Abstract
Study Objectives:
The aim of this study was to evaluate cardiopulmonary exercise performance in lean and obese patients with obstructive sleep apnea (OSA) compared with controls.
Design:
Case-control study.
Setting:
The study was carried out in Sao Paulo Sleep Institute, Sao Paulo, Brazil.
Patients and Participants:
Individuals with similar ages were allocated into groups: 22 to the lean OSA group, 36 to the lean control group, 31 to the obese OSA group, and 26 to the obese control group.
Interventions:
The participants underwent a clinical evaluation, polysomnography, a maximum limited symptom cardiopulmonary exercise test, two-dimensional transthoracic echocardiography, and spirometry.
Measurements and Results:
The apnea-hypopnea index, arousal index, lowest arterial oxygen saturation (SaO2) and time of SaO2 < 90% were different among the groups. There were differences in functional capacity based on the following variables: maximal oxygen uptake (VO2max), P < 0.01 and maximal carbon dioxide production (VCO2max), P < 0.01. The obese patients with OSA and obese controls presented significantly lower VO2max and VCO2max values. However, the respiratory exchange ratio (RER) and anaerobic threshold (AT) did not differ between groups. Peak diastolic blood pressure (BP) was higher among the obese patients with OSA but was not accompanied by changes in peak systolic BP and heart rate (HR). When multiple regression was performed, body mass index (P < 0.001) and male sex in conjunction with diabetes (P < 0.001) independently predicted VO2max (mL/kg/min).
Conclusions:
The results of this study suggest that obesity alone and sex, when associated with diabetes but not OSA, influenced exercise cardiorespiratory function.
Citation:
Rizzi CF; Cintra F; Mello-Fujita L; Rios LF; Mendonca ET; Feres MC; Tufik S; Poyares D. Does obstructive sleep apnea impair the cardiopulmonary response to exercise? SLEEP 2013;36(4):547-553.
Keywords: Exercise, functional capacity, obesity, obstructive sleep apnea
INTRODUCTION
The use of the cardiopulmonary exercise test (CPET) in individuals with obstructive sleep apnea (OSA) has been of interest in recent years. However, these studies have resulted in conflicting data.1–8 There is evidence in the literature that OSA impairs exercise capacity, as shown by diminished peak oxygen uptake (VO2), anaerobic threshold (AT), respiratory exchange ratio (RER) workload capacity, and abnormal cardiovascular responses.1–8 In most of these studies, individuals were overweight or obese. The study published by Hargens et al.,7 however, evaluated young obese men with OSA matched for age, body mass index (BMI), and central adiposity with obese men without OSA (mean age of 22 y). The authors also included a third group of normal weight individuals without OSA (control group) and found only attenuated heart rate (HR) recovery in the obese OSA group. However, their VO2, adjusted for fat-free soft-tissue mass, was similar to other groups without OSA. However, other studies showed conflicting data regarding whether OSA could reduce exercise capacity.6,8
Alonso-Fernández et al.8 evaluated 31 individuals with moderate to severe OSA and 15 controls without OSA. Individuals were overall overweight and obese. The aims of this study were to evaluate the cardiac response to exercise in adults with OSA and normal ejection fraction, and to test the hypothesis that continuous positive airway pressure (CPAP) improves the cardiac response to exercise. Although the authors did not observe differences in workload capacity, maximal oxygen uptake (VO2max), or HR between the groups, cardiac output response was lower in patients with OSA. Three months of CPAP treatment was associated with decreases in peak HR and improvements in the left ventricular systolic response during exercise.
In a study performed by Kaleth et al.,6 there were no differences in VO2 among middle-aged and overweight patients with OSA and controls. When lean patients with OSA were studied, similar exercise performance was found in comparison with lean non-OSA controls. These results together suggest that obesity is a likely predictor of diminished cardiopulmonary capacity observed in patients with OSA.9
Patients with OSA have altered cardiovascular and metabolic capacity, which partially explains their potential impairment in ventilatory and metabolic responses to exercise.10 Obesity is an important cause of impaired exercise performance. In obese subjects, the VO2 adjusted for body weight was lowered11 and the AT12 and ventilatory threshold were achieved at a lower power output.13 An additional study suggested that morbidly obese individuals had severely reduced cardiopulmonary performance, similar to those with systolic heart failure.14
In summary, previous studies have suggested that obesity and OSA alter exercise performance. Most of these studies attempted to compare metabolic or cardiac response to exercise in overweight or obese individuals with OSA to similarly over-weight/obese controls.
Therefore, it is unclear whether OSA or obesity itself impairs exercise capacity. To date, there are no published studies that have evaluated the metabolic and cardiac responses to exercise in lean and obese adults with and without OSA. We hypothesized that obesity but not OSA impairs exercise capacity. Thus, in this study, we aimed to evaluate the exercise performance of lean and obese sedentary patients with OSA compared with lean and obese non-OSA controls.
MATERIALS AND METHODS
Study Population
Fifty-three patients with a recent diagnosis of OSA from the Sleep Clinic of Sao Paulo, Brazil were included. Control individuals without OSA were selected from the community. The inclusion criteria were as follows: male or female, age between 35 and 65 y; lean with BMI > 18.5 to 24.9 kg/m2; obese with BMI > 30 and < 40 kg/m2; sedentary lifestyle for at least 6 mo (exercise activity of less than 3 days per wk for at least 30 min); the ability to undergo a treadmill exercise test and no recent hospitalization or change in drug treatment. The exclusion criteria were as follows: pulmonary disease forced expiratory volume in one second/forced vital capacity (FEV1/FVC) ratio less than 70% of predicted or New York Heart Association class III or IV heart failure, unstable angina, valvular heart disease, life-threatening arrhythmia, atrial fibrillation, left bundle branch block, uncontrolled hypertension, renal disease, neuromuscular conditions, pregnancy, and being under treatment for OSA. An apnea-hypopnea index (AHI) ≥ 10 was used as the cutoff value for OSA. The control group was composed of individuals with AHI < 5.
All of the individuals were instructed to come to the clinic and to avoid drinking coffee or smoking cigarettes on the day before the evaluation. A clinical evaluation, a 12-lead electrocardiogram (ECG), spirometry, a treadmill symptom-limited maximum CPET and bidimensional echocardiogram were performed. Nine subjects were using β-blockers (one lean patient with OSA, one lean control, four obese patients with OSA, and three obese control subjects). The β-blockers were gradually replaced by 20 to 40 mg/day of enalapril the wk before the test. The patients remained on enalapril for another wk after which the test was performed. β-blockers were reintroduced after test completion.
The study was approved by Universidade Federal de São Paulo's Ethics Committee (number 1396/06) and registered at clinicaltrials.gov under number 007686225. All of the subjects signed an informed consent form.
Polysomnography
Polysomnography was conducted in all of the patients and controls using the digital system EMBLA® (17 channels,Natus Neurology, Ontario, Canada). The following variables were monitored: electroencephalogram (four channels: C3-A2, C4-A1, O1-A2, O2-A1), electrooculogram (two channels: LOC– A2, ROC-A1), electromyogram (two channels: submentonian and anterior tibial muscles), ECG (one modified DII channel), snoring, and body position. Nasal flow was monitored using a pressure transducer. Respiratory effort was monitored with abdominal and thoracic sensors. Arterial oxygen saturation (SaO2) and pulse were recorded with a pulse oximeter (Ohmeda 3700, GE Healthcare, Finland). All of the polysomnographies were performed and scored by an experienced sleep technician following the guidelines for sleep studies15 and reviewed by a sleep medicine physician. Arousals were defined using the criteria from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association,16 and respiratory events were rated using the American Academy of Sleep Medicine Task Force criteria.17 An AHI ≥ 5 was considered the threshold for OSA.
Cardiopulmonary Exercise Test
All of the patients fasted for 2 hours before the CPET. The subjects underwent a maximum, symptom-limited CPET on a treadmill (Ergo PC 13, Micromed®, Brazil) in a quiet air-conditioned room with an average temperature of 21°C and full resuscitation facilities. During the tests, the subjects were monitored with a 12-lead ECG, a pulse oximeter (Nonin®, model 9500, Plymouth, Minnesota, USA), and a non-invasive brachial artery blood pressure sphygmomanometer; breath-by-breath measurements were taken of respiratory parameters including VO2, carbon dioxide production (VCO2), pulse oxygen, RER, metabolic equivalents, oxygen and carbon dioxide expiratory fractions, minute ventilation (MV), and ventilatory reserve (VR) using a mask (Vista CPX®, Vacumed, Ventura, CA, USA). A modified BORG scale for dyspnea and leg fatigue was analyzed. After calibration, the CPET was performed on a treadmill with a ramp protocol taking into account the age and gender sex of the subject. The anaerobic threshold was determined by the V-slope and ventilatory equivalents methods.18 RER was used to ensure maximal exercise intensity for all of the participants.
An arterial blood pressure was measured at baseline and every 3 min during exercise and at recovery (until 6 min after completion). Continuous ECG recording was performed during the test and continued for 6 min during recovery. The CPET was stopped by the attending physician if the diastolic blood pressure (BP) was > 120 mm Hg in normotensive subjects or > 140 mm Hg in hypertensive subjects; if the systolic BP was > 260 mm Hg; if there was a sustained decrease in the systolic BP of 10 mm Hg or more; if there were clinical symptoms of chest pain, syncope or near-syncope; or if there were ECG changes consistent with ischemia, complex ventricular arrhythmia, sustained atrial arrhythmia, or a second or third degree atrioventricular block.
Echocardiography
All of the subjects underwent two-dimensional transthoracic echocardiography (iE33O, Philips Electronics, Netherlands) before the CPET. The end-diastolic and end-systolic left ventricle diameters were measured from the short-axis views, and the ejection fraction was derived from these measurements. All of the echocardiograms were analyzed by experienced cardiologist staff member who was blinded to the exercise data.
Spirometry
Lung function testing was performed using a computerized spirometer (Pony FX, Cosmed®, Rome, Italy) following the procedures and predicted values recommended by the American Thoracic Society19 to exclude pulmonary disease. The lung function system was calibrated with a 3-L syringe at different flow rates at least once daily. All of the spirometric measurements were performed while the patient was in a sitting position. The FEV1, FVC, and the FEV1/FVC ratio were measured in each subject and recorded in absolute values and percent predicted. All of the measurements were performed by experienced lung function-testing technicians.
Statistical Analyses
The subjects' characteristics are presented as means and standard deviations. Shapiro-Wilk normality tests were performed for all baseline demographic characteristics. The patient and control groups were compared using one-way analysis of variance, Bonferroni post hoc tests, and chi-square tests. For the variables with abnormal distributions, the Kruskal-Wallis test was used to compare groups. A linear multiple regression model was subsequently built using VO2max (mL/kg/min) as the dependent variable, and BMI, AHI, minimum SaO2, sex, presence of OSA, diabetes, and hypertension as independent variables. P values of 0.05 or less were considered significant. The statistical analyses were performed using Statistic 7.0 software (Statsoft, Tulsa, OK, USA).
RESULTS
One hundred forty-one subjects were evaluated at the sleep clinic between January 2007 and April 2009; of these, 115 participated of the protocol. Twenty-six patients were excluded due to high blood pressure before CPET (n = 5), BMI > 40 kg/m2 (n = 2), AHI > 100 (n = 4), and AHI < 10 (n = 15). Twenty-two patients were allocated to the lean OSA group, 36 to the lean control group, 31 to the obese OSA group, and 26 to the obese control group.
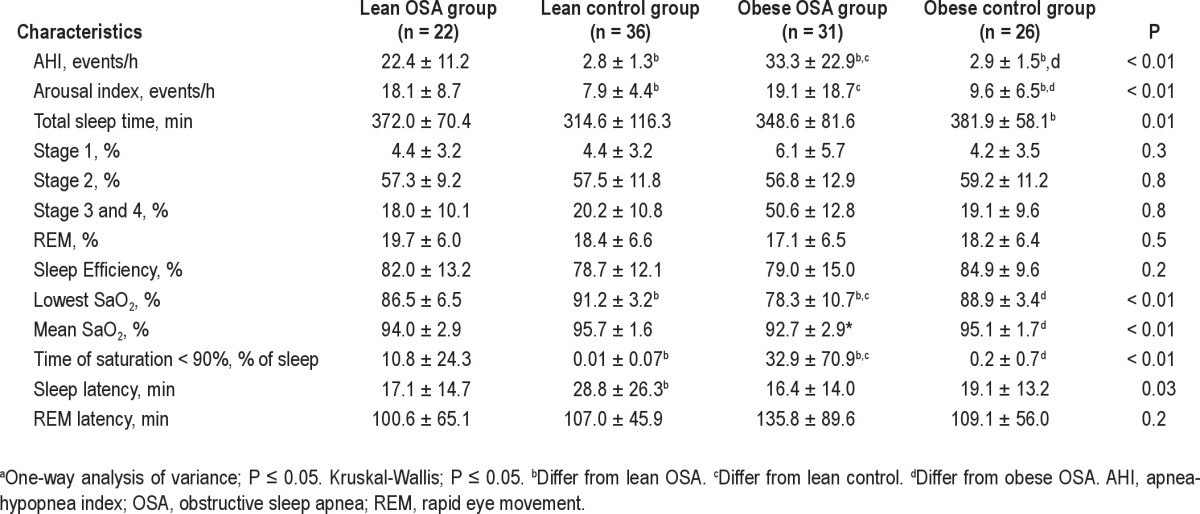
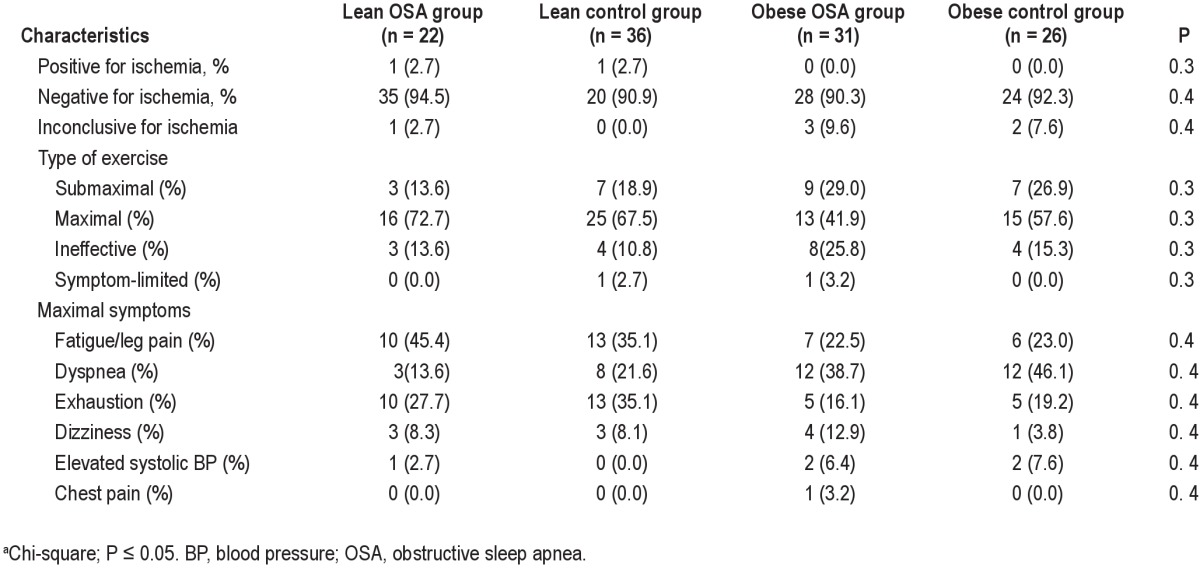
The baseline characteristics of the population are presented in Table 1. There were no differences in the mean age and sex between the groups, and as expected, there were differences in the BMI between the lean and obese groups. At baseline, the systolic BP and diastolic BP were higher in both of the obese groups, and the obese patients with OSA were more hypertensive and diabetic than the other subjects. The polysomnographic parameters showed that the obese patients with OSA had higher AHI (P < 0.01), arousal index (P < 0.01), lowest SaO2 (P < 0.01), and time of SaO2 < 90% (P < 0.01) compared with the lean OSA and control subjects, as shown in Table 2.
Table 1.
Baseline characteristics of the groupsa
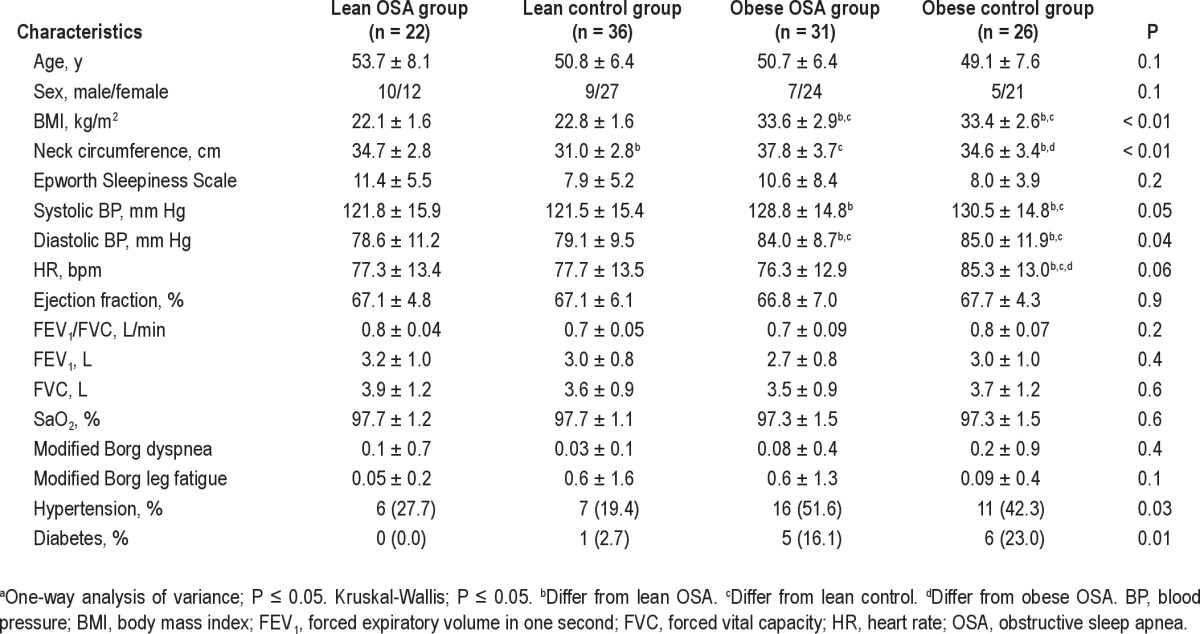
Table 2.
Polysomnographic parameters of the groupsa
The peak exercise data are listed in Table 3. There were differences in the functional capacity between the groups in terms of the VO2max (P < 0.01) and VCO2max (P < 0.01). However, RER (P = 0.6) and AT (P = 0.7) did not differ between the groups. Figure 1 shows the VO2max for the different groups. The peak diastolic BP (P = 0.01) was higher among the obese subjects. With regard to peak systolic BP and HR, the results were similar between the groups. Table 4 shows the general characteristics of the test.
Table 3.
Peak exercise data of groupsa
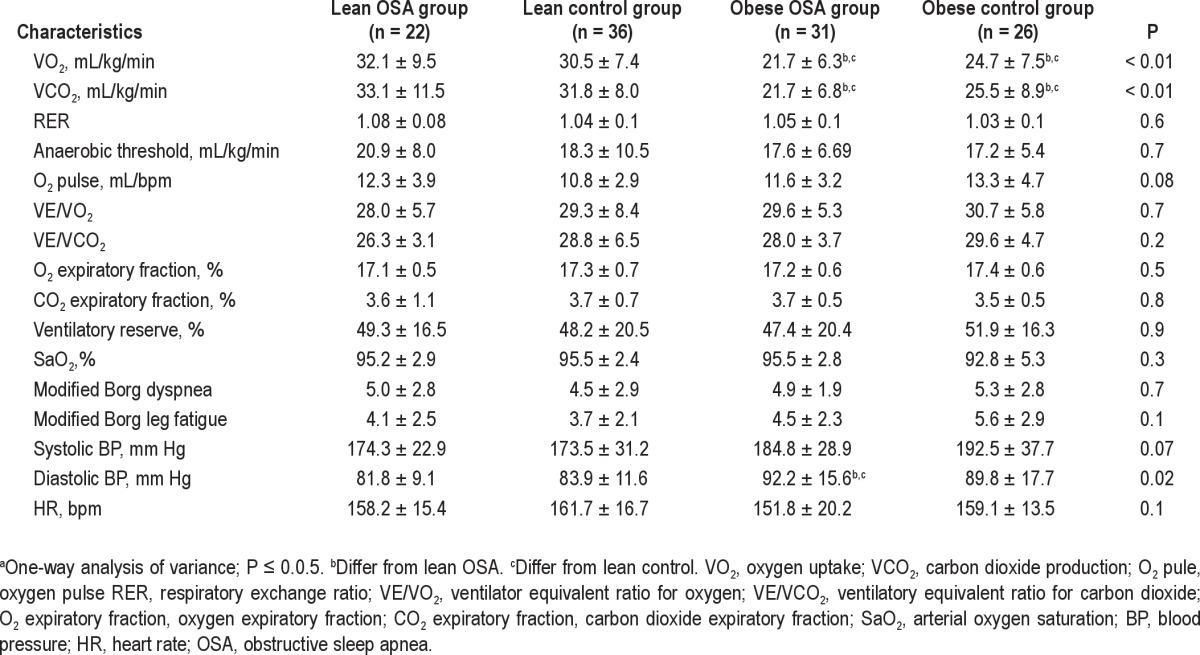
Figure 1.
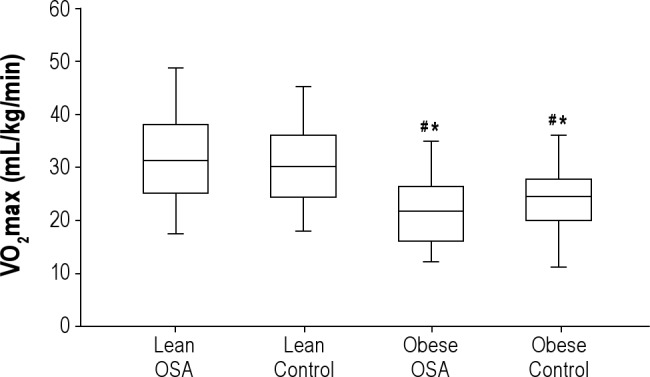
Maximal oxygen uptake (VO2max) data by groups. #Differ from lean OSA. *Differ from lean control. OSA, obstructive sleep apnea.
Table 4.
General characteristics of cardiopulmonary exercise testa
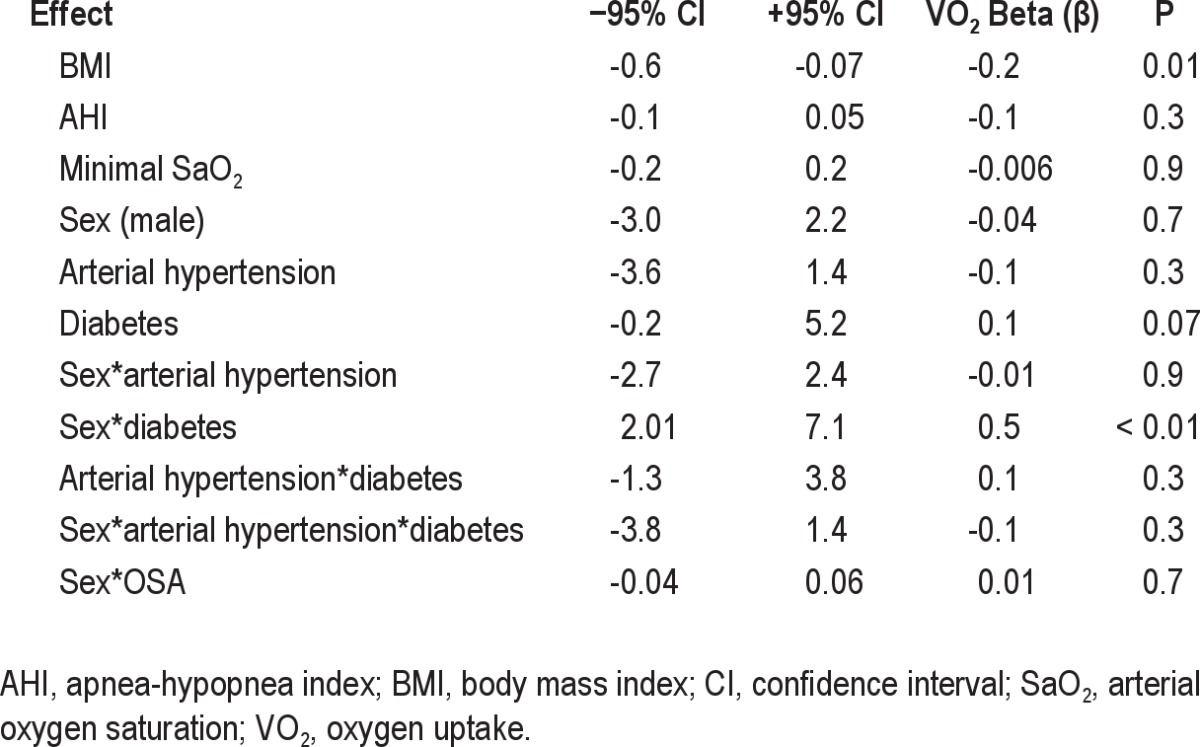
The multiple regression results demonstrated that BMI (P = 0.01) alone and male sex plus diabetes (P < 0.01) were independently associated with VO2max (mL/kg/min) (Table 5). OSA plus sex was not significant (P = 0.7).
Table 5.
Multiple regression analysis
DISCUSSION
Obesity is a confounding factor in studies that evaluate exercise capacity in patients with OSA. This is the only study, to the best of our knowledge, that examined exercise capacity in lean and obese patients with OSA compared with controls without OSA. The current study provides evidence suggesting that OSA alone does not impair functional capacity. Our major findings are that obese patients with OSA and obese controls, as well as lean patients with OSA and lean OSA control had similar exercise performance. However, both obese groups had lower exercise performance compared with both lean groups.
The association between OSA and obesity is very common, and it is considered the most important cofounding factor in clinical studies.1–8 The prevalence of obesity has risen in recent years, and consequently, a concomitant increase in OSA frequency is expected.
Previous studies have evaluated abnormal exercise capacity in patients with OSA, and most of them included overweight and obese individuals. Lin et al.1 analyzed 20 patients with OSA in an ergometer cycle and found that peak VO2 and workload are diminished compared with matched controls. Another study, which attempted to control for obesity, found a lower VO2max in morbidly obese patients with OSA compared with similar obese controls. These authors also found a significant negative correlation between VO2 and AHI in these populations.3 However, oxygen saturation and partial pressure of carbon dioxide (pCO2) values were not reported in this study, which precluded the exclusion of hypoventilation among these morbidly obese patients. Over-weight and obese patients with OSA also have worse hemodynamic response to exercise than non-OSA controls. Blunted HR recovery,6,7 delayed systolic BP recovery, and elevated diastolic BP during exercise were found in these patients.6
Despite an anomalous HR response, Hargens et al.7 found no difference in the VO2max (adjusted for fat-free tissue mass) between overweight patients with OSA and overweight non-OSA controls; however, lean controls had higher VO2 responses at submaximal and peak intensities compared with both groups. These results are similar to those found in lean patients with OSA and lean controls.9 In our previous study, OSA did not impair functional capacity in lean patients in terms of VO2, AT, and RER.9 The current findings suggest that OSA (defined by AHI) does not impair functional capacity in lean and obese subjects in terms of VO2 and VCO2 values. Although obese patients with OSA were more hypertensive than obese controls, they achieved a similar peak HR, which was lower compared with that of lean subjects. We also ensured that all of the groups had similar exercise intensity. We chose to use a treadmill to evaluate exercise because it requires more metabolic demand, cardiac stress, and ventilatory stress. In addition, walking, not cycling, is a natural and comfortable activity, even for sedentary individuals.
The influence of obesity on exercise has been recognized. Large masses of adipose tissue require high energy levels to maintain physical activity. Consequently, obese subjects cannot sustain the same exercise level as lean subjects18 The VO2 adjusted for body weight is lower in obese patients, and they seem to reach only submaximal exercise output, reflecting a low functional capacity.11 In addition, lower AT and oxygen pulse were found in the obese subjects, suggesting reduced cardiopulmonary function.12 The breathing pattern is often characterized by a higher respiratory rate and diminished tidal volume, representing an attempt to minimize the respiratory work due to adipose tissue.20
We acknowledge, however, that there are others factors beyond obesity that could also affect exercise capacity, such as daytime hypersomnolence, fatigue, altered psychological status, and cardiorespiratory comorbidities in patients with OSA.21–24 For instance, Alonso-Fernández et al.8 found that cardiac output during exercise was lower in obese patients with OSA relative to comparably obese individuals without OSA. They also reported that there was an increase in the left ventricular systolic response after 3 mo of CPAP treatment even without BMI changes. This may suggest that OSA alone is responsible for lowering the cardiac performance during exercise, without affecting either metabolic or respiratory exercise variables. We did not evaluate the left ventricular systolic response during exercise, and thus will not be able to determine if this type of response changes after effective OSA treatment, regardless of BMI status. However the lack of difference in metabolic or respiratory variables between patients with OSA and control patients in the study by Alonso-Fernández et al.8 was probably due to the characteristics of their population, obese patients with OSA and obese controls. In this sense, the inclusion of lean patients with OSA and lean controls in our study allowed us to verify the main effect of obesity upon exercise capacity.
Diabetes was more frequent among obese subjects, with higher prevalence in the OSA group. Several studies demonstrated that type I or II diabetes is associated with poor exercise performance in men and women.25–29 It seems that glycemia status, insulin resistance, and mitochondrial capacity, which facilitate oxygen transport, are related to this effect, and vascular and peripheral microcirculation injury can exacerbate this condition.25–27,29 In this study, we have neither evaluated insulin resistance nor controlled for diabetes treatment, but at the time of data collection, the obese and lean subjects had similar glycemic values. In addition, the multiple regression analysis showed that BMI alone and male sex, when associated with diabetes but not with AHI and other polysomnographic parameters, were determinants of decreased exercise capacity. The effects of sex on exercise have been reported.30–32 However, it is interesting to note that, when comorbidities such as diabetes, hypertension, obesity, AHI, and minimal oxygen saturation were controlled for, sex alone was not a significant determinant of low VO2 (mL/kg/min) in our sample. Despite the potential influence of diabetes on sex effect found in our study, we acknowledge that the low number of diabetic individuals, in particular among lean controls and lean patients with OSA, may limit any conclusion.
In summary, we found a significant difference in peak effort among obese and non obese subjects, regardless of OSA and sex. This result suggests that obesity is the major cause of poor functional capacity. In this sense, obesity remains an important clinical issue, particularly when it is associated with diabetes, not only because it increases cardiovascular risk in the OSA population but also because it impairs exercise and ventilatory capacity.
The potential limitations of this study were the lack of adjustment for fat-free tissue mass, and the predominance of females in both of the control groups, although the sex differences were not significant. This relative predominance was taken into account when the data were analyzed. Finally, we acknowledge that OSA among lean subjects was less severe than among the obese subjects, which was expected because OSA tends to be more severe in more obese individuals.33,34 However, AHI did not predict exercise capacity performance, as assessed by VO2max (mL/kg/min). In conclusion, obesity alone and sex with diabetes, but not OSA, affected exercise cardiorespiratory function.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Associaçao Fundo de Incentivo a Psicofarmacologia (AFIP), Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and Centro de Estudos em Psicobiologia e Exercício (CEPE). Dalva Poyares and Sergio Tufik are recipients of CNPQ grants, Brazil. This study was supported by Fundacao Fundo de Amparo a Pesquisa do Estado de Sao Paulo (FAPESP) e Associacao Fundo de Incentivo a Pesquisa (AFIP).
REFERENCES
- 1.Lin CC, Hsieh WY, Chou CS, Liaw SF. Cardiopulmonary exercise testing in obstructive sleep apnea syndrome. Respir Physiol Neurobiol. 2006;150:27–34. doi: 10.1016/j.resp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Tryfon S, Stanopoulos I, Dascalopoulou E, Argyropoulou P, Bouros D, Mavrofridis E. Sleep apnea syndrome and diastolic blood pressure elevation during exercise. Respiration. 2004;71:499–504. doi: 10.1159/000080635. [DOI] [PubMed] [Google Scholar]
- 3.Vanhecke TE, Franklin BA, Zalesin KC, et al. Cardiorespiratory fitness and obstructive sleep apnea syndrome in morbidly obese patients. Chest. 2008;134:539–45. doi: 10.1378/chest.08-0567. [DOI] [PubMed] [Google Scholar]
- 4.Ozturk L, Metin G, Cuhadaroglu C, Utkusavaş A, Tutluoglu B. Cardio-pulmonary responses to exercise in moderate-to-severe obstructive sleep apnea. Tuberkul oz ve Toraks Dergisi. 2005;53:10–8. [PubMed] [Google Scholar]
- 5.Przybylowski T, Bielicki P, Kumor M, et al. Exercise capacity in patients with obstructive sleep apnea syndrome. J Physiol Pharmacol. 2007;58:563–74. [PubMed] [Google Scholar]
- 6.Kaleth AS, Chittenden TW, Hawkins BJ, et al. Unique cardiopulmonary exercise test responses in overweight middle-aged adults with obstructive sleep apnea. Sleep Med. 2007;8:160–8. doi: 10.1016/j.sleep.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Hargens TA, Guill SG, Zedalis D, Gregg JM, Nickols-Richardson SM, Herbert WG. Attenuated heart rate recovery following exercise testing in overweight young men with untreated obstructive sleep apnea. Sleep. 2008;31:104–10. doi: 10.1093/sleep/31.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso-Fernández A, García-Rio F, Arias MA, et al. Obstructive sleep apnoea-hypoapnoea syndrome reversibly depresses cardiac response to exercise. Eur Heart J. 2006;27:207–15. doi: 10.1093/eurheartj/ehi621. [DOI] [PubMed] [Google Scholar]
- 9.Rizzi CF, Cintra F, Risso T, et al. Exercise capacity and obstructive sleep apnea in lean subjects. Chest. 2011;137:109–14. doi: 10.1378/chest.09-1201. [DOI] [PubMed] [Google Scholar]
- 10.Tonini J, Michallet AS, Flore P, et al. Effect of chronic intermittent hypoxia on exercise adaptations in healthy subjects. Respir Physiol Neurobiol. 2011;179:287–93. doi: 10.1016/j.resp.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Serés L, López-Ayerbe J, Coll R, et al. Cardiopulmonary function and exercise capacity in patients with morbid obesity. Rev Esp Cardiol. 2003;56:594–600. doi: 10.1016/s0300-8932(03)76921-8. [DOI] [PubMed] [Google Scholar]
- 12.Salvadori A, Fanari P, Mazza P, Agosti R, Longhini E. Work capacity and cardiopulmonary adaptation of the obese subject during exercise testing. Chest. 1992;101:674–9. doi: 10.1378/chest.101.3.674. [DOI] [PubMed] [Google Scholar]
- 13.Salvadori A, Fanari P, Tovaglieri I, et al. Ventilation and its control during incremental exercise in obesity. Respiration. 2008;75:26–33. doi: 10.1159/000097245. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher MJ, Franklin BA, Ehrman JK, et al. Comparative impact of morbid obesity vs heart failure on cardiorespiratory fitness. Chest. 2005;127:2197–203. doi: 10.1378/chest.127.6.2197. [DOI] [PubMed] [Google Scholar]
- 15.Rechtschaffen A, Kales A. Los Angeles, CA: Los Angeles Brain Information Service, Brain Information Institute; 1968. A manual of standardized terminology, technique and scoring system for sleep stages of human sleep. [Google Scholar]
- 16.EEG arousals: scoring rules and examples. A preliminary report from Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 17.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 18.ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–77. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 19.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 20.Lavie CJ, Milani RV, Mehra MR. Peak exercise oxygen pulse and prognosis in chronic heart failure. Am J Cardiol. 2004;93:588–93. doi: 10.1016/j.amjcard.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Hong S, Dimsdale JE. Physical activity and perception of energy and fatigue in obstructive sleep apnea. Med Sci Sports Exerc. 2003;35:1088–92. doi: 10.1249/01.MSS.0000074566.94791.24. [DOI] [PubMed] [Google Scholar]
- 22.Smith SS, Doyle G, Pascoe T, Douglas JA, Jorgensen G. Intention to exercise in patients with obstructive sleep apnea. J Clin Sleep Med. 2007;3:689–94. [PMC free article] [PubMed] [Google Scholar]
- 23.Basta M, Lin HM, Pejovic S, Sarrigiannidis A, Bixler E, Vgontzas AN. Lack of regular exercise, depression, and degree of apnea are predictors of excessive daytime sleepiness in patients with sleep apnea: sex differences. J Clin Sleep Med. 2008;4:19–25. [PMC free article] [PubMed] [Google Scholar]
- 24.Aguillard RN, Riedel BW, Lichstein KL, Grieve FG, Johnson CT, Noe SL. Daytime functioning in obstructive sleep apnea patients: exercise tolerance, subjective fatigue, and sleepiness. Appl Psychophysiol Biofeedback. 1998;23:207–17. doi: 10.1023/a:1022257514209. [DOI] [PubMed] [Google Scholar]
- 25.Item F, Heinzer-Schweizer S, Wyss M, et al. Mitochondrial capacity is affected by glycemic status in young untrained women with type 1 diabetes but is not impaired relative to healthy untrained women. Am J Physiol Regul Integr Comp Physiol. 2011;301:R60–6. doi: 10.1152/ajpregu.00747.2010. [DOI] [PubMed] [Google Scholar]
- 26.Larsen S, Stride N, Hey-Mogensen M, et al. Increased mitochondrial substrate sensitivity in skeletal muscle of patients with type 2 diabetes. Diabetologia. 2011;54:1427–36. doi: 10.1007/s00125-011-2098-4. [DOI] [PubMed] [Google Scholar]
- 27.Baldi JC, Cassuto NA, Foxx-Lupo WT, Wheatley CM, Snyder EM. Glycemic status affects cardiopulmonary exercise response in athletes with type I diabetes. Med Sci Sports Exerc. 2010;42:1454–9. doi: 10.1249/MSS.0b013e3181d1fdb3. [DOI] [PubMed] [Google Scholar]
- 28.Leite SA, Monk AM, Upham PA, Bergenstal RM. Low cardiorespiratory fitness in people at risk for type 2 diabetes: early marker for insulin resistance. Diabetol Metab Syndr. 2009;1:8. doi: 10.1186/1758-5996-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young JL, Pendergast DR, Steinbach J. Oxygen transport and peripheral microcirculation in long-term diabetes. Proc Soc Exp Biol Med. 1991;196:61–8. doi: 10.3181/00379727-196-43164. [DOI] [PubMed] [Google Scholar]
- 30.Fletcher GF, Froelicher VF, Hartley LH, Haskell WL, Pollock ML. Exercise standards. A statement for health professionals from the American Heart Association. Circulation. 1990;82:2286–322. doi: 10.1161/01.cir.82.6.2286. [DOI] [PubMed] [Google Scholar]
- 31.Shephard RJ. Exercise and training in women, Part II: Influence of menstrual cycle and pregnancy. Can J Appl Physiol. 2000;25:35–54. doi: 10.1139/h00-003. [DOI] [PubMed] [Google Scholar]
- 32.Cintra F, Poyares D, Rizzi CF, et al. Cardiorespiratory response to exercise in men and women with obstructive sleep apnea. Sleep Med. 2009;10:368–73. doi: 10.1016/j.sleep.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]


