr deficit. Further, a change in creatine kinase dynamics would likely produce a decrease in gray matter ATP without necessarily producing a decrease in PCr, as replenishment of high-energy phosphates in nucleotide compounds would be impaired. Therefore, the most likely explanation for the reduction in gray matter PCr observed in this study is a change in load or energy demand. This increased demand due to hyperarousal would also be consistent with both a rise in cerebral glucose metabolism and a reduction in gamma-aminobutyric acid as a function of PI disease severity.6,7
Reduction in energetic capacity, either evidenced by reduced PCr or reduced bNTP, has also been observed in both unipolar18,28,29 and bipolar30,31 depression. Insomnia is both a risk factor for incident depression32–34 and is a common symptom of major depressive disorder (MDD).35 Given the decreased PCr and bNTP in both MDD and PI, this phenotypic similarity might imply a common underlying pathophysiology in the two disorders. However, results of glucose utilization studies using positron emission tomography also indicate that the production of usable energy, in the form of high-energy phosphates from mitochondrial oxidative phosphorylation, is lowered in MDD in many brain areas, but elevated in PI throughout the day,5,6,36–38 indicating that the mechanisms of bioenergetic pathology in these two disorders are distinct.
Our hypothesis that cell membrane turnover would be increased in the insomnia group was not supported, and indeed the evidence strongly suggests the opposite, that cell membrane turnover, particularly that of phosphatidylcholine, is dramatically reduced. Membranes are maintained in a homeostatic equilibrium between synthesis and degradation by the action of a calcium independent phospholipase A2 (iPLA2).39–42 Increasing membrane precursor availability, either by adding exogenous precursor or by increasing the efficiency of the cytidine diphosphate: phosphocholine citydyltransferase has been noted to increase the quantity of GPCho or GPEtn in cell culture39 and healthy tissue.40,43
PCho was lower in the white matter of the PI group than in the control group, where PCho was reduced by 65% of the normal control means. The magnitude of the difference, while quite large, is within range of that seen in a study of chronic schizophrenia.13 Although one interpretation of this finding by itself would be that PCho is depleted due to high turnover, we also found that GPCho was reduced in white matter, demonstrating that both the anabolic precursor and catabolic metabolite of phosphatidylcholine was reduced in PI and that this apparent slowing of turnover occurs in an intensity-dependent fashion (i.e., is correlated with WASO).
These results need to be interpreted carefully in light of several important technical and statistical limitations of the study design, which could affect the generalizability of the results. First, the use of 2D- CSI is not an optimal method for voxel tissue sampling due to the chemical-shift displacement artifact inherent in frequency-selective techniques. The 31P-MRS spectrum is broad, approximately 23 ppm from PEtn to b-NTP. At 4 T, this equates to approximately 1,600 Hz, which introduces a considerable spatial displacement of the 3-cm-thick excited slab between metabolites, with this displacement increasing the further off-resonance each metabolite is from PCr. A quantitative analysis of the effect of this artifact revealed a margin of error in our gray matter and white matter tissue fractions of approximately 13% and 3% for beta-nucleoside triphosphate (bNTP) and PEtn respectively, with bNTP and PEtn representing the most off-resonance metabolites on either side of PCr. Our regression models did not account for this source of measurement error.
Our 2D-CSI slab placement also determined our sampling region. Areas within the sampling region included portions of the thalamus, globus-pallidus, putamen, caudate, temporal cortex, posterior cingulate, and parietal cortex. However, the sampling region did not include areas often assessed in single-voxel MRS, including anterior cingulate, occipital, and frontal cortices and the hippocampus. In future work, three-dimensional (3D)-CSI should be used to broaden the sampling region and negate any displacement artifact, because off-resonance effects are negligible with the phase-encoding method used in 3D-CSI.14
Another important limitation involves the nature of sampling of gray and white matter used in this study. Due to the placement of our sampling grid, which is biased toward the center of the brain and based on the results of our tissue segmentation, we oversample white matter and undersample gray matter such that our mean voxel, after removal of cerebrospinal fluid, is composed of 37.5% gray matter. Our choice of a total tissue voxel containing equal amounts of gray matter and white matter (i.e., 50% gray matter) therefore reflects a compromise between the gray matter and white matter content of an average brain and our sampling results.
As a result of oversampling white matter in this study, we were able to estimate white matter changes with greater precision than gray matter changes. Therefore, differences in statistical significance between tissue types should not be equated with differences in the magnitude of change within the different tissue compartments. It is also important to note that our theoretical voxels of “pure gray” and “pure white” matter are mathematical extensions of the tissue type regression and do not represent real voxels observed in the dataset. Therefore, these theoretical voxels are meant to quantify relative contributions of tissue types in brain regions composed of both gray matter and white matter.
It is unclear if the changes we are seeing in this study are inherent to PI per se, or instead generalize to the effects of chronic sleep restriction. Studies using sleep deprivation in normal patients and making use of different diagnostic subgroups of patients with insomnia could assist in the interpretation of findings from this study. Nevertheless, these findings of reduced PCr lend further support to growing evidence of bioenergetic, neurochemical, neurophysiologic, endocrine, and cognitive hyperarousal in PI.
DISCLOSURE STATEMENT
This study was partially supported by a research grant from Sepracor Inc. Dr. Buxton has received research support from Sepracor, Inc. and research materials and/or equipment from Phillips Respironics. Dr. Renshaw is a consultant and equity holder in Ridge Diagnostics. Dr. Winkelman has received research support from GlaxoSmithKline. He also serves as a consultant and/or is on the advisory board of Impax Laboratories, Pfizer, Inc., UCB, Zeo Inc., and Sunovion, Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the research volunteers for their participation, and the Brigham and Women's Hospital General Clinical Research Center and the McLean Hospital Brain Imaging Center technical staff for their assistance with data collection. This work was supported by The Frank Gillis Fund, The Florence Petrlik Charitable Foundation, The American Sleep Medicine Foundation, a research grant from Sepracor Inc., GCRC grant M01-RR02635, and NIH grants MH58681 and AG20654. The work was performed at McLean Hospital and Brigham and Women's Hospital. There are no conflicts of interest for any of the authors of this work.
SUPPLEMENTAL MATERIAL
A point of confusion can arise between the biochemical and the spectroscopic nomenclature for adenosine triphosphate (ATP). (A) ATP is composed of an adenosine molecule, R and three high-energy phosphate groups referred to as α, β, and γ according to the distance from adenosine. Adenosine diphosphate (ADP) possesses two high-energy phosphates referred to as α and β. Therefore, in this construct, the phosphate group unique to ATP is the γ group. (B) Spectroscopic nomenclature of high-energy phosphates differs from the biochemical description of ATP because of two important properties of spectroscopic imaging. The first is that quantification of triphosphate molecules with purine or pyrimidine (R) molecules other than adenosine (cytidine, guanosine, uridine) occur; therefore the triphosphate resonances are referred to as deriving from nucleoside triphosphate (NTP). Second, in 31P spectroscopy, chemical shift of each phosphate is determined by the different groups around each individual phosphate with the α phosphate (green) being bound by an R group and a phosphate group, the γ phosphate (blue) being bound by a phosphate group and a hydroxyl group, and the β phosphate (red) being bounded by 2 phosphate groups. Nucleoside diphosphate (NDP) possesses two phosphate groups. The first (green) is similar to the α phosphate in NTP being bound by an R group and a phosphate group. The second (blue) is similar to γ in NTP being bound by a phosphate group and a hydroxyl group. The chemical shift of the β phosphate therefore is unique to NTP and the resonance is quantified as representative of the level of NTP without contamination from NDP.
REFERENCES
- 1.Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417–23. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 3.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psycho-physiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 4.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 6.Nofzinger EA, Nissen C, Germain A, et al. Regional cerebral metabolic correlates of WASO during NREM sleep in insomnia. J Clin Sleep Med. 2006;2:316–22. [PubMed] [Google Scholar]
- 7.Winkelman JW, Buxton OM, Jensen JE, et al. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS) Sleep. 2008;31:1499–506. doi: 10.1093/sleep/31.11.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renshaw PF, Parow AM, Hirashima F, et al. Multinuclear magnetic resonance spectroscopy studies of brain purines in major depression. Am J Psychiatry. 2001;158:2048–55. doi: 10.1176/appi.ajp.158.12.2048. [DOI] [PubMed] [Google Scholar]
- 9.Iosifescu DV, Bolo NR, Nierenberg AA, Jensen JE, Fava M, Renshaw PF. Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biol Psychiatry. 2008;63:1127–34. doi: 10.1016/j.biopsych.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Hoang TQ, Bluml S, Dubowitz DJ, et al. Quantitative proton-decoupled 31P MRS and 1H MRS in the evaluation of Huntington's and Parkinson's diseases. Neurology. 1998;50:1033–40. doi: 10.1212/wnl.50.4.1033. [DOI] [PubMed] [Google Scholar]
- 11.Klunk WE, Xu C, Panchalingam K, McClure RJ, Pettegrew JW. Quantitative 1H and 31P MRS of PCA extracts of postmortem Alzheimer's disease brain. Neurobiol Aging. 1996;17:349–57. doi: 10.1016/0197-4580(96)00035-8. [DOI] [PubMed] [Google Scholar]
- 12.Kato T, Shioiri T, Murashita J, et al. Lateralized abnormality of high energy phosphate metabolism in the frontal lobes of patients with bipolar disorder detected by phase-encoded 31P-MRS. Psychol Med. 1995;25:557–66. doi: 10.1017/s003329170003347x. [DOI] [PubMed] [Google Scholar]
- 13.Jensen JE, Al-Semaan YM, Williamson PC, et al. Region-specific changes in phospholipid metabolism in chronic, medicated schizophrenia: (31) P-MRS study at 4.0 Tesla. Br J Psychiatry. 2002;180:39–44. doi: 10.1192/bjp.180.1.39. [DOI] [PubMed] [Google Scholar]
- 14.Jensen JE, Drost DJ, Menon RS, Williamson PC. In vivo brain (31)PMRS: measuring the phospholipid resonances at 4 Tesla from small voxels. NMR Biomed. 2002;15:338–47. doi: 10.1002/nbm.776. [DOI] [PubMed] [Google Scholar]
- 15.Jensen JE, Daniels M, Haws C, et al. Triacetyluridine (TAU) decreases depressive symptoms and increases brain pH in bipolar patients. Exp Clin Psychopharmacol. 2008;16:199–206. doi: 10.1037/1064-1297.16.3.199. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 17.Venables WN, Ripley BD. Modern applied statistics with S. 4 ed. New York: Springer; 2002. [Google Scholar]
- 18.Forester BP, Harper DG, Jensen JE, et al. 31Phosphorus magnetic resonance spectroscopy study of tissue specific changes in high energy phosphates before and after sertraline treatment of geriatric depression. Int J Geriatr Psychiatry. 2009;24:788–97. doi: 10.1002/gps.2230. [DOI] [PubMed] [Google Scholar]
- 19.Doyle TJ, Bedell BJ, Narayana PA. Relative concentrations of proton MR visible neurochemicals in gray and white matter in human brain. Magn Reson Med. 1995;33:755–9. doi: 10.1002/mrm.1910330603. [DOI] [PubMed] [Google Scholar]
- 20.Mason GF, Chu WJ, Vaughan JT, et al. Evaluation of 31P metabolite differences in human cerebral gray and white matter. Magn Reson Med. 1998;39:346–53. doi: 10.1002/mrm.1910390303. [DOI] [PubMed] [Google Scholar]
- 21.Hetherington HP, Spencer DD, Vaughan JT, Pan JW. Quantitative (31)P spectroscopic imaging of human brain at 4 Tesla: assessment of gray and white matter differences of phosphocreatine and ATP. Magn Reson Med. 2001;45:46–52. doi: 10.1002/1522-2594(200101)45:1<46::aid-mrm1008>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 22.Erecinska M, Silver IA. ATP and brain function. J Cereb Blood Flow Metab. 1989;9:2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- 23.King LJ, Lowry OH, Passonneau JV, Venson V. Effects of convulsants on energy reserves in the cerebral cortex. J Neurochem. 1967;14:599–611. doi: 10.1111/j.1471-4159.1967.tb09563.x. [DOI] [PubMed] [Google Scholar]
- 24.Blennow G, Folbergrova J, Nilsson B, Siesjo BK. Cerebral metabolic and circulatory changes in the rat during sustained seizures induced by DL-homocysteine. Brain Res. 1979;179:129–46. doi: 10.1016/0006-8993(79)90497-9. [DOI] [PubMed] [Google Scholar]
- 25.Meyer RA, Sweeney HL, Kushmerick MJ. A simple analysis of the “phosphocreatine shuttle”. Am J Physiol. 1984;246:C365–77. doi: 10.1152/ajpcell.1984.246.5.C365. [DOI] [PubMed] [Google Scholar]
- 26.Sokoloff L, Reivich M, Kennedy C, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 27.Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Some-ren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67:182–5. doi: 10.1016/j.biopsych.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Moore CM, Christensen JD, Lafer B, Fava M, Renshaw PF. Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: a phosphorous-31 magnetic resonance spectroscopy study. Am J Psychiatry. 1997;154:116–8. doi: 10.1176/ajp.154.1.116. [DOI] [PubMed] [Google Scholar]
- 29.Volz HP, Rzanny R, Riehemann S, et al. 31P magnetic resonance spectroscopy in the frontal lobe of major depressed patients. Eur Arch Psychiatry Clin Neurosci. 1998;248:289–95. doi: 10.1007/s004060050052. [DOI] [PubMed] [Google Scholar]
- 30.Kato T, Takahashi S, Shioiri T, Inubushi T. Brain phosphorous metabolism in depressive disorders detected by phosphorus-31 magnetic resonance spectroscopy. J Affect Disord. 1992;26:223–30. doi: 10.1016/0165-0327(92)90099-r. [DOI] [PubMed] [Google Scholar]
- 31.Kato T, Takahashi S, Shioiri T, Murashita J, Hamakawa H, Inubushi T. Reduction of brain phosphocreatine in bipolar II disorder detected by phosphorus-31 magnetic resonance spectroscopy. J Affect Disord. 1994;31:125–33. doi: 10.1016/0165-0327(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 32.Turek FW. Insomnia and depression: if it looks and walks like a duck. Sleep. 2005;28:1362–3. [PubMed] [Google Scholar]
- 33.Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76:255–9. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 34.Riemann D, Workshop P. Does effective management of sleep disorders reduce depressive symptoms and the risk of depression? Drugs. 2009;69:43–64. doi: 10.2165/11531130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28:1457–64. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]
- 36.Biver F, Goldman S, Delvenne V, et al. Frontal and parietal metabolic disturbances in unipolar depression. Biol Psychiatry. 1994;36:381–8. doi: 10.1016/0006-3223(94)91213-0. [DOI] [PubMed] [Google Scholar]
- 37.Kimbrell TA, Ketter TA, George MS, et al. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry. 2002;51:237–52. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- 38.Hosokawa T, Momose T, Kasai K. Brain glucose metabolism difference between bipolar and unipolar mood disorders in depressed and euthymic states. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:243–50. doi: 10.1016/j.pnpbp.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Morash SC, Cook HW, Spence MW. Phosphatidylcholine metabolism in cultured cells: catabolism via glycerophosphocholine. Biochim Biophys Acta. 1988;961:194–202. doi: 10.1016/0005-2760(88)90114-2. [DOI] [PubMed] [Google Scholar]
- 40.Baburina I, Jackowski S. Cellular responses to excess phospholipid. J Biol Chem. 1999;274:9400–8. doi: 10.1074/jbc.274.14.9400. [DOI] [PubMed] [Google Scholar]
- 41.Balsinde J, Balboa MA. Cellular regulation and proposed biological functions of group VIA calcium-independent phospholipase A2 in activated cells. Cell Signal. 2005;17:1052–62. doi: 10.1016/j.cellsig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Barbour SE, Kapur A, Deal CL. Regulation of phosphatidylcholine homeostasis by calcium-independent phospholipase A2. Biochim Biophys Acta. 1999;1439:77–88. doi: 10.1016/s1388-1981(99)00078-5. [DOI] [PubMed] [Google Scholar]
- 43.Babb SM, Wald LL, Cohen BM, et al. Chronic citicoline increases phosphodiesters in the brains of healthy older subjects: an in vivo phosphorus magnetic resonance spectroscopy study. Psychopharmacology (Berl) 2002;161:248–54. doi: 10.1007/s00213-002-1045-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A point of confusion can arise between the biochemical and the spectroscopic nomenclature for adenosine triphosphate (ATP). (A) ATP is composed of an adenosine molecule, R and three high-energy phosphate groups referred to as α, β, and γ according to the distance from adenosine. Adenosine diphosphate (ADP) possesses two high-energy phosphates referred to as α and β. Therefore, in this construct, the phosphate group unique to ATP is the γ group. (B) Spectroscopic nomenclature of high-energy phosphates differs from the biochemical description of ATP because of two important properties of spectroscopic imaging. The first is that quantification of triphosphate molecules with purine or pyrimidine (R) molecules other than adenosine (cytidine, guanosine, uridine) occur; therefore the triphosphate resonances are referred to as deriving from nucleoside triphosphate (NTP). Second, in 31P spectroscopy, chemical shift of each phosphate is determined by the different groups around each individual phosphate with the α phosphate (green) being bound by an R group and a phosphate group, the γ phosphate (blue) being bound by a phosphate group and a hydroxyl group, and the β phosphate (red) being bounded by 2 phosphate groups. Nucleoside diphosphate (NDP) possesses two phosphate groups. The first (green) is similar to the α phosphate in NTP being bound by an R group and a phosphate group. The second (blue) is similar to γ in NTP being bound by a phosphate group and a hydroxyl group. The chemical shift of the β phosphate therefore is unique to NTP and the resonance is quantified as representative of the level of NTP without contamination from NDP.