Abstract
Study Objectives:
Respiratory cycle-related electroencephalographic (EEG) changes (RCREC), especially in delta and sigma frequencies, are thought to reflect subtle, breath-to-breath inspiratory microarousals that are exacerbated in association with increased work of breathing in obstructive sleep apnea (OSA). We wondered whether snoring sounds could create these microarousals, and investigated whether earplugs, anticipated to alter snoring perception, might affect RCREC.
Design:
Randomized controlled trial.
Setting:
An accredited, academic sleep laboratory.
Patients:
Adults (n = 400) referred for suspected OSA.
Interventions:
Subjects were randomly assigned to use earplugs or not during a night of diagnostic polysomnography.
Results:
Two hundred three of the participants were randomized to use earplugs. Earplug use was associated with lower RCREC in delta EEG frequencies (0.5-4.5 Hz), although not in other frequencies, after controlling for potential confounds (P = 0.048). This effect of earplug use was larger among men in comparison with women (interaction term P = 0.046), and possibly among nonobese subjects in comparison with obese subjects (P = 0.081). However, the effect of earplug use on delta RCREC did not differ significantly based on apnea severity or snoring prominence as rated by sleep technologists (P > 0.10 for each).
Conclusions:
This randomized controlled trial is the first study to show that perception of snoring sounds, as modulated by earplugs, can influence the cortical EEG during sleep. However, the small magnitude of effect, lack of effect on RCREC in EEG frequencies other than delta, and absence of effect modulation by apnea severity or snoring prominence suggest that perception of snoring is not the main explanation for RCREC.
Citation:
Chirakalwasan N; Ruzicka DL; Burns JW; Chervin RD. Do snoring sounds arouse the snorer? SLEEP 2013;36(4):565-571.
Keywords: Polysomnography, obstructive sleep apnea, computer-assisted signal processing, respiratory cycle-related EEG changes, RCREC, earplugs, snoring, sound
INTRODUCTION
In epidemiological studies, snoring is associated with adverse health outcomes including excessive daytime sleepiness and cardiovascular disease.1 The assumption is often that snoring is a biomarker for underlying obstructive sleep apnea (OSA), in many if not most snorers, and that OSA drives the associations with adverse outcomes. However, some evidence suggests that snoring could have consequences even after OSA is taken into account.2,3 Whether snoring sounds have any effect on sleep or health of the snorer has never been investigated. During sleep, the reticular nucleus of the thalamus plays a gating function that is believed to screen from cortical perception much of the sensory information that would otherwise pass unhindered during wakefulness.4–6 Nonetheless, particularly loud sounds or those with significance, such as the sleeper's own name or a child's voice, are “heard” and lead to awakening. However, the widespread assumption that internally generated, monotonous, and recurrent snoring sounds are habituating, ignored by the brain, and incapable of arousing the sleeper has remained untested in existing relevant reports.7,8
One challenge in addressing this question has been that most interventions to remove snoring, and test the effects, also remove underlying OSA. A second challenge has been to separate effects of snoring, on a breath-to-breath basis, from those of discrete apneas and hypopneas. We aimed to address these challenges through an innovative combination of “low-tech” and “high-tech” solutions. To address the first challenge, we studied the sleep of patients, at risk for OSA, with and without use of earplugs. Earplugs could decrease snoring sound perception through aural air conduction, or potentially increase it through bone conduction in the absence of external “white noise”, but a critical advantage of earplugs is that any change in sleep and breathing when they are used can be attributed more readily to a change in sound perception than to a change in the patency of the upper airway.
To address the second challenge, we took advantage of an electronic algorithm that we developed previously to demonstrate and quantify cortical arousal that occurs on a breath-to-breath basis during sleep, outside time occupied by apneas or hypopneas.9 The generated metric, respiratory cycle-related electroencephalographic (EEG) changes (RCREC), is more prominent in individuals with sleep apnea than in those without sleep apnea10; correlates to some extent with work of breathing as measured by esophageal pressure monitoring11; improves after adenotonsillectomy or during administration of continuous positive airway pressure (CPAP)10,12; and predicts subjectively or objectively measured daytime sleepiness even after rates of apneas and hypopneas are taken into account.13,14 Increases in sigma (high frequency) EEG components of RCREC, and decreases in delta (low frequency) components during each labored inspiration suggest that RCREC are likely to reflect subtle but quite numerous transient inspiratory microarousals.14 However, the mechanisms that cause these inspiratory micro-arousals have never been identified. Could the sound of the snorer's own snoring play a role?
We recruited adult patients scheduled for diagnostic polysomnography, because of suspected OSA, to participate in a randomized controlled trial to determine what effects use of earplugs may have, if any, on RCREC. Although we could not know for certain whether earplugs would decrease or increase perception of snoring sounds, we reasoned that any change in RCREC with use of earplugs would suggest some breath-to-breath effect of snoring sound perception on cortical activity during sleep. A secondary aim was to assess whether earplugs, if they do modulate RCREC, might also affect subjective ratings of morning sleepiness. Finally, we also explored whether earplugs might have any positive or negative influence on sleep apnea severity or quality of sleep.
METHODS
Subjects
Adults age 18 yr or older who were suspected to have OSA and referred for clinical purposes to either of two accredited University of Michigan sleep centers for laboratory-based, full-night diagnostic polysomnography were invited to participate in this randomized, controlled but unmasked clinical trial. Individuals who had known sensitivities to foam earplugs, who were unable to provide written informed consent for any reason, or who were anticipated to be unable to complete a Stanford Sleepiness Scale on the morning after polysomnography were excluded. Subjects with a history of hearing loss were excluded, though hearing of participants was not tested. This study was approved by the University of Michigan Medical School Institutional Review Board (IRBMED).
Protocol
In an initial, first phase of recruitment for this protocol, each potential subject received an invitation by mail to participate, well in advance of the sleep study. Each packet also contained foam earplugs identical to those that would be used during polysomnography (Howard Leight, Max Ear Plugs NRR33 Uncorded, with Noise Reduction Rating 33 dB). Individuals interested in participation were asked to use the earplugs for at least 5 nights prior to the appointment in the sleep laboratory. This was to ensure that participants could become habituated to wearing earplugs before testing, and thereby reduce any potential effect of novel earplug use on sleep continuity. Several days before the scheduled polysomnogram, clinic staff called subjects to confirm their studies, and also reminded them to wear their earplugs at home. Upon arrival at the sleep laboratory for polysomnography, each participant was randomized to use earplugs during the recording or to not use earplugs. On the morning after the diagnostic polysomnogram, each subject was asked to complete the Stanford Sleepiness Scale. This validated, single-item instrument asks subjects to rate their own instantaneous level of sleepiness on a seven-point scale.15,16 Subjects who completed the protocol received a $5.00 gift card to a local coffee shop.
In the initial execution of this protocol, some subjects wanted to participate but neglected to habituate to use of earplugs for at least 3 of the 5 previous nights. Others had their sleep study appointments changed too rapidly to allow the habituation period. To accommodate such subjects, the protocol was amended approximately midcourse to allow inclusion of these individuals in a second phase of recruitment. Statistical comparison and adjustment to account for any effects of habituation to earplug use was then added to analytic plans, as described below.
Polysomnography
All polysomnograms were performed using six EEG channels (F3-A2, F4-A1, C3-A2, C4-A1, O1-A2, O2-A1 of the 10-20 international electrode placement system), two electro-oculogram channels, chin and bilateral anterior tibialis surface electromyogram (EMG), two electrocardiogram (EKG) leads, a snoring sensor, finger oximetry, nasal pressure monitoring, oronasal thermocouples, and plethysmography belts at the chest and abdomen.17 Polysomnograms were scored for clinical purposes following standard criteria,17 by experienced technologists who were masked to group assignment (earplug use versus no earplug use) of the subjects. Hypopneas were defined using the American Academy of Sleep Medicine 2007 Manual “alternative” rule, which accepts an arousal or ≥ 3% oxyhemoglobin desaturation as a defining consequence of the event. Respiratory event-related arousals (RERAs) were scored following the same reference.17 The number of apneas, hypopneas, and RERAs per hour of sleep were calculated and reported as the respiratory disturbance index.
Computation of RCREC
The RCREC were computed with an algorithm implemented in MATLAB (MathWorks, Natick, MA) and described previously.9,12,14 Briefly, the C4-M1 EEG channel was band-pass filtered digitally to form five time series corresponding to the delta (0.5-4.5 Hz), theta (4.5-8.5 Hz), alpha (8.5-12.5 Hz), sigma (12.5-15.5 Hz), or beta frequencies (15.5-30.5 Hz). Filtering of the C4-M1 EEG data in each case was performed with a fifth-order Butterworth digital filter, implemented using a zero-phase forward and reverse filtering technique (MATLAB filtfilt function) that has zero phase distortion. The EEG and thermocouple signals during the first 3 hours of recorded sleep were analyzed. Limitation of analyses to the first 3 hours has generally been used9,10,14 to focus on a portion of the night that may be most relevant to next-day sleepiness,18,19 to enhance comparability across subjects who sleep for different durations, and because RCREC results during the first 3 hours tend to resemble results for the entire night.9 Respiratory cycles corresponding to respiratory rates higher than 30 or lower than eight cycles per minute were considered unlikely to be physiologic and were filtered out. Then, only respiration cycles with airflow amplitudes and durations between the fifth and 95th percentile were used in the calculations, to screen out residual apneas, hypopneas, and airflow signal artifacts.
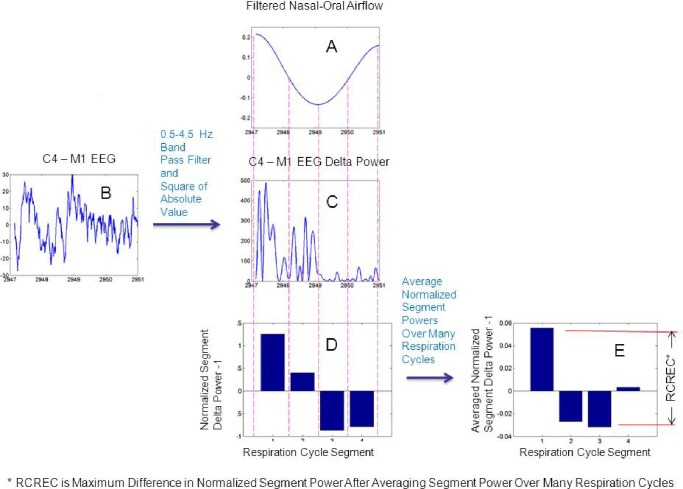
To define respiratory cycles and component phases, the signal from an oronasal thermocouple was used. Maxima, minima, and intervening temporal midpoints for each respiratory cycle were used to identify four time segments: early expiration, late expiration, early inspiration, and late inspiration. For each specific EEG frequency band, the mean EEG intensity over each respiratory cycle segment was calculated and divided by the mean intensity over the entire relevant respiratory cycle. In this manner, the EEG frequency-specific, normalized average power was computed for each of the four segments of the respiration cycle. The RCREC for a given sleep study was computed as the maximum difference between the mean EEG powers associated with each of the four respiratory cycle segments (Figure 1).
Figure 1.
Computation of respiratory cycle-related electroencephalogram (EEG) changes (RCREC). The variation of the EEG signal power is computed for a specific frequency band during each respiratory cycle. The nasal-oral thermistor signal is filtered (A) and divided into four time segments based on maxima, minima, and their midpoints. A digital band-pass filter is applied to the measured EEG signal (B) to produce a time series for the targeted frequency band. This signal is squared to produce a time series that shows variation of the relevant EEG power (C). The mean frequency-specific EEG power is then computed for each of the four respiratory cycle segments as defined in (A). The mean power for each segment is normalized by the mean frequency-specific power over the entire respiratory cycle. One is subtracted from each result to derive the measures shown for each of the four segments (D). The segment-specific EEG powers are averaged over many respiratory cycles to obtain the average measure for each of the four respiratory segments (E). The subject's RCREC, on this sleep study, is computed as the difference between the maximum and minimum mean relative EEG powers for each of the four respiratory segments. The RCREC thus reflects the average extent to which EEG power varies in synchrony with the respiratory cycle.
Analyses
As the main variables often showed distributions that were not normal, summary descriptive statistics were provided as medians and first and third quartiles. Primary outcome variables, for comparison between subjects randomized to use earplugs during polysomnography and those who were not, included RCREC in each of the five physiologic frequency bands that have been the focus of previous reports (delta, theta, alpha, sigma, and beta). Secondary outcome variables included the Stanford Sleepiness Scale score; three standard measures of disrupted sleep (arousal index [arousals per hour of sleep], stage shift index, percent Stage N1 sleep); and two standard measures of sleep apnea severity (respiratory disturbance index [RDI], minimum oxygen saturation). The primary explanatory variable was use of earplugs, or not, during the polysomnogram.
Initial bivariate associations were tested with the Wilcoxon rank-sum test. Then, multiple linear regression models were constructed to adjust for several potential confounding variables, including age, sex, body mass index, and respiratory disturbance index. After the requirement for habituation to the earplugs was removed, pre-polysomnography habituation to the earplugs (present versus absent) was also planned as a covariate in all multiple regression models. In regression models, where appropriate, transformations were applied to variables that did not show normal distributions. Effect modification was explored using stratification for each potential confounding variable. Suspected differences were confirmed by addition of interaction terms to the relevant regression models.
The level of significance was set at P < 0.05. The sample size of n = 400 provided a power of at least 0.88 to detect an incremental r-square statistic associated with earplug use of 0.02 or greater. This study was registered at http://clinicaltrials.gov/ (NCT01062854).
RESULTS
Subjects
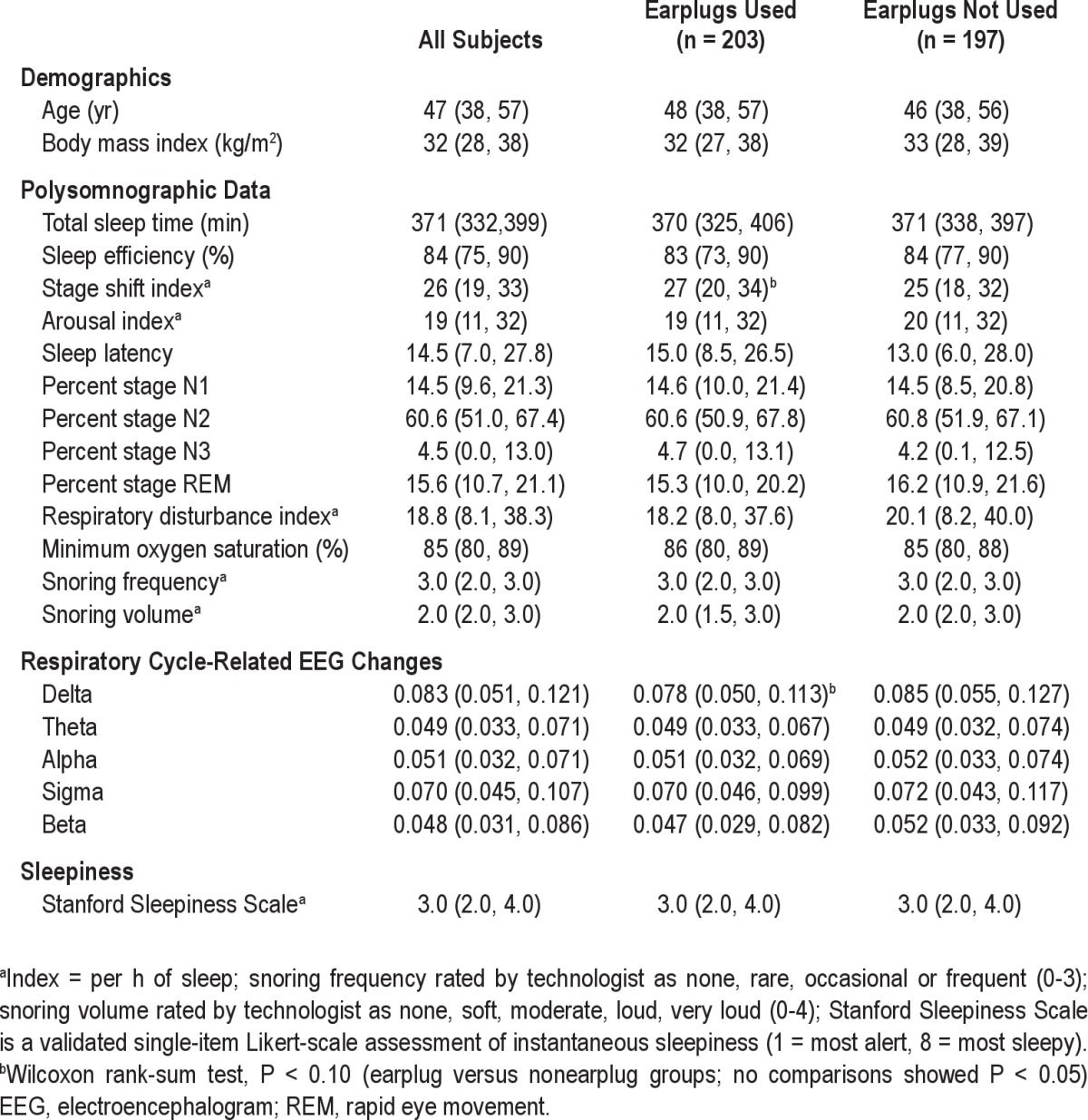
Among 404 enrolled subjects, 400 subjects (including 202 women) had complete polysomnographic data, slept at least 90 minutes (the approximate length of one complete sleep cycle), and provided the data for the current analyses. These subjects represented approximately 26% of those approached about possible participation in the study. Demographic, sleep, RCREC, and sleepiness data are summarized in Table 1. Subjects randomized to use earplugs (n = 203) or not use earplugs (n = 197) during polysomnography showed no significant difference in several potential confounders, including age and body mass index (Table 1), and no significant difference in sex, race/ethnicity, or likelihood of having been habituated to earplug use (n = 192) before the study (chi-square P > 0.05 for each).
Table 1.
Medians (first and third quartiles) for demographic, sleep, respiratory cycle-related EEG changes, and sleepiness measures
RCREC and Standard Sleep Measures, With and Without Earplugs
In bivariate, unadjusted analyses, subjects who used ear-plugs in comparison with those who did not showed a trend toward diminished RCREC in delta EEG frequencies (Table 1). In contrast, earplug users and nonusers showed no differences in RCREC for other EEG frequencies. The two groups also showed no differences in standard measures of sleep stages, sleep disruption, technician-characterized snoring, or apnea severity, except that the earplug users did show a trend toward a higher stage shift index. Results were essentially identical whether the respiratory disturbance index or the apneahypopnea index was used as a measure of apnea severity.
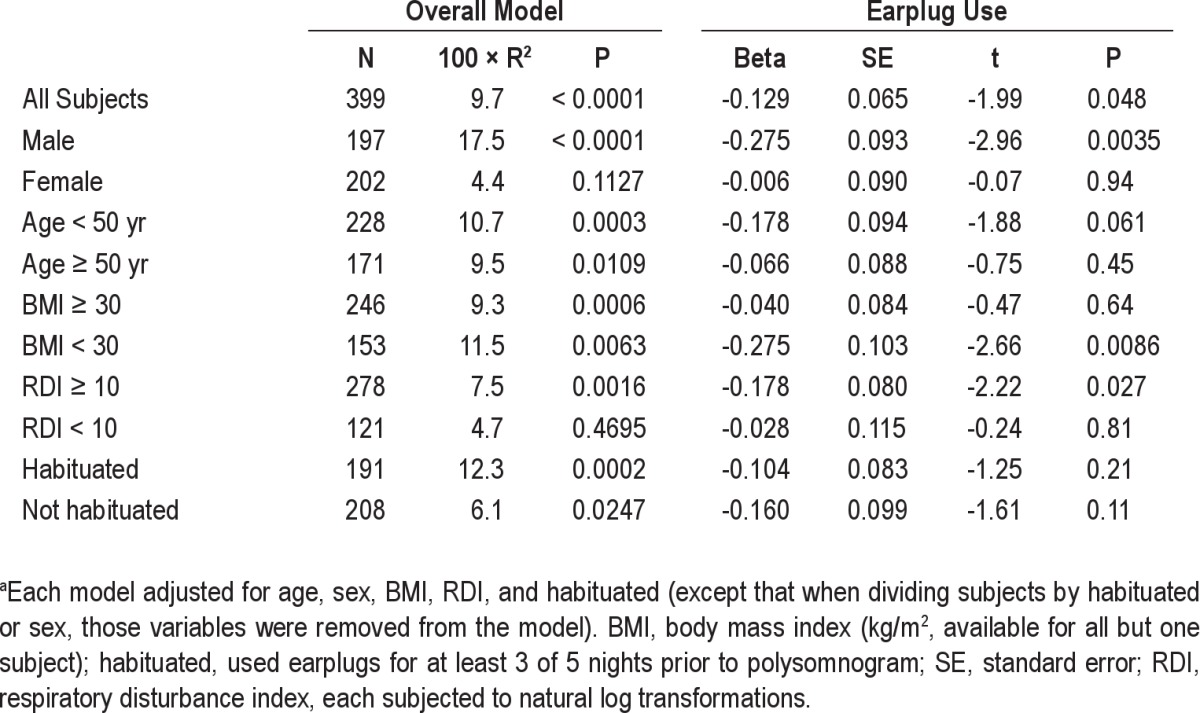
Multiple linear regression of delta RCREC (natural log transformation) on earplug use—along with the planned covariables of age, sex, body mass index, respiratory disturbance index (natural log transformation), and earplug habituation as potential confounds—produced the results in Table 2 (first row). In this model, earplug use predicted lower delta RCREC after taking the potential confounders into account. In contrast, multiple linear regression of the stage shift index (natural log transformation) on earplug use after adjustment for the same covariables showed little change in the strength of the trend between the stage shift index and earplug use (total model R2 = 0.27, P < 0.0001; earplug beta = 0.061, SE = 0.034, t = 1.79, P = 0.075).
Table 2.
Multiple linear regression of delta respiratory cycle-related EEG changes (RCREC) on earplug use and several potential confounding variables, for all subjects and subsets as indicateda
Earplug Use and RCREC in Subsets of Subjects
Fully adjusted analyses among men and women separately suggested that the association observed among all subjects between earplug use and delta RCREC derived exclusively from men (Table 2). A regression model with an earplug*sex interaction term confirmed this effect modification (P = 0.046, Figure 2). Analyses among younger and older subjects separately suggested that the association may have derived more from the younger patients, but the interaction term was not significant. Analyses among obese and non-obese subjects separately suggested that the earplug-RCREC association derived mainly from leaner subjects (interaction term P = 0.081). Analyses among subjects with significant sleep apnea (respiratory disturbance index ≥ 10) or among remaining subjects separately suggested that the earplug-RCREC association may have derived more from subjects with significant sleep apnea, but the interaction term was not significant. Similarly, prominent snoring according to recording technologists (defined as frequent and loud or very loud snoring [Table 1] and present in 31% of subjects) produced no significant interaction term (data not shown). Separate analyses among subjects who were or were not habituated to earplug use prior to polysomnography suggested no effect modification for this variable.
Figure 2.
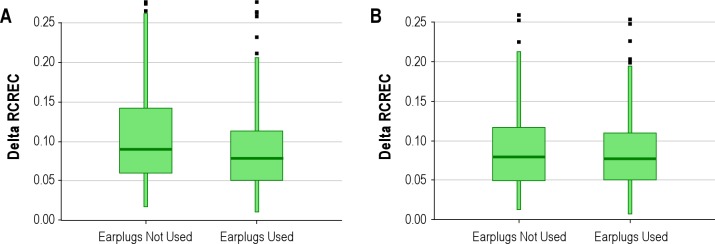
Delta respiratory cycle-related electroencephalographic (EEG) changes (RCREC) was lower when earplugs were used than when they were not used by men (A), but among women no such association emerged (B). Plots show median and 10th, 25th, 75th, and 90th percentiles, as well as some (not all) outliers.
Earplug Use and Subjective Morning Sleepiness
Stanford Sleepiness Scale scores on the morning after polysomnography were greater than 3 for 110 subjects; 3 or less for 250 subjects; and missing in 40 subjects. Earplug use was not associated with high sleepiness scores (> 3) in bivariate analysis (chi-square P = 0.49), or in a logistic regression model that adjusted for age, sex, body mass index, habituation, and respiratory disturbance index (odds ratio = 1.67, 95% confidence interval [0.73, 1.87]).
DISCUSSION
This randomized clinical trial, among 400 patients referred to a sleep laboratory for suspected OSA, represents an innovative attempt to test for the first time whether snoring sounds themselves may arouse the snorer from sleep. The main findings were that use of ear-plugs diminishes respiratory cycle-related EEG changes in delta EEG frequency ranges, but not theta, alpha, sigma, or beta ranges. Earplug use was particularly likely to diminish delta RCREC in subjects who were male, non-obese, or more affected by OSA (RDI ≥ 10), although effect modification could be confirmed statistically only for gender. Secondary outcomes defined by more standard sleep measures, including percent time spent in each sleep stage, arousal index, sleep efficiency, and next-morning subjective sleepiness, generally showed no difference between 203 subjects who used ear-plugs and 197 who did not.
The primary question that motivated this study—whether snoring sounds can have a measurable effect on sleep of the snorer—appears to have a positive answer. Previous evidence suggests that RCREC in delta and sigma ranges are likely to be most salient physiologically, at least with respect to potential effects on daytime sleepiness.10,13,14 In the current study, delta if not sigma RCREC showed sensitivity to earplug use. The findings that positive delta RCREC results arose from men rather than women, perhaps from non-obese subjects more than obese subjects, and possibly from more severe apneics rather than less severe apneics, together also suggest that the delta RCREC findings could have a physiological basis. This is because male sex is well known to be associated with worse OSA20; obesity may well change the nature of OSA and its consequences;21 and patients who have more severe OSA might be anticipated to have more baseline RCREC,10 with more potential for modulation by earplugs.
Although the delta RCREC results could still conceivably have been spurious, none of the other, standard polysomno-graphic measures targeted in this study showed statistically significant differences between the randomized groups. These negative findings may be interesting to any clinician who has heard a patient complain that he or she wakes himself or herself up with loud snores or snorts. To our knowledge, this is the first research paradigm designed in a manner that might assess whether the snoring sound itself disturbs standard sleep measures. Previous research has suggested that increased work of breathing rather than hypoxia or hypercarbia is likely to result in arousal after an obstructive sleep apnea event,22 but this protocol did not assess for any possible effects of snoring sounds themselves. Other studies have shown that sleep can be disrupted by snoring of a bed partner, but again did not assess any effects on the snorer.23
Nonetheless, the current conclusion that snoring also appears to arouse the snorer—as assessed by RCREC but not standard sleep measures—cannot be drawn without qualifications. The level of significance overall (P = 0.048) for diminished delta RCREC in association with earplug use was marginal in the total sample, which was sizeable. The sample size permitted initial plans to test five RCREC frequency ranges without adjustment for simultaneous comparisons, which in retrospect would have rendered the delta RCREC findings, in the total sample if not some of the stratified groups, non-significant. Exactly why an effect of earplugs on RCREC should be smaller for women or more obese subjects, after adjustment for RDI, is not readily obvious. Perhaps most perplexing are two additional questions: why did RCREC in only one frequency band, as opposed to all of them, show sensitivity to use of earplugs, and why did snoring prominence (as rated by technologists, if not objective means) fail to modify earplug effects on delta RCREC?
One consideration is that the experimental paradigm may have had only limited ability to show that either RCREC or standard sleep measures are affected by snoring sounds. Whereas it was reasonable to hypothesize that earplugs could change snoring perception—and critically, do so without anticipated direct effect on the upper airway—we cannot know whether earplugs augment or diminish perception of snoring sounds generated internally, and so close to the ears. Air conduction of noise may be diminished, while perception of internal sounds is augmented by an “occlusion effect” often reported by hearing aid users.24,25 Some frequencies may have been augmented while others were attenuated.26 Thus, opposing influences of earplugs may have combined to diminish our overall ability to measure any effect of snoring sound on RCREC, nocturnal sleep, and next-morning sleepiness. In short, we had no way to know in advance the direction or magnitude of changes in snoring perception that would be afforded by earplug use. We therefore planned the study to look for any small difference in RCREC, be it augmentation or diminution, between the two randomized arms.
Additional limitations of this study included the inability to mask subjects or monitoring technologists to treatment assignments. However, all outcome measures with the exception of morning sleepiness were objectively calculated from electronic signals or were derived from scoring performed by technologists masked to earplug status. Other uncontrolled influences could have arisen from underlying inter-individual differences in inherent RCREC, or chronic exposure at home for some participants to loud snoring from a bed partner. Comparisons within subjects on and off earplugs, on successive nights in random order, and for more than one night in each condition, might have shown RCREC differences more readily. Finally, objective sleepiness assessments or others less focused than the Stanford Sleepiness Scale on the immediate sensation of sleepiness might have proven more sensitive to earplug use. In contrast to past studies that found associations between RCREC and objective or chronic subjective daytime sleepiness, adjusted logistic regression models for the current data did not reveal associations between delta or sigma RCREC and the Stanford Sleepiness Scale (results not shown).
Despite these limitations that may explain why we failed to detect an effect of earplugs more easily, the positive results for delta EEG frequencies do suggest that snoring sound perception, as modulated by earplugs, can contribute to cortical microarousals (RCREC) during sleep. During non-rapid eye movement (NREM) sleep, hyperpolarization of thalamocortical neurons blocks afferent input to the cortex. In parallel with these cellular changes, sleep spindles decrease the ability of auditory input to generate event-related potentials.27 An opportunity for future research could be to quantify the auditory characteristics of snoring sounds that might overcome thalamocortical sensory gaiting.28,29 Another paradigm of interest might be to record snoring, control airway narrowing with continuous positive airway pressure, and then assess whether RCREC can be re-enhanced by playing back the snorer's original noise, although the external source might not completely simulate internal generation of the noise. At this time, however, lack of associations in our study between earplug use and RCREC in most frequencies, together with the lack of effect modification by snoring prominence on the association between earplug use and delta RCREC, suggest that snoring sounds are not the main cause of RCREC. Respiratory effort as assessed by esophageal pressure similarly explains some but not most of the variance in RCREC.11 Although the possibility remains that RCREC arise from multiple pathways, available data indicate that a physiological explanation for what generates RCREC remains to be clarified.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Chervin serves on the board of directors for the AASM, ABSM, ASMF, and International Pediatric Sleep Association and on the advisory board of Sweet Dreamzzz. He has received research support from Philips Respironics and Fisher Paykel and has consulted for the NIH, Proctor & Gamble, and Zansors. He serves as a section editor for UpToDate and receives remuneration. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Susan L. Garetz, MD, and Ralph Lydic, PhD, for thoughtful assistance on the topics of earplug occlusion effects and thalamic function with respect to auditory stimuli during sleep. This work was performed at the University of Michigan and Michigan Tech Research Institute, Ann Arbor, Michigan. Support: R01 HL080941, R01 HL105999, and UL1RR024986.
REFERENCES
- 1.Partinen M, Hublin C. Epidemiology of sleep disorders. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. St. Louis: Elsevier Saunders; 2011. pp. 694–715. [Google Scholar]
- 2.Gottlieb DJ, Yao Q, Redline S, Ali T, Mahowald MW. Does snoring predict sleepiness independently of apnea and hypopnea frequency? Am J Respir Crit Care Med. 2000;162:1512–7. doi: 10.1164/ajrccm.162.4.9911073. [DOI] [PubMed] [Google Scholar]
- 3.Lee SA, Amis TC, Byth K, et al. Heavy snoring as a cause of carotid artery atherosclerosis. Sleep. 2008;31:1207–13. [PMC free article] [PubMed] [Google Scholar]
- 4.McCormick DA, Bal T. Sensory gating mechanisms of the thalamus. Curr Opin Neurobiol. 1994;4:550–6. doi: 10.1016/0959-4388(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 5.Cairns BE, McErlane SA, Fragoso MC, Jia WG, Soja PJ. Spontaneous discharge and peripherally evoked orofacial responses of trigemino-thalamic tract neurons during wakefulness and sleep. J Neurosci. 1996;16:8149–59. doi: 10.1523/JNEUROSCI.16-24-08149.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Model of thalamo-cortical slow-wave sleep oscillations and transitions to activated states. J Neurosci. 2002;22:8691–704. doi: 10.1523/JNEUROSCI.22-19-08691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastuji H, Garcia-Larrea L, Franc C, Mauguiere F. Brain processing of stimulus deviance during slow-wave and paradoxical sleep: a study of human auditory evoked responses using the oddball paradigm. J Clin Neurophysiol. 1995;12:155–67. doi: 10.1097/00004691-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Afifi L, Guilleminault C, Colrain IM. Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS. Respir Physiol Neurobiol. 2003;136:221–34. doi: 10.1016/s1569-9048(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 9.Chervin RD, Burns JW, Subotic NS, Roussi C, Thelen B, Ruzicka DL. Method for detection of respiratory cycle-related EEG changes in sleep-disordered breathing. Sleep. 2004;27:110–5. doi: 10.1093/sleep/27.1.110. [DOI] [PubMed] [Google Scholar]
- 10.Chervin RD, Burns JW, Subotic NS, Roussi C, Thelen B, Ruzicka DL. Correlates of respiratory cycle-related EEG changes in children with sleep-disordered breathing. Sleep. 2004;27:116–21. doi: 10.1093/sleep/27.1.116. [DOI] [PubMed] [Google Scholar]
- 11.Chervin RD, Malhotra RK, Burns JW. Respiratory cycle-related EEG changes during sleep reflect esophageal pressures. Sleep. 2008;31:1713–20. doi: 10.1093/sleep/31.12.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chervin RD, Shelgikar AV, Burns JW. Respiratory cycle-related EEG changes: response to CPAP. Sleep. 2012;35:203–9. doi: 10.5665/sleep.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chervin RD, Weatherly RA, Ruzicka DL, et al. Subjective sleepiness and polysomnographic correlates in children scheduled for adenotonsillectomy vs. other surgical care. Sleep. 2006;29:495–503. [PMC free article] [PubMed] [Google Scholar]
- 14.Chervin RD, Burns JW, Ruzicka DL. Electroencephalographic changes during respiratory cycles predict sleepiness in sleep apnea. Am J Respir Crit Care Med. 2005;171:652–8. doi: 10.1164/rccm.200408-1056OC. [DOI] [PubMed] [Google Scholar]
- 15.Hoddes E, Dement W, Zarcone V. The development and use of the Stanford Sleepiness Scale (SSS) Psychophysiology. 1972;9:150. [Google Scholar]
- 16.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 17.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. 1st ed. Westchester, Illinois: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- 18.Heinzer R, Gaudreau H, Décary A, et al. Slow-wave activity in sleep apnea patients before and after continuous positive airway pressure treatment: contribution to daytime sleepiness. Chest. 2001;119:1807–13. doi: 10.1378/chest.119.6.1807. [DOI] [PubMed] [Google Scholar]
- 19.Morisson F, Décary A, Petit D, Lavigne G, Malo J, Montplaisir J. Daytime sleepiness and EEG spectral analysis in apneic patients before and after treatment with continuous positive airway pressure. Chest. 2001;119:45–52. doi: 10.1378/chest.119.1.45. [DOI] [PubMed] [Google Scholar]
- 20.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occur-rence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 21.Gozal D, Kheirandish-Gozal L. Obesity and excessive daytime sleepiness in prepubertal children with obstructive sleep apnea. Pediatrics. 2009;123:13–8. doi: 10.1542/peds.2008-0228. [DOI] [PubMed] [Google Scholar]
- 22.Gleeson K, Zwillich CW, White DP. The influence of increasing ventila-tory effort on arousal from sleep. Am Rev Respir Dis. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- 23.Beninati W, Harris CD, Herold DL, Shepard JW. The effect of snoring and obstructive sleep apnea on the sleep quality of bed partners. Mayo Clin Proc. 1999;74:955–8. doi: 10.4065/74.10.955. [DOI] [PubMed] [Google Scholar]
- 24.Dempsey JJ. The occlusion effect created by custom canal hearing aids. Am J Otol. 1990;11:44–6. [PubMed] [Google Scholar]
- 25.Jespersen CT, Groth J, Kiessling J, Brenner B, Jensen OD. The occlusion effect in unilateral versus bilateral hearing aids. J Am Acad Audiol. 2006;17:763–73. doi: 10.3766/jaaa.17.10.7. [DOI] [PubMed] [Google Scholar]
- 26.Le Cocq C, Laville F, Gargour C. Subjective quantification of earplug occlusion effect using external acoustical excitation of the mouth cavity. J Acoust Soc Am. 2010;128:763–70. doi: 10.1121/1.3458843. [DOI] [PubMed] [Google Scholar]
- 27.Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37:563–76. doi: 10.1016/s0896-6273(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 28.Rohrmeier C, Herzog M, Haubner F, Kuehnel TS. The annoyance of snoring and psychoacoustic parameters: a step towards an objective measurement. Eur Arch Otorhinolaryngol. 2012;269:1537–43. doi: 10.1007/s00405-011-1878-2. [DOI] [PubMed] [Google Scholar]
- 29.Bruck D, Ball M, Thomas I, Rouillard V. How does the pitch and pattern of a signal affect auditory arousal thresholds? J Sleep Res. 2009;18:196–203. doi: 10.1111/j.1365-2869.2008.00710.x. [DOI] [PubMed] [Google Scholar]