Abstract
Study Objective:
To determine whether corticotropin-releasing factor (CRF) in the basolateral amygdala (BLA) modulated sleep and fear-conditioned alterations in sleep.
Design:
After 2 days of habituation to recording procedures, baseline sleep recordings were obtained. The animals were then habituated to the handling procedure necessary for microinjections over 2 consecutive days. In experiment 1, rats received microinjections of 0.5 μL antalarmin (1.61 or 4.82 mM), a CRF receptor 1 antagonist, or distilled water once a week for 3 wk. In experiment 2, rats received a microinjection of either antalarmin or vehicle prior to inescapable shock training (ST; 20 shocks; 0.8 mA, 0.5 sec; 1 min interstimulus interval). The animals were placed back in the context 7 days later for 30 min without shock (CR; context re-exposure). Sleep was recorded for 8 h after each manipulation.
Setting:
NA.
Subjects:
Outbred Wistar rats.
Interventions:
The rats were surgically implanted with electrodes for recording the electroencephalogram and electromyogram for determining arousal state and with bilateral guide cannulae directed at BLA.
Measurements and Results:
Antalarmin microinjected into BLA did not significantly alter sleep under undisturbed conditions. However, antalarmin microinjected bilaterally into BLA prior to ST blocked reductions in rapid eye movement sleep that ST normally produces. Further, the single microinjection prior to ST blocked the reduction in rapid eye movement typically seen after subsequent CR. Behavioral freezing, an indicator of fear memory, was not altered.
Conclusions:
CRF in BLA is involved in regulating stress-induced alterations in sleep and it plays a role in modulating how stressful memories influence sleep.
Citation:
Wellman LL; Yang L; Ambrozewicz MA; Machida M; Sanford LD. Basolateral amygdala and the regulation of fear-conditioned changes in sleep: role of corticotropin-releasing factor. SLEEP 2013;36(4):471-480.
Keywords: Amygdala, corticotropin-releasing factor, fear conditioning, sleep
INTRODUCTION
Contextual fear conditioning is a variant of classic conditioning in which initially neutral environments come to elicit fear responses through association with an unconditioned, fear-inducing stressor (usually inescapable foot shock).1 Subsequently, the fearful context alone elicits behavioral and physiologic responses indicative of fear and anxiety. Much of the work on contextual fear has focused on immediate responses to the fearful context including behavioral freezing, an indicator of fear memory,2–4 autonomic activation,5–7 and fear-potentiated startle.1 Foot shock stress and fearful contexts also can produce alterations in sleep that can persist for several hours even after an animal has been returned to its home cage, a safe environment. Changes in sleep after training with inescapable foot shock and reexposure to related contexts include a prominent reduction in rapid eye movement (REM) sleep that occurs in the first few hours after exposure.8–11 Nonrapid eye movement (NREM) sleep also can be altered.12–14
The basolateral amygdala (BLA) has an established, although not fully understood, role in the formation and consolidation of memories for emotional or stressful events. A variety of manipulations that damage or inactivate BLA prior to or after training15–20 or prior to context re-exposure21,22 attenuate freezing. BLA also has a role in the regulation of both NREM23 and REM,24 but its role in regulating the effects of fear on sleep has not been established.
Corticotropin-releasing factor (CRF) plays a significant role in mediating central nervous system responses to stressors25–30 and has roles in anxiety and conditioned fear. Intracerebroventricular (ICV) administration of CRF in rats produces many of the signs associated with anxiety in humans, including increased wakefulness,31–34 altered locomotor activity, and an exaggerated startle response.35,36 Both fear conditioning1 and ICV administration of CRF1,36 can enhance the amplitude of acoustic startle whereas ICV administration of the nonselective CRF antagonist α-helical CRF (α-HelCRF)9–41 blocks both CRF- and fear-potentiated startle, but does not alter lower baseline startle amplitude.36
CRF also plays a role in fear-conditioned alterations in sleep. In mice, ICV administration of CRF enhances the reduction in REM after fearful contexts associated with inescapable foot shock whereas ICV administration of the nonspecific CRF antagonist, astressin, attenuates fear-induced reductions in REM.37 In addition, we have found that microinjections of the CRF1 receptor antagonist, antalarmin (ANT), into the central nucleus of the amygdala (CNA) in rats block fear-induced reductions in REM and attenuate Fos expression, a marker of neural activation, in regions important in stress and REM regulation including the hypothalamic paraventricular nucleus, locus coeruleus, and dorsal raphe nucleus.38 However, ANT microinjected into CNA does not alter freezing in the fearful context, a finding consistent with other work demonstrating that fear behavior in waking can be dissociated from post-fear changes in sleep.39
Most of the work on CRF regulation of the amygdala has focused on CNA; however, the BLA has greater densities of CRF1 receptors40,41 and there are indications that they play a role in conditioned fear.42 Their potential role in regulating sleep has not been examined. In this study, we examined the effects of microinjections of ANT into the BLA on spontaneous sleep. We also microinjected ANT into the BLA prior to inescapable foot shock training to determine potential roles of CRF in fear acquisition, and we tested for potential effects on contextual fear in a subsequent drug-free exposure to the training context alone. In this second experiment, we examined freezing as an index of fear and examined sleep after training and after context reexposure alone. Thus, we assessed the role of CRF in the BLA in regulating spontaneous sleep and we examined the potential role of the BLA in mediating the relationship between waking fear behavior and sleep.
METHODS
Study Subjects
The 25 study subjects were 90-day-old Wistar rats obtained from Harlan (Indianapolis, IN). Upon arrival, the rats were individually housed in polycarbonate cages and given ad libitum access to food and water. The rooms were kept on a 12:00:12:00 light:dark cycle with lights on from 07:00 to 19:00 h. Light intensity during the light period was 100–110 lux and less than 1 lux during the dark period. Ambient temperature was maintained at 24.5 ± 0.5°C.
Surgery
Beginning 1 week after arrival, the rats were anesthetized with isoflurane (5% induction; 2% maintenance) and implanted with skull screw electrodes for recording the electroencephalogram (EEG) and stainless-steel wire electrodes sutured to the dorsal neck musculature for recording the electromyogram (EMG). Leads from the recording electrodes were routed to a nine-pin miniature plug that mated to one attached to a recording cable. Bilateral guide cannulae (26 ga) for microinjections into the BLA were implanted with their tips aimed 1.0 mm above BLA (2.6 posterior, 4.8 lateral, and 8.0 ventral to bregma).43 The recording plug and cannulae were affixed to the skull with dental acrylic and stainless-steel anchor screws. Ibuprofen (15 mg/kg) was made available in the water supply for relief of postoperative pain. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by Eastern Virginia Medical School's Animal Care and Use Committee (Protocol # 07-005).
Drugs
The CRF1 receptor antagonist ANT was obtained from Sigma-Aldrich, St. Louis, MO, USA. All dosages were prepared in pyrogen-free distilled water (dW) and were sonicated for 20 min to ensure that the drug was dissolved completely. A fresh solution was prepared for each experimental day.
Procedures
All experimental manipulations were conducted during the fourth hour of the light period such that sleep recording would begin at the start of the fifth hour. This resulted in 8-h of light period recording on each experimental day.
Home cages were changed at least 3 days prior to injection day. The same room was used for animal housing and sleep recording. The microinjections and behavioral testing were conducted in a room separate from that used for recording.
Sleep Recording
For recording sleep, each animal, in its home cage, was placed on a rack outfitted for electrophysiologic recording and a lightweight, shielded cable was connected to the miniature plug on the rat's head. The cable was attached to a swivel that permitted free movement of the rat within its cage. EEG and EMG signals were processed by a Grass, Model 12 polygraph equipped with model 12A5 amplifiers (West Warwick, RI) and routed to an analog-to-digital board (Eagle PC30) housed in a Pentium class personal computer. The signals were digitized at 128 Hz and collected in 10-sec epochs using a custom sleep data collection program.
The rats were allowed a postsurgery recovery period of 14 days prior to beginning the experiment. Once recovered, the animals were randomly assigned to one of three groups: injection only (n = 8) for studies of effects of ANT on undisturbed sleep or to ANT prior to shock training (ANT-ST; n = 10) or dW prior to shock training (dW-ST; n = 7) for studies of its effects on shock training and fear. All rats were habituated to the recording cable and chamber over 3 consecutive days. Then the rats were habituated to the 5-min handling procedure necessary for microinjections over 2 consecutive days and a baseline following handling (BH) was recorded.
Microinjections
For microinjections, injection cannulae (33 ga) were secured in place within the guide cannulae, projecting 1.0 mm beyond the tip of the guide cannulae for delivery of drug into the target region. The injection cannulae were connected to one end of lengths of polyethylene tubing that had the other end connected to 5.0 μL Hamilton syringes. The injection cannulae and tubing were prefilled with the solution to be injected. Once the cannulae were in place, 0.5 μL of either drug or vehicle was bilaterally infused over 3-min. The cannulae were left in place 1-min preinjection and postinjection to allow for maximal absorption of the solution.
Fear Conditioning
Each ST session lasted 30-min. During this procedure, individual rats were placed in shock chambers (Coulbourn (Street Whitehall, PA) Habitest cages equipped with grid floors (Model E10-18RF) that were housed in Coulbourn Isolation Cubicles (Model H10-23)) and were allowed to freely explore for 5-min. Over the next 20 min, they were presented with 20 foot shocks (0.8 mA, 0.5-sec duration) at 1.0-min intervals. Shock was produced by Coulbourn Precision Regulated Animal Shockers (Model E13-14) and presented via the grid floor of the shock chamber. Five min after the last shock, the rats were returned to their home cages. On the CR day, the rats were returned to the shock chambers and allowed to explore freely for 30-min (no shock presented) before being returned to their home cage. The shock chamber was thoroughly cleaned with diluted alcohol following each session. Each session was videotaped using mini video cameras (Weldex (Cypress, CA), WDH-2500BS, 3.6-mm lens) attached to the center of the ceiling of the shock chamber for subsequent visual scoring of freezing.
Experimental Design
Experiment 1
Experiment 1 assessed the effects of microinjections of ANT into the BLA on otherwise undisturbed sleep. The rats received an injection of either ANT (1.61 or 4.82 mM) or dW before being returned to the home cage for sleep recording. Injections were administered in a pseudo-randomized order at 7 day intervals over a period of 3 wk.
Experiment 2
Experiment 2 assessed the effects of microinjections of ANT into the BLA on changes in sleep induced by ST and on subsequent fear-induced alterations in sleep. On experimental day 1, animals in the ANT-ST and dW-ST groups received micro-injections of drug (4.82 mM) or dW, respectively, prior to ST. Following ST, the animals were returned to their home cage for sleep recording. Seven days later, the animals underwent CR and were then returned to their home cage for sleep recording. The animals did not receive a microinjection on the CR day.
Data Analyses
Sleep
Computerized EEG and EMG records were visually scored by trained observers blind to drug condition in 10-sec epochs to determine wakefulness, NREM, and REM. Wakefulness was scored based on the presence of low-voltage, fast EEG and high amplitude, tonic EMG levels. NREM was characterized by the presence of spindles interspersed with slow waves, lower muscle tone, and no gross body movements. REM was scored continuously during the presence of low voltage, fast EEG, theta rhythm, and muscle atonia. Data were collapsed into 4-h blocks (Block 1 and Block 2) and total 8-h light period. The following sleep parameters were examined in the data analyses: total NREM (min), total REM (min); total sleep (REM + NREM), REM% (REM/total sleep*100) and number and average duration of NREM and REM episodes (defined as contiguous 10-sec epochs of a given state).
The sleep data for Experiment 1 were analyzed with repeated-measures analyses of variance (ANOVAs). The data in Experiment 2 was analyzed with two-way mixed factors (Group (dW-ST and ANT-ST) × Treatment (days)) ANOVAs with repeated measures on Treatment. Post-hoc Tukey tests were used to determine differences among means as appropriate.
Freezing
Videotapes of the ST and CR sessions were scoring for freezing, defined as the absence of body movement except for respiration.44,45 Freezing was scored by a trained observer blind to condition in 5-sec intervals during 1.0-min observation periods over the course of the 30-min the rats were in the shock chamber. The percentage of time spent freezing was calculated (FT%: freezing time/ observed time × 100) for each animal for each observation period.
The freezing data for ST were analyzed in three periods: the 5-min preshock period, the following 20-min shock period (during which shock was experienced) and the 5-min postshock period. Freezing data for the CR day were analyzed in three 10-min blocks and compared with the preshock period on the ST day. The data for the preshock, shock, and postshock periods were analyzed with separate two-way mixed factors (Group (dW-ST and ANT-ST) × Period (Pre, Shock and Post for ST; Pre, Blocks1-3 for CR)) ANOVAs with repeated measures on Period. Post-hoc comparisons were conducted with Tukey tests. All reported sleep and freezing data were examined for and passed the normality test prior to conducting the relevant ANOVA.
RESULTS
Experiment 1
Histology
In the injection-only group, one animal had to be excluded in the middle of the study due to signal loss and another was excluded due to improperly placed cannulae. Therefore, statistical analysis was completed on six animals whose cannulae were bilaterally located in the BLA (Figure 1A).
Figure 1.
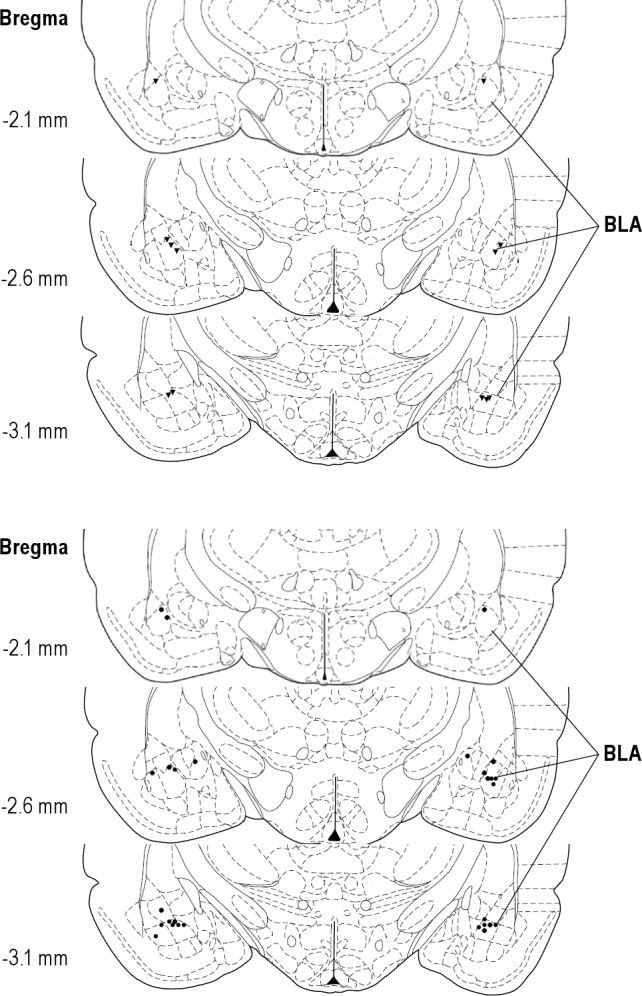
Drawing showing microinjection sites in (A) experiment 1 (n = 7) and (B) experiment 2 (n = 14; diamond: dW, distilled water control group; circle: ANT, antalarmin group). BLA, basolateral amygdala. Note: two dW treated rats had unilateral placements with one cannula in BLA and the other in the central nucleus. Because their data did not differ from rats with bilateral placements, they were retained in the analysis.
Sleep
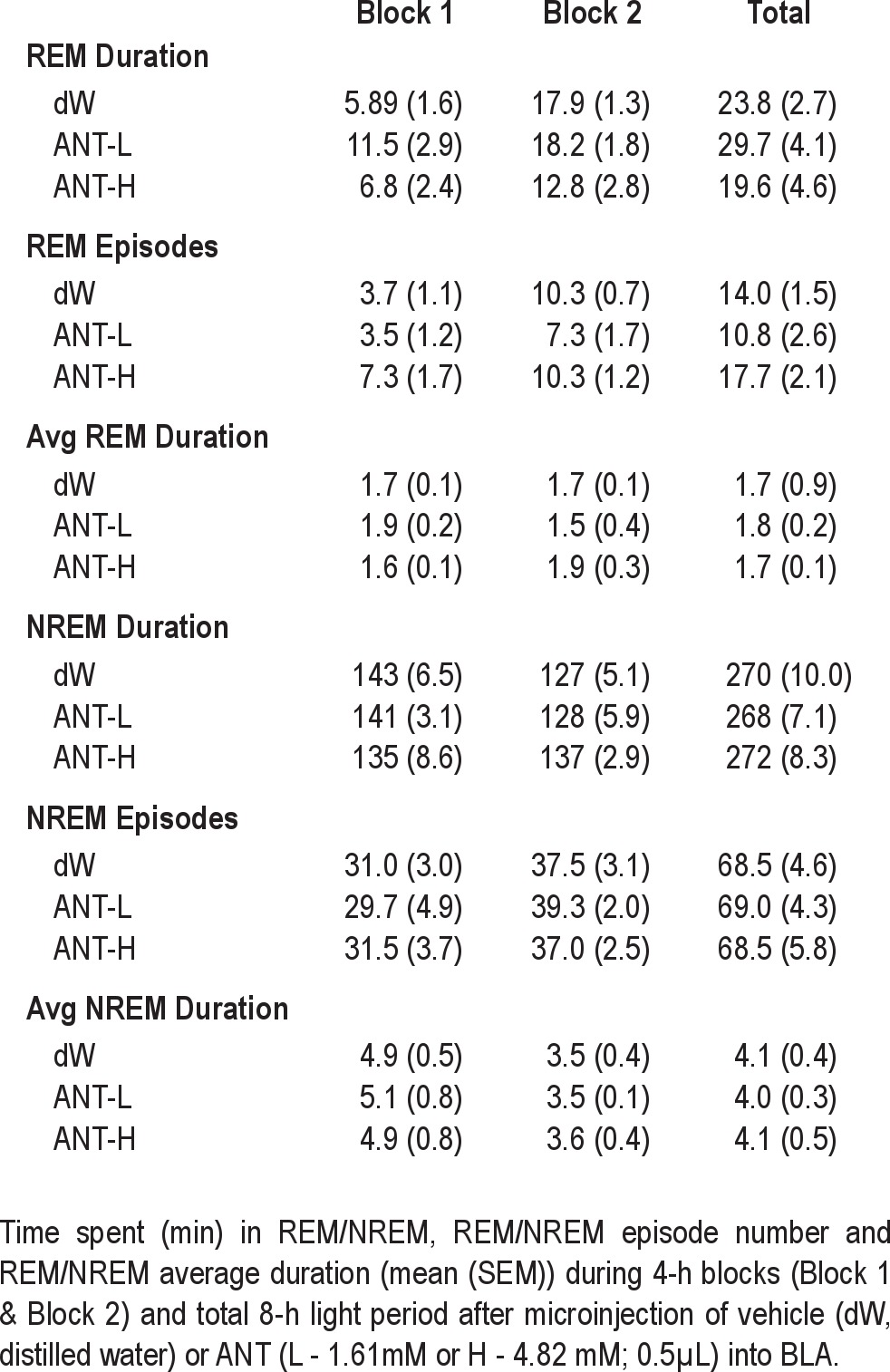
There were no significant differences between dW and either the 1.62 mM or 4.82 mM concentrations of ANT of any of the sleep measures (REM, NREM, or total sleep) we examined over the 8-h recording period (Table 1).
Table 1.
Experiment 2
Histology
In the dW-ST group, three of seven animals had one cannula located in the area between the BLA and the CNA. We ran twoway ANOVA and t-tests comparing the sleep parameters between the animals with bilateral BLA cannulae and the animals with unilateral BLA cannulae and did not find any significant differences. Therefore, all animals in the dW-ST group were used for further statistical analyses (Figure 1B).
In the ANT-ST group, three animals had one cannula located in the area between the BLA and CNA. Analyses using two-way ANOVA and t-tests showed significant differences between animals with bilateral BLA cannulae and the animals with unilateral BLA cannula. These data indicated that bilateral administration of ANT into the BLA was required to produce significant effects on REM. Therefore, data for the seven animals with bilateral BLA cannulae were used in the full statistical analyses (Figure 1B).
Freezing
On the ST day, there was a main effect of period (F2,24 = 30.8, P < 0.001). FT% was significantly increased during the shock (P < 0.001) and postshock (P < 0.001) periods compared with the preshock period (Figure 2A). However, the two groups did not differ in FT%.
Figure 2.
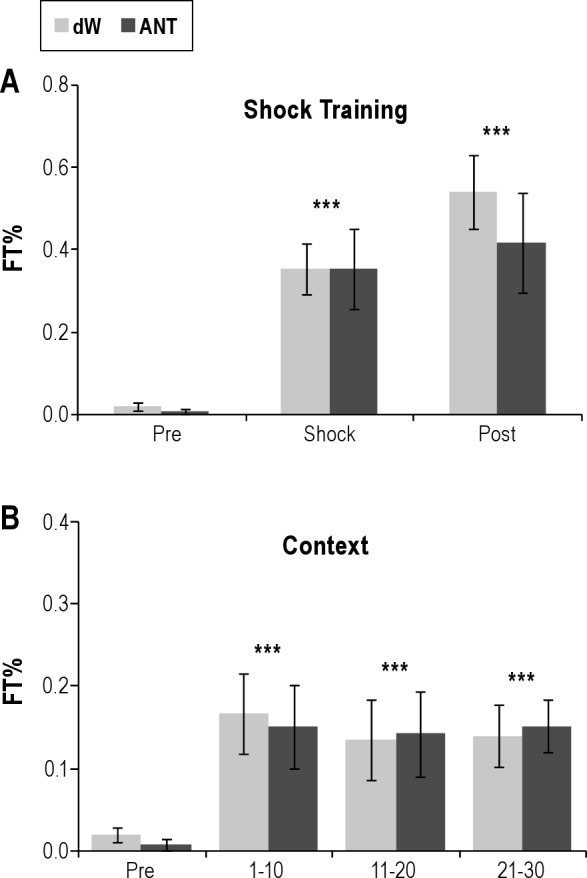
Percent time freezing (%Freezing) plotted for (A) the 5-min preshock period (Pre) when the rats were naïve, the shock period (Shock), and the postshock period (Post) and for (B) the 30-min context reexposure (CR) divided into three 10-min blocks (1-10, 11-20, and 21-30). No shocks were presented in CR. dW, distilled water; ANT, antalarmin (4.82 mM). Error bars are ± standard error of the mean. ***P < 0.001 compared with Pre.
On the CR day, there was a main effect of block for period (F3,36 = 7.1, P = < 0.001). FT% was significantly increased during each 10-min block compared with preshock values (Figure 2B). There were no significant differences in FT% between groups or across blocks on the CR day.
REM Sleep
The analysis of total REM during Block 1 was characterized by a significant Group × Treatment interaction (F2,24 = 17.8, P < 0.001). Post-hoc analyses for each recording day found no difference in sleep on BH, but REM was reduced in the dW-ST group compared with the ANT-ST group (Figure 3) on the ST day (P < 0.001) and on the CR day (P < 0.001). Comparisons within groups for the dW-ST rats (Figure 4) found significantly reduced REM during ST (P < 0.001) and CR (P < 0.002) compared with BH. REM was also significantly reduced on ST compared with CR (P < 0.032). Total REM in the ANT-ST rats did not significantly differ across recording days (Figure 5).
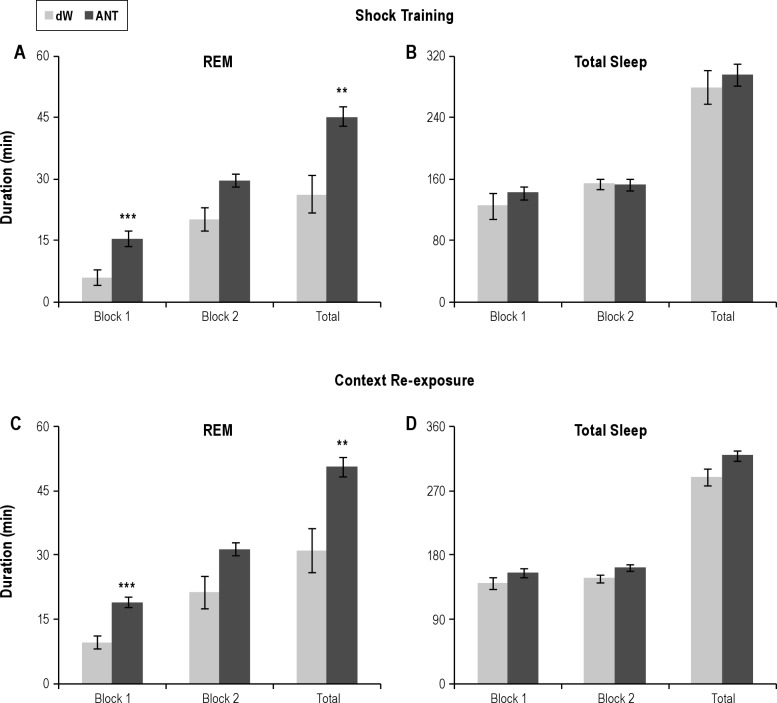
Figure 3.
Direct comparisons of total sleep amounts for dW and ANT plotted in 4-h blocks (Block 1 and Block 2) and the total 8-h light period. (A) Total REM duration during shock training. (B) Total sleep duration during shock training. (C) Total REM duration during context reexposure. (D) Total sleep duration during context reexposure. Error bars are ± standard error of the mean. **P < 0.01; and ***P < 0.001 compared with dW. ANT, antalarmin; dW, distilled water; REM, rapid eye movement.
Figure 4.
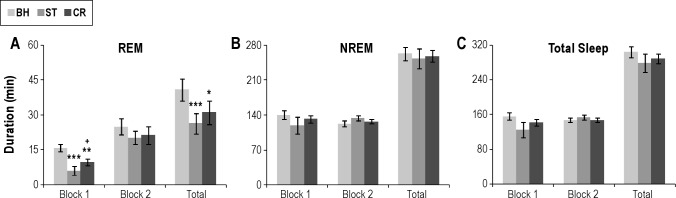
Total sleep amounts plotted in 4-h blocks (Block 1 and Block 2) and the total 8-h light period. (A) Total REM duration for handling BH, ST, and CR in the dW control group. (B) Total NREM duration for handling BH, ST, and CR in the dW group. (C) Total sleep duration for handling BH, ST, and CR in the dW group. Error bars are ± standard error of the mean. *P < 0.05; **P < 0.01; and ***P < 0.001 compared with BH. +P < 0.05 compared with ST. BH, control; CR, context reexposure; dW, distilled water; NREM, nonrapid eye movement; REM, rapid eye movement; ST, shock training.
Figure 5.
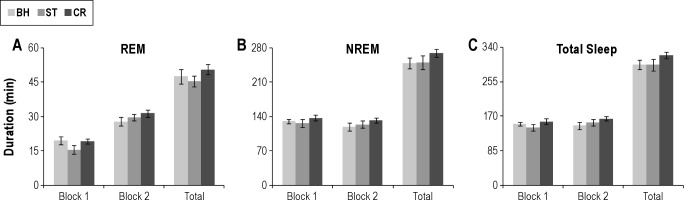
Total sleep amounts plotted in 4-h blocks (Block 1 and Block 2) and the total 8-h light period. (A) Total REM duration for handling BH, ST, and CR in the antalarmin (ANT; 4.82 mM) control group. (B) Total NREM duration for handling BH, ST, and CR in the ANT group. (C) Total sleep duration for handling BH, ST, and CR in the ANT group. Error bars are ± standard error of the mean. BH, control; CR, context reexposure; dW, distilled water; NREM, nonrapid eye movement; REM, rapid eye movement; ST, shock training.
The analysis of total REM during Block 2 was characterized by a significant Group Main Effect (F1,12 = 5.4, P < 0.04). Total REM was greater in the ANT-ST rats, but the comparison across treatment days was not significant.
The analysis of total REM for the 8-h recording period revealed a significant Group × Treatment interaction (F2,24 = 5.2, P < 0.015). Post-hoc analyses for each recording day found no difference in sleep on BH, but REM was reduced in the dW-ST group compared with the ANT-ST group on the ST day (P < 0.005) and on the CR day (P < 0.005). Comparisons within groups for the dW-ST rats found significantly reduced REM during ST (P < 0.001) and CR (P < 0.02) compared with BH. REM on ST and CR did not significantly differ. The ANT-ST rats did not significantly differ across recording days.
The analysis of REM% during Block 1 revealed significant Group (F1,12 = 14.5, P < 0.003) and Treatment (F2,24 = 10.7, P < 0.001) main effects. REM% was greater in the ANT-ST rats, but did not differ across treatment days. There was no difference in Block 2. However, when the entire 8-h recording period was considered, there were significant differences between groups and within days. The analysis for total 8-h REM% reveal a significant Group × Treatment interaction (F2,24 = 4.4, P < 0.025). REM% did not differ between groups on BH (dW = 10.3% ± 1.1%; ANT = 13.1% ± 1.1%), but was significantly greater in the ANT-ST rats after ST (P = 0.005; 11.1% ± 1.5%) and after CR (P = 0.015; 12.2% ± 0.5%) than the dW rats (ST = 4.3% ± 1.4% and CR = 7.1% ± 1.1%). REM% was decreased in the dW-ST rats on ST (P < 0.001) and CR (P < 0.008) compared with BH, whereas the ANT-ST rats did not differ across days.
The analysis of REM episodes (Table 2) during Block 1 was characterized by a significant Group × Treatment interaction (F2,24 = 3.628, P = 0.042). Post-hoc analyses for each recording day found no difference in sleep on BH, but REM episodes were reduced in the dW-ST group compared with the ANT-ST group on the ST day (P = 0.001) and on the CR day (P = 0.048). Comparisons within groups for the dW-ST rats found significantly reduced REM episodes during ST (P = 0.002) compared with BH. The ANT-ST rats did not significantly differ across recording days. Comparisons for Block 2 were not significant.
Table 2.
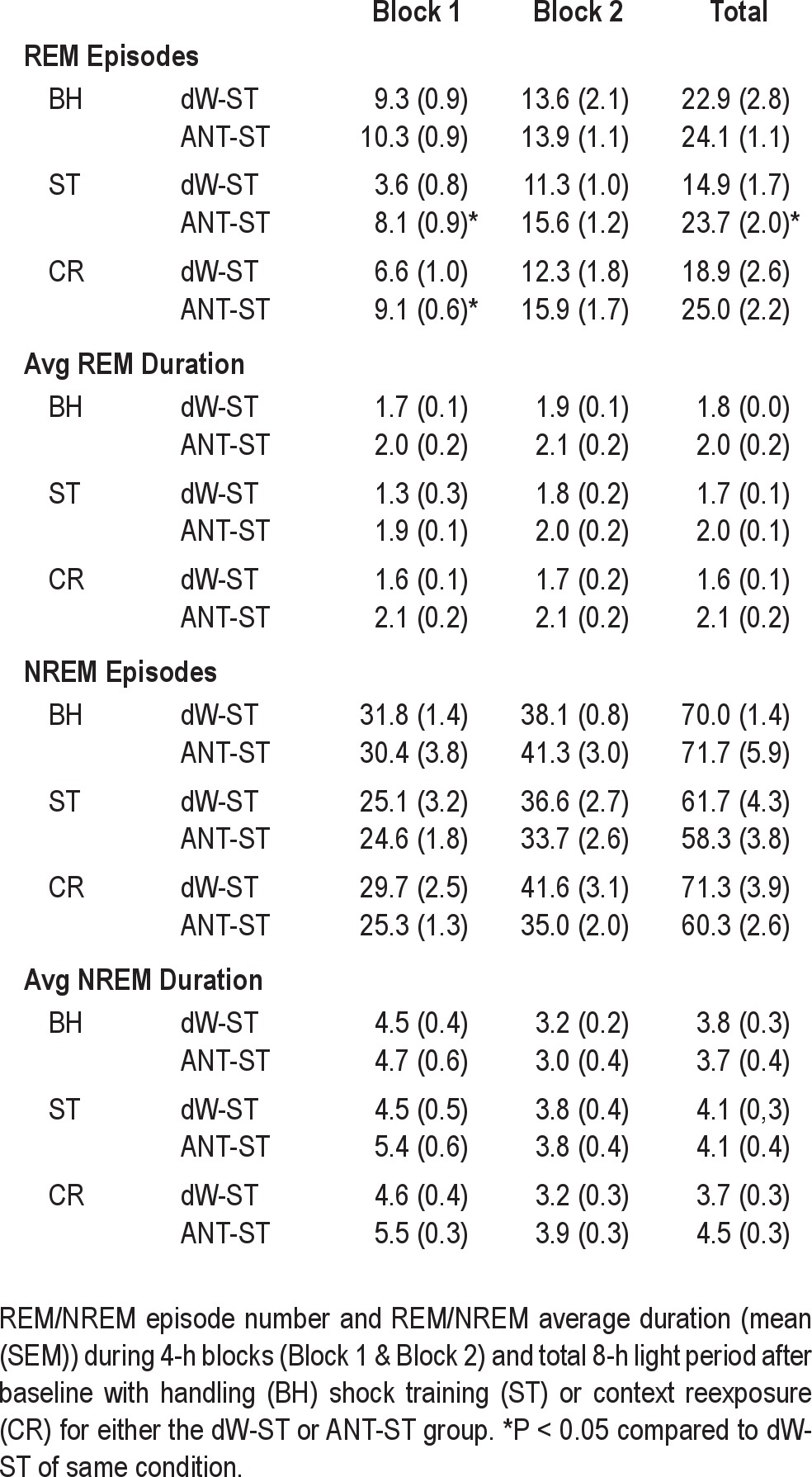
The analysis of REM episodes for the 8-h recording period revealed a significant Group × Treatment interaction (F2,24 = 3.911, P = 0.034). Post-hoc analyses for each recording day found no difference in sleep on BH, but REM episodes were reduced in the dW-ST group compared with the ANT-ST group on the ST day (P = 0.008) and there was a trend toward a reduction on the CR day (P = 0.054). Comparisons within groups for the dW-ST rats found significantly reduced REM episodes during ST (P = 0.018) compared with BH. The ANT-ST rats did not significantly differ across recording days.
Total Sleep and NREM Sleep
The analyses for total sleep amount revealed a significant Treatment Main Effect for Block 1 (F2,24 = 4.2, P = 0.03). Total sleep was decreased during Block 1 after ST compared with BH (P = 0.04). The difference between BH and CR did not reach significance (P = 0.08). There was no significant difference between groups. The analysis of total sleep in Block 2 or the total 8-h recording period did not find differences for either groups or treatments.
The analysis did not reveal any significant differences in total NREM or NREM episode duration for either 4-h block or total 8-h period.
DISCUSSION
The current study demonstrates that microinjecting ANT into the BLA does not significantly alter sleep under baseline, undisturbed conditions, but can significantly attenuate stress-and fear-induced reductions in REM. Consistent with previous work on fear conditioning in rats,8 training with inescapable foot shock and reexposure to the shock context alone reduced REM in animals receiving vehicle microinjections. By comparison, bilateral (but not unilateral) microinjections of ANT into BLA before training blocked reductions in REM on both ST and CR. Moreover, the effect of ANT on sleep was observed even though it did not significantly alter freezing compared with a control group on either the ST or CR day. These results demonstrate a significant role for CRF1 receptors in the BLA in regulating stress- and fear-induced changes in REM and provide further evidence that fear memory, as indicated by freezing, can be dissociated from the effects of stress and fear on sleep.
CRF and Spontaneous Sleep
We found no effect of microinjections of ANT into the BLA on spontaneous light period sleep. This result is generally consistent with the findings of other studies administering CRF antagonists during the light period in nonstressed conditions. For example, ICV administration into rats with two specific CRF-receptor antagonists, αHelCRF and astressin, did not alter waking when administered before the light period.33 However, the specific effects of CRF antagonists on sleep may vary with the time of administration. For instance, Chang and Opp33 found that ICV administration of CRF antagonists reduced wakefulness when administered before the dark period, although others have reported no effect of αHelCRF-administered ICV before the dark period on sleep and waking.46 Strains with genetic differences in the CRF system may also respond differently to CRF antagonists. For example, differences in the CRF system have been reported for C57BL/6J mice and BALB/cJ mice47 and we found that astressin-administered ICV did not significantly alter wakefulness or sleep in C57BL/6J mice but decreased active wakefulness and significantly increased REM at some dosages in BALB/cJ mice,48 who show increased activity and less spontaneous sleep during the light period. Thus, most data suggest that antagonizing CRF could increase sleep during periods of high spontaneous arousal, as suggested by Chang and Opp,33 but would have minimal effects when sleep was already high.
CRF and Stress-Induced Alterations in Sleep
Microinjections of ANT into the BLA blocked the reduction in REM normally produced by inescapable foot shock stress. CRF has been implicated in stress-induced alterations in sleep,49,50 particularly in the control of REM.46 Our results indicate that CRF in the BLA plays a role in inhibiting REM during stress; however, there are hypotheses that CRF promotes REM after stress. These hypotheses are primarily based on studies that applied CRF antagonists in association with a stress paradigm. For example, findings that ICV administration of αHelCRF before restraint stress prevented the increase in REM46 that can follow restraint administered at dark onset51 led to the suggestion that CRF mediates the increase. However, other investigators found no increase in REM after dark-onset restraint and no effect of the CRF antagonist, astressin, on sleep after restraint,49 but did find that astressin attenuated the increase in wakefulness over a 5-h period immediately after light period restraint.49
A recent study52 examined baseline and recovery sleep after sleep deprivation in conditional mouse mutants that overexpress CRF in the entire central nervous system or only in the forebrain. In baseline recordings, homozygous mice with either global or forebrain overexpression of CRF showed increased REM compared with control mice and both homozygous and heterozygous mice with global overexpression of CRF showed enhanced recovery REM after sleep deprivation. Enhanced REM recovery, but not NREM recovery, was blocked by oral administration of the CRF1 receptor antagonist, DMP696, 1-hour before the end of sleep deprivation. Peripheral stress hormone levels were not elevated during baseline and did not differ across genotypes after sleep deprivation. The authors concluded that enhanced REM in these mice was most likely induced through the activation of CRF1 receptors. Consistent with this conclusion is a report that repeated administration of αHelCRF in rats during sleep deprivation also reduced the amount of REM recovery.50 However, conflicting evidence is found in a study in sleep-deprived humans reporting that repeated administration of CRF during the actual recovery period blocked the increase in REM.53 Additionally, in rats, administration of CRF and αHelCRF during extended REM deprivation produced similar effects on initial recovery REM, primarily reduced REM episode duration.54 Thus, the timing of the treatment appears to be a significant factor in whether antagonizing CRF blocks enhanced REM.
Other work consistent with CRF playing an inhibitory role in regulating REM comes from studies of escapable shock. Training with escapable shock and reminders of escapable shock can produce significant enhancements in REM.55 Microinjections of either saline or astressin prior to training produce similar, significant enhancements in post-stress REM relative to a non-shocked handling control condition whereas the increases in REM are blocked by pretreatment with CRF.56 The effect of CRF was relatively specific for REM as changes in NREM and wakefulness were minimal.
Conditioned Fear and Sleep
Behavioral freezing has been used to evaluate fear and fear memory, with greater FT% being interpreted as indicating stronger fear reactions.2–4,44,45 Minimal if any freezing occurred in either group during the preshock period. ST resulted in similar significant increases in FT% compared with the preshock period in control and ANT-treated rats. Compared with the pre-shock period, the control and ANT-treated rats also showed similar FT% during context re-exposure (no shock presented). Thus, based on freezing, microinjections of ANT into the BLA did not alter behavioral fear responses.
We and others have demonstrated that training with inescapable foot shock and subsequent presentation of fearful cues and contexts associated with inescapable foot shock produce very similar alterations in post-stress sleep, primarily a reduction in REM. We also have demonstrated that continued freezing in a fearful context is associated with reduced REM, whereas extinguished fear is associated with normalized REM.14 Thus, given this relationship between waking fear behavior and REM, the apparent disassociation of freezing and later REM amounts after microinjections of ANT into the BLA may appear surprising. However, mice trained with controllable and uncontrollable stress, modeled by escapable and inescapable foot shock, show statistically equivalent amounts of freezing when reex-posed to the chamber used for training, but directionally different amounts of REM.39 Mice trained with escapable shock can show increased REM whereas mice trained with inescapable shock show decreased REM. We recently showed that injections of ANT into the CNA prior to context reexposure attenuates subsequent REM reductions normally observed without affecting freezing.38 Thus, these evolving lines of evidence indicate that fear behavior in wakefulness (as indicated by freezing) is not predictive of subsequent REM, and that CRF in the amygdala plays a significant role in regulating the relationship between fear and sleep. Indices of acute stress (corticosterone39 and stress-induced hyperthermia57) are also similar for animals trained with escapable and inescapable stress, though post-stress alterations in REM may be directionally different.
One of the more puzzling results in this study was the finding that antagonizing CRF1 receptors in the BLA prior to ST blocked fear-induced reductions in REM after a subsequent exposure to the ST context alone, but did not alter fear memory, as indicated by freezing. That is, microinjections of ANT into the BLA blocked the reduction in REM that is normally produced by both the initial stressor and contextual reminders of the stressors, but did not block waking fear behavior. This finding is consistent with the potential for dissociation between waking fear behavior and sleep, but also suggests that specific aspects of the stressful experience and stressful memory responsible for the reductions in REM were altered by antagonizing CRF1 receptors in the BLA. Support for the specificity of CRF1 receptors in our results is based on in vitro findings that ANT displaced 125I-oCRF binding in rat pituitary, frontal cortex, and cerebellum, which have greater amounts of CRF1 receptors, but not in the heart, which has greater amounts of CRF2 receptors.58
Amygdala, Conditioned Fear, and the Regulation of Sleep
To our knowledge, there has been limited examination of the potential role of CRF in the BLA in regulating contextual fear. A previous study reported that bilateral microinjections of DMP696, a nonpeptide CRF1 receptor antagonist, into the BLA did not significantly alter the acquisition of conditioned fear but did reduce freezing on reexposure to the fearful context.42 We also found no effect of microinjections of ANT into the BLA on fear acquisition, but our results for context reexposure are dramatically different as we saw equivalent FT% in the control and drug groups. One potential reason for the discrepancy is differences in the training paradigms. The study reporting an effect of antagonizing CRF1 receptors in the BLA on freezing used five foot shocks (1 mA, 1-sec duration) delivered at 2.0-min intervals whereas we used 20 foot shocks (0.8 mA, 0.5-sec duration) at 1.0-min intervals. Overtraining can overcome the effects of BLA lesions on contextual fear,59–61 indicating that circuitry outside the BLA can be involved in contextual fear under some circumstances.59 Thus, it is possible that the more extensive training paradigm (with respect to number of trials) we used may have engaged other regions involved with memory of contextual fear, though we must note that pretraining lesions of the BLA can impair acquisition with up to 25 training trials.59
The involvement of the BLA in the control of sleep is indicated by reports that bilateral electrolytic and chemical lesions of the BLA increase NREM and total sleep time in rats23 and that bilateral chemical lesions of the amygdala produce more consolidated sleep in chair-restrained Rhesus monkeys.62 Electrical and chemical stimulation of the BLA also increase low-voltage, high- frequency activity in the cortical EEG and decrease NREM and total sleep time, respectively.23,63 Recently we found that microinjections into the BLA of the Group II metabotropic glutamate (mGlu) receptor agonist, LY379268, selectively reduced REM without significantly altering wakefulness or NREM.24 Thus, though limited, current data demonstrate roles for BLA in regulating both NREM and REM. The influence on REM is likely enacted via influences on the CNA, which has projections to brainstem REM regulatory/generator regions and an established role in regulating REM64 and possibly through the bed nucleus of the stria terminals, which has brainstem targets similar to those of CNA.65
CRF, Conditioned Fear, Sleep, and Psychopathology
The CRF system,66–70 fear conditioning,71–75 and stress-induced alterations in sleep76–79 are implicated in the etiology of anxiety disorders, including posttraumatic stress disorder (PTSD). The amygdala has a critical role in the acquisition and expression of conditioned fear59–61,74,75,80 and recent evidence suggests that it is a strong regulator of conditioned alterations in sleep.38,81 It also plays a significant role in anxiety and PTSD.82 However, fear memory, as indicated by freezing, can be acquired with very few training trials whereas conditioned alterations in sleep require relatively extensive training procedures.11 As discussed previously, several lines of evidence now indicate that behavioral fear and subsequent sleep also can dissociate even with relatively extensive fear training and that the amygdala is important for regulating the relationship between waking fear behavioral and subsequent sleep. The current work demonstrates that the neural processes underlying conditioned behavioral fear and sleep can be separated and indicates that a full understanding of fear conditioning and its relevance for disease will require determining its effects on sleep and well as fear behavior in wakefulness. The importance of understanding the neural substrate underlying fear-conditioned changes in sleep is found in the association of significant sleep disturbances with anxiety, PTSD, and virtually all emotional disorders.
CONCLUSION
ANT microinjected into the BLA prior to fear conditioning attenuated reductions in REM that occur following foot shock training as well those following reexposure to the fearful context. This attenuation occurred without any change to fear-dependent freezing. Several lines of work now indicate that freezing can dissociate from stress- and fear-induced alterations in sleep. The current data suggest that CRF1 receptors within the BLA are important for regulating stress-induced alterations in REM, and that they play a role in modulating how stressful memories influence sleep. However, additional work is needed to establish the specific role of the BLA in mediating the effects of fear memory on sleep to determine whether CRF alters fear learning or actual memory formation. Given the role of CRF in the stress response, delineating the processes by which it regulates how stressful memories affect sleep could have significance for understanding the mechanisms underlying the sleep disturbances associated with emotional disorders. The effects we observed with manipulations in the BLA suggest that these mechanisms may be located in a very circumscribed region in the brain.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by NIH research grants MH64827 and Eastern Virginia Medical School institutional funds.
Footnotes
A commentary on this article appears in this issue on page 455.
REFERENCES
- 1.Liang KC, Melia KR, Miserendino MJ, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J Neurosci. 1992;12:2303–12. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 3.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 4.Paylor R, Tracy R, Wehner J, Rudy J. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci. 1994;108:810–7. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- 5.Nijsen M, Croiset G, Diamant M, et al. Conditioned fear-induced tachycardia in the rat: vagal involvement. Eur J Pharmacol. 1998;350:211–22. doi: 10.1016/s0014-2999(98)00261-1. [DOI] [PubMed] [Google Scholar]
- 6.Hode Y, Ratomponirina C, Gobaille S, Maitre M, Kopp C, Misslin R. Hypoexpression of benzodiazepine receptors in the amygdala of neophobic BALB/c mice compared to C57BL/6 mice. Pharmacol Biochem Behav. 2000;65:35–8. doi: 10.1016/s0091-3057(99)00131-8. [DOI] [PubMed] [Google Scholar]
- 7.Stiedl O, Tovote P, Ogren SO, Meyer M. Behavioral and autonomic dynamics during contextual fear conditioning in mice. Auton Neurosci. 2004;115:15–27. doi: 10.1016/j.autneu.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Pawlyk AC, Jha Sk, Brennan FX, Morrison AR, Ross RJ. A rodent model of sleep disturbances in posttraumatic stress disorder: the role of context after fear conditioning. Biol Psychiatry. 2005;57:268–77. doi: 10.1016/j.biopsych.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Sanford LD, Tang X, Ross RJ, Morrison AR. Influence of shock training and explicit fear-conditioned cues on sleep architecture in mice: strain comparison. Behav Genet. 2003;33:43–58. doi: 10.1023/a:1021051516829. [DOI] [PubMed] [Google Scholar]
- 10.Sanford LD, Yang L, Tang X. Influence of contextual fear on sleep in mice: a strain comparison. Sleep (Abstract Supplement) 2003;26:527–40. doi: 10.1093/sleep/26.5.527. [DOI] [PubMed] [Google Scholar]
- 11.Sanford LD, Fang J, Tang X. Sleep after differing amounts of conditioned fear training in BALB/cJ mice. Behav Brain Res. 2003;147:193–202. doi: 10.1016/s0166-4328(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 12.Tang X, Yang L, Sanford LD. Interactions between brief restraint, novelty and footshock stress on subsequent sleep and EEG power in rats. Brain Res. 2007;1142:110–8. doi: 10.1016/j.brainres.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang X, Yang L, Sanford LD. Rat strain differences in freezing and sleep alterations associated with contextual fear. Sleep (Abstract Supplement) 2005;28:1235–44. doi: 10.1093/sleep/28.10.1235. [DOI] [PubMed] [Google Scholar]
- 14.Wellman LL, Yang L, Tang X, Sanford LD. Contextual fear extinction ameliorates sleep disturbances found following fear conditioning in rats. Sleep. 2008;31:1035–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Koo JW, Han JS, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci. 2004;24:7654–62. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cousens G, Otto T. Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behav Neurosci. 1998;112:1092–103. doi: 10.1037//0735-7044.112.5.1092. [DOI] [PubMed] [Google Scholar]
- 17.Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996;110:718–26. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- 18.Maren S. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J Neurosci. 1998;18:3088–97. doi: 10.1523/JNEUROSCI.18-08-03088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacchetti B, Lorenzini CA, Baldi E, Tassoni G, Bucherelli C. Auditory thalamus, dorsal hippocampus, basolateral amygdala, and perirhinal cortex role in the consolidation of conditioned freezing to context and to acoustic conditioned stimulus in the rat. J Neurosci. 1999;19:9570–8. doi: 10.1523/JNEUROSCI.19-21-09570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilensky AE, Schafe GE, LeDoux JE. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J Neurosci. 2000;20:7059–66. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav Neurosci. 1994;108:1005–9. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- 22.Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–91. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- 23.Zhu GQ, Zhong MK, Zhang JX, Zhao LZ, Ke DP, Wang M, et al. [Role of basolateral amygdaloid nuclei in sleep and wakeful state regulation] Sheng Li Xue Bao. 1998;50:688–92. [PubMed] [Google Scholar]
- 24.Dong E, Wellman LL, Yang L, Sanford LD. Group II metabotropic gluta-mate (mGlu) receptors in the basal amygdala (BA) regulate rapid eye movement sleep (REM) Sleep (Abstract Supplement) 2009;32:A8. [Google Scholar]
- 25.Deussing JM, Wurst W. Dissecting the genetic effect of the CRH system on anxiety and stress-related behaviour. C R Biol. 2005;328:199–212. doi: 10.1016/j.crvi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–80. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 27.Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–52. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 28.Bakshi VP, Kalin NH. Corticotropin-releasing hormone and animal models of anxiety: gene-environment interactions. Biol Psychiatry. 2000;48:1175–98. doi: 10.1016/s0006-3223(00)01082-9. [DOI] [PubMed] [Google Scholar]
- 29.Heinrichs SC, Menzaghi F, Merlo Pich E, Britton KT, Koob GF. The role of CRF in behavioral aspects of stress. Ann N Y Acad Sci. 1995;771:92–104. doi: 10.1111/j.1749-6632.1995.tb44673.x. [DOI] [PubMed] [Google Scholar]
- 30.Koob G, Bloom F. Corticotropin-releasing factor and behavior. Fed Proc. 1985;44:259–63. [PubMed] [Google Scholar]
- 31.Ehlers CL, Reed TK, Henriksen SJ. Effects of corticotropin-releasing factor and growth hormone-releasing factor on sleep and activity in rats. Neuroendocrinology. 1986;42:467–74. doi: 10.1159/000124489. [DOI] [PubMed] [Google Scholar]
- 32.Marrosu F, Gessa GL, Giagheddu M, Fratta W. Corticotropin-releasing factor (CRF) increases paradoxical sleep (PS) rebound in PS-deprived rats. Brain Res. 1990;515:315–8. doi: 10.1016/0006-8993(90)90614-h. [DOI] [PubMed] [Google Scholar]
- 33.Chang FC, Opp MR. Blockade of corticotropin-releasing hormone receptors reduces spontaneous waking in the rat. Am J Physiol. 1998;275:R793–802. doi: 10.1152/ajpregu.1998.275.3.R793. [DOI] [PubMed] [Google Scholar]
- 34.Chang FC, Opp MR. Pituitary CRH receptor blockade reduces waking in the rat. Physiol Behav. 1999;67:691–6. doi: 10.1016/s0031-9384(99)00139-0. [DOI] [PubMed] [Google Scholar]
- 35.Heilig M, Koob G, Ekman R, Britton K. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17:80–5. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 36.Swerdlow NR, Britton KT, Koob GF. Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by alpha-helical CRF (9-41) Neuropsychopharmacology. 1989;2:285–92. doi: 10.1016/0893-133x(89)90033-x. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, Tang X, Wellman LL, Liu X, Sanford LD. Corticotropin releasing factor (CRF) modulates fear-induced alterations in sleep in mice. Brain Res. 2009;1276:112–22. doi: 10.1016/j.brainres.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Wellman LL, Yang L, Ambrozewicz MA, Tang X, Sanford LD. Antagonizing corticotropin-releasing factor in the central nucleus of the amygdala attenuates fear-induced reductions in sleep but not freezing. Sleep (Abstract Supplement) 2011;34:1539–49. doi: 10.5665/sleep.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machida M, Lonart G, Yang L, Sanford LD. Effects of stressor controllability on neural plasticity associated mRNA levels in mouse amygdala and me-dial prefrontal cortex (mPFC) Sleep (Abstract Supplement) 2011;34:A16. [Google Scholar]
- 40.De Souza EB, Insel TR, Perrin MH, Rivier J, Vale WW, Kuhar MJ. Corticotropin-releasing factor receptors are widely distributed within the rat central nervous system: an autoradiographic study. J Neurosci. 1985;5:3189–203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Pett K, Viau V, Bittencourt JC, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 42.Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150:818–28. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kruger L, Saporta S, Swanson L. Photographic atlas of the rat brain. New York: Cambridge University Press; 1995. [Google Scholar]
- 44.Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68:129–35. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- 45.Doyere V, Gisquet-Verrier P, de Marsanich B, Ammassari-Teule M. Age-related modifications of contextual information processing in rats: role of emotional reactivity, arousal and testing procedure. Behav Brain Res. 2000;114:153–65. doi: 10.1016/s0166-4328(00)00223-0. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez MM, Valatx JL. Effect of intracerebroventricular administration of alpha-helical CRH (9-41) on the sleep/waking cycle in rats under normal conditions or after subjection to an acute stressful stimulus. J Sleep Res. 1997;6:164–70. doi: 10.1046/j.1365-2869.1997.00042.x. [DOI] [PubMed] [Google Scholar]
- 47.Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003;23:700–7. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanford LD, Yang L, Wellman LL, Dong E, Tang X. Mouse strain differences in the effects of corticotropin releasing hormone (CRH) on sleep and wakefulness. Brain Res. 2008;1190:94–104. doi: 10.1016/j.brainres.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang FC, Opp MR. Role of corticotropin-releasing hormone in stressor-induced alterations of sleep in rat. Am J Physiol Regul Integr Comp Physiol. 2002;283:R400–7. doi: 10.1152/ajpregu.00758.2001. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez MM, Valatx JL. Involvement of stress in the sleep rebound mechanism induced by sleep deprivation in the rat: use of alpha-helical CRH (9-41) Behav Pharmacol. 1998;9:655–62. doi: 10.1097/00008877-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Rampin C, Cespuglio R, Chastrette N, Jouvet M. Immobilisation stress induces a paradoxical sleep rebound in rat. Neurosci Lett. 1991;126:113–8. doi: 10.1016/0304-3940(91)90532-x. [DOI] [PubMed] [Google Scholar]
- 52.Kimura M, Muller-Preuss P, Lu A, et al. Conditional corticotropin-releasing hormone overexpression in the mouse forebrain enhances rapid eye movement sleep. Mol Psychiatry. 2010;15:154–65. doi: 10.1038/mp.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schussler P, Yassouridis A, Uhr M, et al. Growth hormone-releasing hormone and corticotropin-releasing hormone enhance non-rapid-eye-movement sleep after sleep deprivation. Am J Physiol Endocrinol Metab. 2006;291:E549–56. doi: 10.1152/ajpendo.00641.2005. [DOI] [PubMed] [Google Scholar]
- 54.Machado RB, Tufik S, Suchecki D. Modulation of sleep homeostasis by corticotropin releasing hormone in REM sleep-deprived rats. Int J Endocrinol. 2010;2010:326151. doi: 10.1155/2010/326151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanford LD, Yang L, Wellman LL, Liu X, Tang X. Differential effects of controllable and uncontrollable footshock stress on sleep in mice. Sleep (Abstract Supplement) 2010;33:621–30. doi: 10.1093/sleep/33.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang L, Wellman LL, Tang X, Sanford LD. Effects of corticotropin releasing factor (CRF) on sleep and body temperature following controllable foot-shock stress in mice. Physiol Behav. 2011;104:886–92. doi: 10.1016/j.physbeh.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang L, Wellman LL, Ambrozewicz MA, Sanford LD. Effects of stressor predictability and controllability on sleep, temperature, and fear behavior in mice. Sleep (Abstract Supplement) 2011;34:759–71. doi: 10.5665/SLEEP.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology. 1996;137:5747–50. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- 59.Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999;19:8696–703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zimmerman JM, Maren S. The bed nucleus of the stria terminalis is required for the expression of contextual but not auditory freezing in rats with basolateral amygdala lesions. Neurobiol Learn Mem. 2011;95:199–205. doi: 10.1016/j.nlm.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem. 2007;14:634–44. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benca RM, Obermeyer WH, Shelton SE, Droster J, Kalin NH. Effects of amygdala lesions on sleep in rhesus monkeys. Brain Res. 2000;879:130–8. doi: 10.1016/s0006-8993(00)02761-x. [DOI] [PubMed] [Google Scholar]
- 63.Dringenberg HC, Vanderwolf CH. Cholinergic activation of the electrocorticogram: an amygdaloid activating system. Exp Brain Res. 1996;108:285–96. doi: 10.1007/BF00228101. [DOI] [PubMed] [Google Scholar]
- 64.Sanford LD, Parris B, Tang X. GABAergic regulation of the central nucleus of the amygdala: implications for sleep control. Brain Res. 2002;956:276–84. doi: 10.1016/s0006-8993(02)03552-7. [DOI] [PubMed] [Google Scholar]
- 65.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 66.Bremner J, Licinio J, Darnell A, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–9. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baker DG, West SA, Nicholson WE, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with post-traumatic stress disorder. Am J Psychiatry. 1999;156:585–8. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- 68.Sautter FJ, Bissette G, Wiley J, et al. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003;54:1382–8. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- 69.de Kloet CS, Vermetten E, Geuze E, et al. Elevated plasma corticotrophin-releasing hormone levels in veterans with posttraumatic stress disorder. Prog Brain Res. 2007;167:287–91. doi: 10.1016/S0079-6123(07)67025-3. [DOI] [PubMed] [Google Scholar]
- 70.Neylan TC, Otte C, Yehuda R, Marmar CR. Neuroendocrine regulation of sleep disturbances in PTSD. Ann N Y Acad Sci. 2006;1071:203–15. doi: 10.1196/annals.1364.015. [DOI] [PubMed] [Google Scholar]
- 71.Grillon C, Southwick SM, Charney DS. The psychobiological basis of post-traumatic stress disorder. Mol Psychiatry. 1996;1:278–97. [PubMed] [Google Scholar]
- 72.Shalev AY, Ragel-Fuchs Y, Pitman RK. Conditioned fear and psychological trauma. Biol Psychiatry. 1992;31:863–5. doi: 10.1016/0006-3223(92)90113-e. [DOI] [PubMed] [Google Scholar]
- 73.Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychol Bull. 1992;112:218–38. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- 74.Davis M. The role of the amygdala in fear and anxiety. Ann Rev Neurosci. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 75.Davis M. Animal models of anxiety based on classical conditioning: the conditioned emotional response (CER) and the fear-potentiated startle effect. Pharmacol Ther. 1990;47:147–65. doi: 10.1016/0163-7258(90)90084-f. [DOI] [PubMed] [Google Scholar]
- 76.Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159:855–7. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- 77.Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159:1696–701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- 78.Mellman TA, Pigeon WR, Nowell PD, Nolan B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress. 2007;20:893–901. doi: 10.1002/jts.20246. [DOI] [PubMed] [Google Scholar]
- 79.Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345:1825–32. doi: 10.1056/NEJMra012893. [DOI] [PubMed] [Google Scholar]
- 80.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 81.Liu X, Yang L, Wellman LL, Tang X, Sanford LD. GABAergic antagonism of the central nucleus of the amygdala attenuates reductions in rapid eye movement sleep after inescapable footshock stress. Sleep (Abstract Supplement) 2009;32:888–96. doi: 10.1093/sleep/32.7.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]