Abstract
Study Objectives:
Obesity is a recognized risk factor for obstructive sleep apnea syndrome (OSAS). We evaluated whether total trunk and central fat mass (CFM) is associated with OSAS in elderly subjects.
Design:
Cross-sectional.
Setting:
Body composition assessment by dual-energy X-ray absorbsiometry (DEXA).
Participants:
749 volunteers aged 67.2 ± 0.8 years (59.4% women).
Intervention:
All participants underwent evaluation of their body composition by DEXA in parallel with clinical and polygraphic assessments. The presence of OSAS was defined by an apnea plus hypopnea index (AHI) ≥ 15.
Measurements and Results:
A total of 44.8% of the population had an AHI < 15, and 55.2% presented OSAS. OSAS subjects were more frequently overweight and had a higher total trunk fat mass and central fat mass (CFM). Correlation analyses revealed that body mass index (r = 0.27, P < 0.001), neck circumference (r = 0.35, P < 0.001), and CFM (r = 0.23, P < 0.001) were significantly related to AHI. Logistic regression analysis indicated that in mild OSAS cases (> 15AHI < 30), BMI (OR: 1.10; 95% CI: 1.03-1.18; P = 0.008), and male gender (OR: 1.49; 95% CI: 1.05-2.12, P = 0.03) were key factors explaining an AHI between 15 and 30. In severe cases (AHI > 30), male gender (OR: 3.65; 95% CI: 2.40-5.55; P < 0.001) and CFM (OR: 1.10; 95% CI: 1.03-1.19; P = 0.009) were significant independent predictors of OSAS.
Clinical Trial Registration:
NCT 00759304 and NCT 00766584.
Conclusions:
Although central fat mass plays a role in the occurrence of severe OSAS in men older than 65 years of age, its low discriminative sensitivity in mild OSAS cases does not warrant systematic use of DEXA for the diagnosis of OSAS.
Citation:
Degache F; Sforza E; Dauphinot V; Celle S; Garcin A; Collet P; Pichot V; Barthélémy JC; Roche F. Relation of central fat mass to obstructive sleep apnea in the elderly. SLEEP 2013;36(4):501-507.
Keywords: Sleep-related breathing disorders, obstructive sleep apnea, obesity, fat mass, body composition, elderly
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is the most frequent sleep-related breathing disorder (SRBD), with an observed prevalence of 2% to 4% in middle-aged adults2,3 and up to 20% in the elderly.4 Characterized by repeated collapse of the pharynx during sleep, OSAS leads to oxygen desaturation, fragmented sleep, daytime sleepiness, and cardiovascular complications. Hypertension,1 coronary heart disease, and chronic heart failure are all significantly associated with OSAS. More than age, obesity has been found to be the major risk factor for OSAS occurrence, with a 10% weight gain beyond the normal range being associated with an increased risk of developing this disorder.5
The mechanisms linking obesity with risk of OSAS include diminished pharyngeal lumen diameter, due to fatty tissue in the lateral airway walls, decreased upper airway muscle force due to fatty deposits in the muscles, and reduced upper airway diameter secondary to a mass effect of the enlarged abdomen on the chest walls and tracheal traction.6–8 Moreover, previous studies have demonstrated that rather than a generalized increase in fat mass, it is central obesity, for which abdominal obesity is used as a surrogate, and upper body fat distribution, for which neck circumference is used as a marker, that play a key role in the development of OSAS.9–11
Several reports have suggested that central abdominal fat rather than total body fat is most closely associated with metabolic syndrome,12 and cardiovascular risk in both adults13,14 and children,15–17 suggesting that assessment of body fat distribution is an important factor in the control of risk factors associated with OSAS. This has led to increasing interest in the evaluation of methods for assessing body fat distribution, such as measurement of waist circumference (WC), determination of body mass index (BMI), computed tomography (CT), magnetic resonance imaging (MRI) and dual energy X-ray absorptiometry (DEXA). DEXA is a validated technique capable of accurately determining the cross-sectional mass of discrete fat deposits.18–20 Even though DEXA cannot distinguish between intra-abdominal visceral and subcutaneous adipose tissue, it provides an inexpensive tool for exploring in large populations the clinical significance of central obesity and its associated risks.21
A relationship between anthropometric data and central obesity has been proposed in OSAS, but only few studies in small patient samples have measured visceral fat distribution.22–26 Moreover, no data have been published concerning the possible role of obesity in elderly people, in whom OSAS is highly prevalent.4 In this study, we analyzed the role of central obesity in OSAS occurrence in a large population-based cohort of healthy elderly people. In this way, we aimed to evaluate whether: (1) central obesity is associated with an increased risk of OSAS in the elderly, and (2) whether DEXA measurements of central obesity provide more information than BMI, or waist and neck circumferences, in terms of increased risk of OSAS.
METHODS
Population
The study sample was recruited from the population included in the PROOF (PROgnostic indicator OF cardiovascular and cerebrovascular events) survey.27 This was a longitudinal study in a population-based cohort of 1,011 volunteers aged over 65 years, living in the city of Saint-Etienne (France) designed to assess the influence of autonomic nervous system activity on cardiovascular and cerebrovascular morbidity and mortality. An ancillary study addressing the association between OSAS, assessed by ambulatory polygraphic recording, and cardiovascular and cerebrovascular morbidity during a 7-year follow-up, was proposed to participants (the Synapse study). The criteria for inclusion in the Synapse study comprised: absence of previous myocardial infarction, stroke, and atrial fibrillation; previous OSAS diagnosis or treatment; acceptance to undergo polygraphy, 24-h monitoring of blood pressure and heart rate, and withdrawal of blood samples for analysis. A total of 854 subjects (58.5% women), meeting all these inclusion criteria and none of the exclusion criteria, were enrolled in the Synapse study. All subjects underwent a clinical assessment including a questionnaire on demographic characteristics, medical history and medication, and an evaluation of sleepiness according to the Epworth Sleepiness Scale (ESS). Within this group of volunteers, 749 subjects (59.4% women), aged 67.2 ± 0.8 years, accepted to undergo a DEXA evaluation of their body composition at the same time as the clinical and polygraphic assessments. These subjects constituted the final sample for the study described here.
This study was approved by the local Ethics Committee (CCPPRB Rhône-Alpes Loire), and a written consent to participation was obtained from all participants. Some of the DEXA data presented here were also used in a previous paper focusing on the influence of DEXA measurements on the effect of gender in OSAS.28
Anthropometric Measurements
BMI was calculated as weight/height squared (kg/m2). Neck circumference (NC) was measured at the middle of the neck between the mid-cervical spine and the mid-anterior neck 0.5 cm below the laryngeal prominence, and waist circumference (WC) was measured midway between the lower rib margin and the iliac crest.
Measurement of Body Composition by DEXA
Regional (arms, legs, trunk, and head) body fat and lean body mass were measured using a whole-body DEXA scanner (Ho-logic QDR-2000, software version V5.67A, Hologic Inc., Bedford, MA). The standard procedures described in the literature for DEXA measurements were employed,19,20,29,30 the Hologic instrument using a single scan mode for all adult subjects.15,16,31 The coefficient of variation of the instrument was < 1% for in vivo measurements.
The body regions were delineated by means of specific anatomical landmarks. Peripheral fat mass (PFM) was calculated by adding the fat mass of the legs to that of the arms, and peripheral lean mass by adding the lean mass of the legs to that of the arms. Central fat mass (CFM) was calculated either as the trunk fat mass (TFM) or as the sum of the TFM and the fat mass of the head (HFM). In our sample no significant differences were found between TFM and CFM defined as the sum of TFM and HFM.
Sleep Study
An unattended nocturnal study was performed at home in all subjects using a polygraphic recording system (HypnoPTT, Tyco Healthcare, Puritan Bennett). The following parameters were recorded: sound emission, electrocardiogram, pulse transit time, R-R interval, airflow based on nasal pressure, respiratory effort, and body position. Oxygen saturation (SpO2) was measured by pulse oximetry. A software package was used for downloading and analysis of tracings. A recording duration ≥ 5 h was required for validation, and monitoring was repeated on a second night if subjective sleep latency exceeded 2 h on the first night or if respiratory parameters were missing. All recordings were visually validated and manually scored for respiratory events and nocturnal SpO2. Hypopnea was defined as ≥ 50% reduction in airflow from the baseline value, lasting ≥ 10 s and associated with ≥ 3% oxygen desaturation. Apnea was defined as an absence of airflow through the nasal cannula lasting > 10 s. The absence of rib cage movements during apnea defined the event as central, whereas a progressive increase in rib cage movements and pulse transit time defined the event as obstructive. To minimize potential overestimation of sleep duration, subjects completed a sleep diary to set the analysis between lights-off and lights-on. The apnea+hypopnea index (AHI) was established as the ratio of the number of apneas and hypopneas per hour of reported sleep time. Indices of nocturnal hypoxemia were as follows: mean SpO2, percentage of recording time spent with SpO2 < 90%, minimal SpO2 value recorded during sleep, and oxygen desaturation index (ODI), defined as the number of episodes of oxyhemoglobin desaturation/h of reported sleep time during which blood oxygen level fell ≥ 3%. According to recent data in elderly subjects,32 an AHI ≥ 15 with ≥ 85% of events scored as obstructive may be considered diagnostic of OSAS; > 15AHI < 30 indicated mild OSAS, while an AHI ≥ 30 indicated severe OSAS. The presence of daytime sleepiness was assessed using a French version of the ESS,33 sleepiness being defined by an ESS score > 10.
Statistical Analysis
The characteristics of the study population in terms of DEXA data and anthropometric measurements were compared amon the 3 AHI groups, using a univariate ANOVA or χ2 test as appropriate. The results are presented as means ± standard deviation (SD) for continuous variables and percentages for categorical variables. The strength of the relationships between DEXA measurements, anthropometric data, and continuous values of AHI were estimated using Pearson correlation coefficient.
Logistic regression analyses were performed to assess the relationship between each risk factor and AHI group. Multivariate models were used to adjust for potential confounders, namely gender and hypertension, and different models were employed to estimate the specific impact of DEXA measurements and anthropometric data. Likelihood ratio tests were then performed to compare the fit between the different models. For each model, the proportional odds assumption was assessed using a parallel-line test. The results were summarized as crude and adjusted odds ratios with the corresponding 95% confidence intervals (CI). All reported P values are two-sided, with a P value < 0.05 being considered statistically significant.
Data were analyzed using the Statistical Package for the Social Sciences version 17 for Windows (SPSS Inc., Chicago, Illinois, USA) and Stata release 11 (Stat Corp, College Station, Texas, USA).
RESULTS
Characteristics of the Study Population
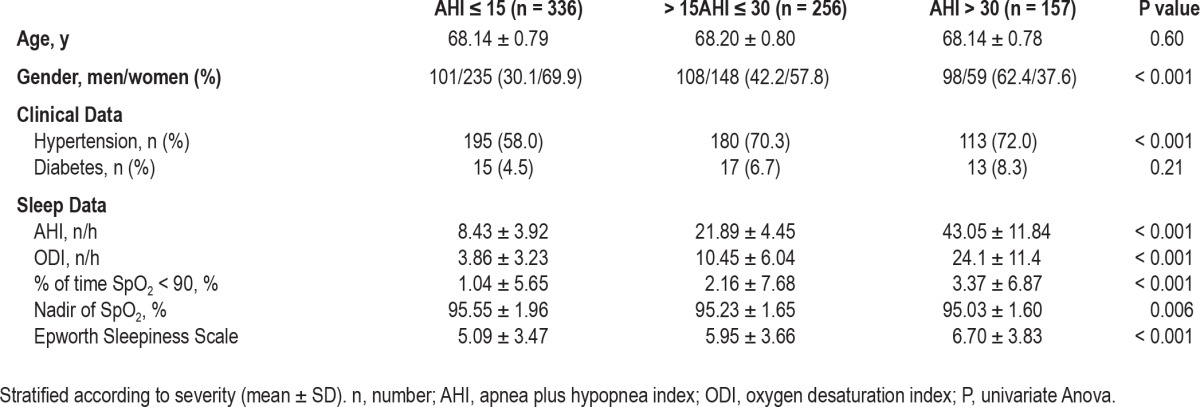
The clinical and polygraphic characteristics of the sample, stratified according to the absence or presence and severity of OSAS are shown in Table 1. In total, 44.8% of the population had an AHI < 15, with OSAS present in 55.2%. Within the OSAS group, 62% presented a mild form of OSAS (> 15AHI < 30) and 38% had a severe form (AHI ≥ 30). While in the group without OSAS and in subjects with mild OSAS women outnumbered men, the majority of subjects with severe OSAS were male. Hypertension and diabetes were the most prevalent cardiovascular risk factors observed in subjects with OSAS. As expected, the groups differed with respect to the severity of AHI and hypoxemia without significant differences in daytime sleepiness, the ESS score being within the normal range in all 3 study groups.
Table 1.
Clinical and polygraphic characteristics of subjects with and without (AHI ≤ 15) OSAS
Anthropometric Data and DEXA Measurements
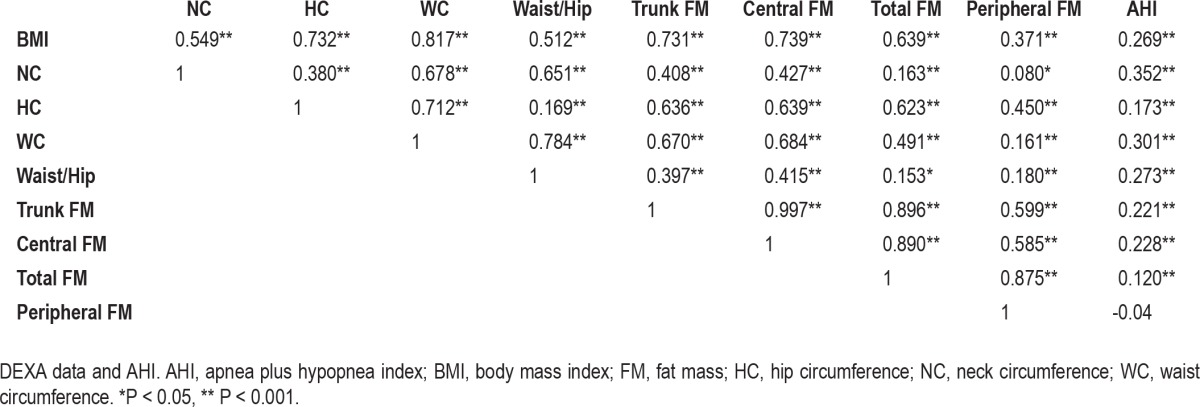
The anthropometric and DEXA data of subjects with an AHI < 15 and those with mild and severe OSAS are presented in Table 2. The subjects manifesting OSAS were more likely to be overweight (59%) or obese and have a significantly higher NC, HC, WC, and waist/hip ratio. The DEXA data demonstrated that body mass, total lean mass, and trunk and central fat mass were the parameters differing significantly between subjects with and without OSAS (P < 0.001), their values tending to increase progressively with the severity of the disorder. Simple linear correlation analysis between anthropometric measurements, DEXA indices, and AHI showed a significant relationship between all measurements (Table 3)—BMI, NC, WC, and CFM being the variables most strongly correlated with AHI. Correlation coefficients between CFM and AHI, and between CFM and ODI reached values of 0.27 (P < 0.001) and 0.34 (P < 0.001), respectively, indicating a stronger relationship between CFM and severity of hypoxemia.
Table 2.
Anthropometric and body composition DEXA measurements in subjects with and without (AHI ≤ 15) OSAS
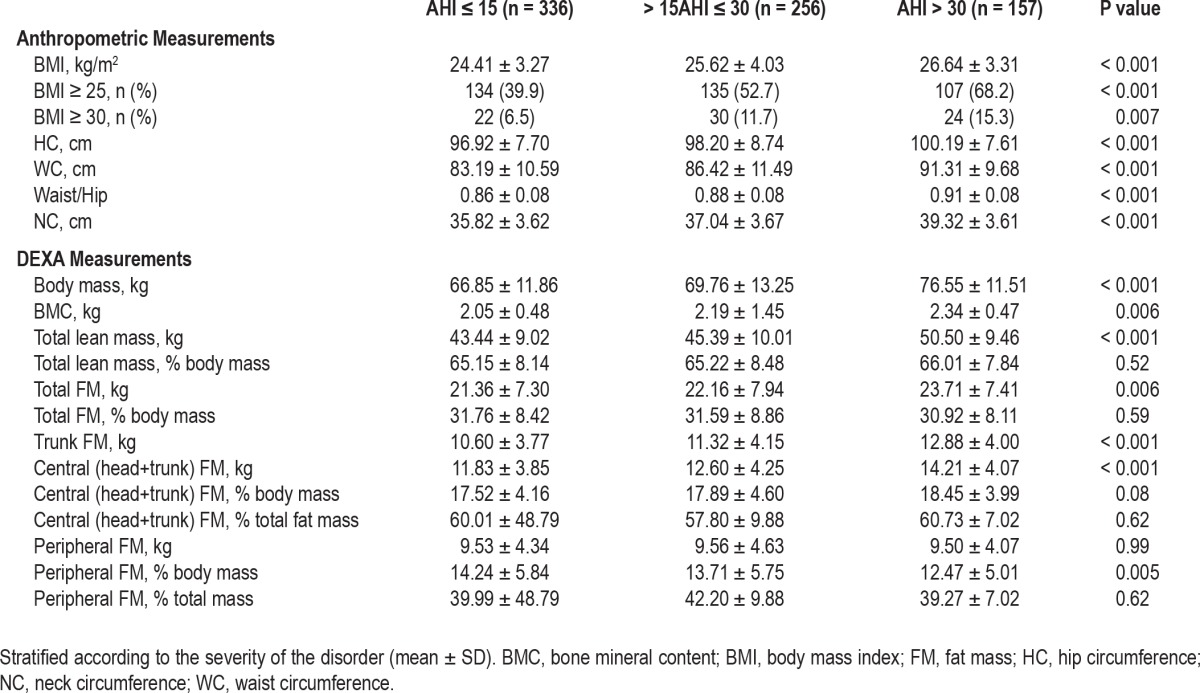
Table 3.
Analysis of the correlations between anthropometric data
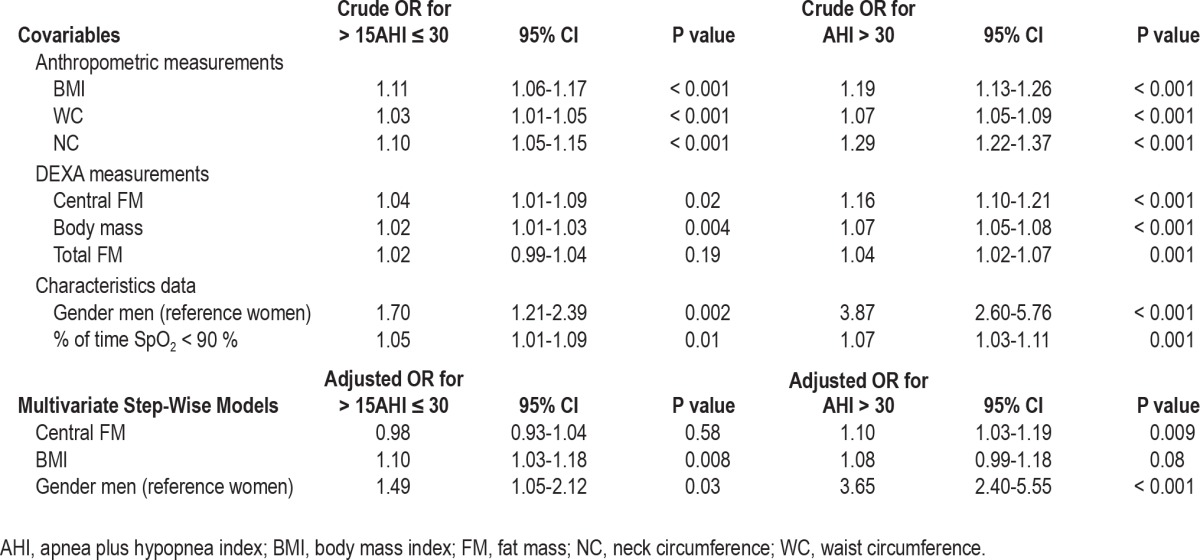
Univariate and multivariate logistic regression analyses were performed to assess the relationship between AHI groups and gender, BMI, NC, and CFM, respectively, in order to evaluate the independent contribution of DEXA measurements. For the total OSAS group (Table 4), NC, BMI, and CFM were the key factors explaining the presence of an AHI ≥ 15, with the role of BMI being predominant. Table 5 shows the results of multinomial logistic regression analysis for the groups presenting mild and severe OSAS, respectively. In the multiple logistic regression model, male gender and CFM remained independent predictors of severe OSAS (OR: 1.10; 95% CI: 1.03-1.19; P = 0.009); BMI, in contrast, being the most important factor in mild cases (OR: 1.10; 95% CI: 1.03-1.18; P = 0.008).
Table 4.
Factors related to an AHI ≥ 15 revealed by logistic regression analysis
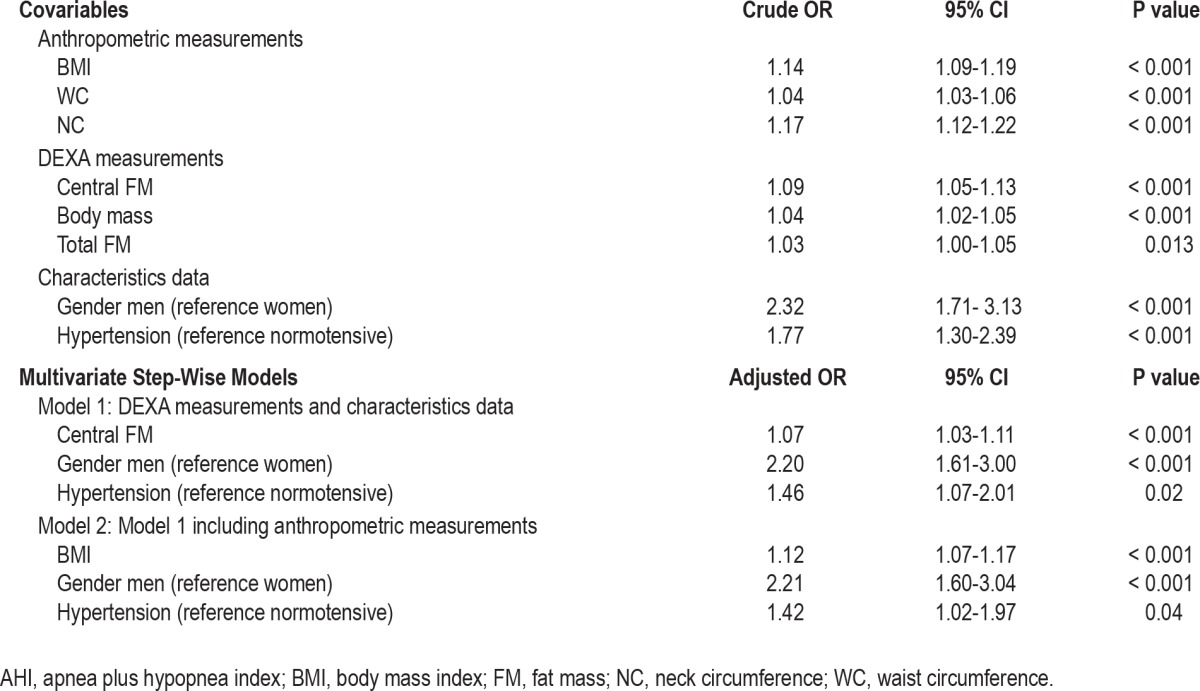
Table 5.
Multinomial logistic regression analysis for mild (> 15AHI ≤ 30) and severe (AHI > 30) OSAS
DISCUSSION
To the best of our knowledge, this is the first study to have investigated whether DEXA measurements are useful for predicting OSAS in the elderly and to analyze the relationship between fat mass distribution and OSAS. Analyzing anthropometric and DEXA data in a large sample of elderly subjects, we found that, whereas BMI more accurately identified the presence of a mild form of OSAS, male gender and central fat distribution better defined the presence of severe cases. We can therefore conclude that in elderly subjects, anthropometric data such as BMI and NC may allow the diagnosis of OSAS, while specific regional fat distribution (namely CFM) and male gender are prognostic factors for the severity of this sleep disorder.
During recent years, a wealth of evidence has indicated the importance of a bidirectional and feed-forward association between OSAS, metabolic syndrome, and obesity.34 Obesity and visceral fat contribute to cardiovascular risk and OSAS occurrence,35,36 while OSAS, via sympathetic hyperactivity and insulin resistance,24,26,34,37 exacerbates obesity. Although BMI and NC are commonly used to define the severity of obesity, measures of central adiposity such as WC have been adopted as more accurate predictors of cardiovascular14 and OSAS risk.11 However, the discriminatory sensitivity and specificity of these measures to detect OSAS are similar, and no systematic study has suggested the superiority of one measure over the others.18 More sophisticated methods such as DEXA have been introduced to determine whether distribution of fat mass rather than BMI is more sensitive to detect an OSAS risk.22,23,25 Since the studies conducted so far have been performed in children and middle-aged patients,17,20,22,25 we analyzed the power of anthropometric and DEXA measures to discriminate OSAS in a large sample of elderly subjects. In accordance with the results reported in middle-aged patients, we found that indirect measures of obesity and fat distribution, namely BMI, NC, and WC, showed a strong and significant correlation with AHI and nocturnal hypoxemia. Interestingly, whereas BMI was the strongest predictor of mild OSAS cases, CFM and male gender were more accurate predictors of severe OSAS. These findings suggest that whereas a simple anthropometric measure such as BMI is alone sufficient to discriminate the presence of OSAS, CFM more accurately identifies severe cases.
Two important questions were raised by our results. The first is whether a DEXA examination should be introduced in clinical practice to evaluate the effect of obesity on older subjects with OSAS; the second is whether the measurement of fat distribution and obesity in the elderly may be affected by confounding factors such as aging and gender. With regard to the first question, CFM was only slightly more powerful than anthropometric measures in severe cases, suggesting that DEXA examinations should not be used routinely to diagnose OSAS. Whereas BMI, WC, and HC are sufficient to clinically stratify the risk of OSAS in elderly subjects, CFM may be more useful for understanding the link between fat distribution, cardiovascular risk, and OSAS severity. The low discriminatory power of CFM in OSAS detection may be explained by the physiological, age-related increase in AHI,35,38 and the lack of clear criteria to define the stage at which AHI becomes clearly pathological in the elderly.39
When we consider confounding factors, we know that gender and aging may interact to increase the risk of OSAS, as demonstrated by the observation that OSAS is more frequent in men and in older subjects.3,4,39,40 This gender effect is related more to differences in fat distribution than to obesity per se.18,31,41 Whereas in men, fat typically accumulates in the upper part of the body, in women, adipose tissue accumulates subcutaneously, particularly over the thighs.6,9,11 Fat deposition in the upper pharyngeal regions may lead to upper airway narrowing during sleep, predisposing men with higher TFM and CFM to more severe OSAS. An interesting finding is that in our sample, no significant gender differences were found for CFM, the mean values being similar in men (12.7 ± 4.0) and women (12.5 ± 4.2). This might be explained by the approximately 12% to 18% increase in visceral fat deposition in people over 65 years old42 and by the shift towards upper body fat deposition occurring in women after menopause.43,44
Some strengths and limitations of our study need to be considered. The major strength is the analysis of a well-defined and large cohort of older people undergoing a systematic clinical and instrumental evaluation and homogeneity in terms of age, allowing a more accurate estimation of the role of OSAS with respect to associated risk factors without the confounding effect of age. The first limitation of the study is that participants were recruited from a cross-sectional community-based survey of a homogenous group of elderly subjects enrolled on the basis of strict inclusion criteria. Therefore, we cannot exclude the possibility that our cohort is not fully representative of a general population, in which case our results cannot be extrapolated to patients seen in routine clinical practice. Second, our cohort comprised subjects aged over 65 years, who could be considered as “young” elderly persons, precluding the application of our results to very “old-old” subjects. Finally, we used an ambulatory polygraphic system for the sleep study, which did not allow assessment of sleep structure or the relationship of respiratory events to sleep stages. However, ambulatory polygraphy is currently considered to be a useful tool for the routine assessment and screening of OSAS in the elderly, facilitating epidemiological evaluations.45
In conclusion, even if central fat mass plays a role in the occurrence of severe OSAS in men aged over 65 years, its low discriminative sensitivity in mild cases does not allow a systematic use of DEXA for the diagnosis of OSAS. Further studies in large clinical populations and in middle-aged patients are needed to assess the pathogenetic role of CFM in the occur-rence and severity of OSAS.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Drs. Jean-Claude Barthélémy and Frédéric Roche are equally contributing senior authors. The PROOF study group thanks all the subjects included in the present study, as well as Mr. Olivier Grataloup, Mrs. Delphine Maudoux, Dr. Stéphane Chomienne (CHU Saint-Etienne, France), Prof. Thierry Thomas and Prof. Christian Alexandre (Service de Rhumatologie, CHU, INSERM EMI 366, Faculté de Médecine Jacques Lisfranc, Université Jean Monnet, Saint Etienne, France, PRES Université de Lyon, France) for their expert help in data acquisition.
Footnotes
A commentary on this article appears in this issue on page 457.
REFERENCES
- 1.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occur-rence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 4.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154:50–9. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Horner RL, Mohiaddin RH, Lowell DG, et al. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur Respir J. 1989;2:613–22. [PubMed] [Google Scholar]
- 7.Mohsenin V. Gender differences in the expression of sleep-disordered breathing: role of upper airway dimensions. Chest. 2001;120:1442–7. doi: 10.1378/chest.120.5.1442. [DOI] [PubMed] [Google Scholar]
- 8.Mortimore IL, Marshall I, Wraith PK, Sellar RJ, Douglas NJ. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am J Respir Crit Care Med. 1998;157:280–3. doi: 10.1164/ajrccm.157.1.9703018. [DOI] [PubMed] [Google Scholar]
- 9.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 10.Grunstein R, Wilcox I, Yang TS, Gould Y, Hedner J. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes. 1993;17:533–40. [PubMed] [Google Scholar]
- 11.Hoffstein V, Mateika S. Differences in abdominal and neck circumferences in patients with and without obstructive sleep apnoea. Eur Respir J. 1992;5:377–81. [PubMed] [Google Scholar]
- 12.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 13.Lavie P, Herer P, Peled R, Berger I, Yoffe N, Zomer J, Rubin AH. Mortality in sleep apnea patients: a multivariate analysis of risk factors. Sleep. 1995;18:149–57. doi: 10.1093/sleep/18.3.149. [DOI] [PubMed] [Google Scholar]
- 14.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61:646–53. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Brambilla P, Bedogni G, Moreno LA, et al. Crossvalidation of anthropometry against magnetic resonance imaging for the assessment of visceral and subcutaneous adipose tissue in children. Int J Obes. 2006;30:23–30. doi: 10.1038/sj.ijo.0803163. [DOI] [PubMed] [Google Scholar]
- 16.Slyper AH. Childhood obesity, adipose tissue distribution, and the pediatric practitioner. Pediatrics. 1998;102:e4. doi: 10.1542/peds.102.1.e4. [DOI] [PubMed] [Google Scholar]
- 17.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch Dis Child. 92:205–8. doi: 10.1136/adc.2006.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K, Lee S, Kim YJ. Waist circumference, dual-energy X-ray absortiometrically measured abdominal adiposity, and computed tomographically derived intra-abdominal fat area on detecting metabolic risk factors in obese women. Nutrition. 2008;24:625–31. doi: 10.1016/j.nut.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Paradisi G, Smith L, Burtner C, et al. Dual energy X-ray absorptiometry assessment of fat mass distribution and its association with the insulin resistance syndrome. Diabetes Care. 1999;22:1310–7. doi: 10.2337/diacare.22.8.1310. [DOI] [PubMed] [Google Scholar]
- 20.Wong WW, Hergenroeder AC, Stuff JE, Butte NF, Smith EO, Ellis KJ. Evaluating body fat in girls and female adolescents: advantages and disadvantages of dual-energy X-ray absorptiometry. Am J Clin Nutr. 2002;76:384–9. doi: 10.1093/ajcn/76.2.384. [DOI] [PubMed] [Google Scholar]
- 21.Bestetti A, Castini D, Bigi R, et al. Truncal fat determined by dual-energy X-ray absorptiometry is an independent predictor of coronary artery disease extension. Eur J Cardiovasc Prev Rehab. 2008;15:428–33. doi: 10.1097/HJR.0b013e3282fb2e05. [DOI] [PubMed] [Google Scholar]
- 22.Bruno E, Alessandrini M, Napolitano B, De Padova A, Di Daniele N, De Lorenzo A. Dual-energy X-ray absorptiometry analysis of body composition in patients affected by OSAS. Eur Arch Otorhinolaryngol. 2009;266:1285–90. doi: 10.1007/s00405-008-0844-0. [DOI] [PubMed] [Google Scholar]
- 23.Chin K, Shimizu K, Nakamura T, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–12. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 24.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–41. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Ogretmenoglu O, Suslu AE, Yucel OT, Onerci TM, Sahin A. Body fat composition: a predictive factor for obstructive sleep apnea. Laryngo-scope. 2005;115:1493–8. doi: 10.1097/01.mlg.0000172204.82314.c3. [DOI] [PubMed] [Google Scholar]
- 26.Schafer H, Pauleit D, Sudhop T, Gouni-Berthold I, Ewig S, Berthold HK. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 2002;122:829–39. doi: 10.1378/chest.122.3.829. [DOI] [PubMed] [Google Scholar]
- 27.Barthelemy JC, Pichot V, Dauphinot V, et al. Autonomic nervous system activity and decline as prognostic indicators of cardiovascular and cerebrovascular events: the ‘PROOF’ Study. Study design and population sample. Associations with sleep-related breathing disorders: the ‘SYNAPSE’ Study. Neuroepidemiology. 2007;29:18–28. doi: 10.1159/000108914. [DOI] [PubMed] [Google Scholar]
- 28.Sforza E, Chouchou F, Collet P, Pichot V, Barthelemy JC, Roche F. Sex differences in obstructive sleep apnoea in an elderly French population. Eur Respir J. 2011;37:1137–43. doi: 10.1183/09031936.00043210. [DOI] [PubMed] [Google Scholar]
- 29.Forbes GB. Body composition: overview. J Nutr. 1999;129:270S–2S. doi: 10.1093/jn/129.1.270S. [DOI] [PubMed] [Google Scholar]
- 30.Salamone LM, Fuerst T, Visser M, et al. Measurement of fat mass using DEXA: a validation study in elderly adults. J Appl Physiol. 2000;89:345–52. doi: 10.1152/jappl.2000.89.1.345. [DOI] [PubMed] [Google Scholar]
- 31.Yeo SE, Hays NP, Dennis RA, et al. Fat distribution and glucose metabolism in older, obese men and women. J Gerontol A Biol Sci Med Sci. 2007;62:1393–401. doi: 10.1093/gerona/62.12.1393. [DOI] [PubMed] [Google Scholar]
- 32.Pavlova MK, Duffy JF, Shea SA. Polysomnographic respiratory abnormalities in asymptomatic individuals. Sleep. 2008;31:241–8. doi: 10.1093/sleep/31.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Billiard M. Le sommeil normal et pathologique. Paris: 1998. [PubMed] [Google Scholar]
- 34.Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005;9:211–24. doi: 10.1016/j.smrv.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Pillar G, Shehadeh N. Abdominal fat and sleep apnea: the chicken or the egg? Diabetes Care. 2008;31(Suppl 2):S303–9. doi: 10.2337/dc08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinohara E, Kihara S, Yamashita S, et al. Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med. 1997;241:11–8. doi: 10.1046/j.1365-2796.1997.63889000.x. [DOI] [PubMed] [Google Scholar]
- 37.Peled N, Kassirer M, Shitrit D, et al. The association of OSA with insulin resistance, inflammation and metabolic syndrome. Respir Med. 2007;101:1696–701. doi: 10.1016/j.rmed.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 38.Ancoli-Israel S, Ayalon L. Diagnosis and treatment of sleep disorders in older adults. Am J Geriatr Psychiatry. 2006;14:95–103. doi: 10.1097/01.JGP.0000196627.12010.d1. [DOI] [PubMed] [Google Scholar]
- 39.Launois SH, Pepin JL, Levy P. Sleep apnea in the elderly: a specific entity? Sleep Med Rev. 2007;11:87–97. doi: 10.1016/j.smrv.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Mant A, Saunders N, Eyland E. Sleep habits and sleep related respiratory disturbance in an older population. In: Horne J, et al., editors. Sleep ‘88. Stuttgart: Gustav Fischer Verlag; 1989. pp. 260–1. [Google Scholar]
- 41.Millman RP, Carlisle CC, McGarvey ST, Eveloff SE, Levinson PD. Body fat distribution and sleep apnea severity in women. Chest. 1995;107:362–6. doi: 10.1378/chest.107.2.362. [DOI] [PubMed] [Google Scholar]
- 42.Seidell JC, Oosterlee A, Deurenberg P, Hautvast JG, Ruijs JH. Abdominal fat depots measured with computed tomography: effects of degree of obesity, sex, and age. Eur J Clin Nutr. 1988;42:805–15. [PubMed] [Google Scholar]
- 43.Aloia JF, Vaswani A, Russo L, Sheehan M, Flaster E. The influence of menopause and hormonal replacement therapy on body cell mass and body fat mass. Am J Obst Gynecol. 1995;172:896–900. doi: 10.1016/0002-9378(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 44.Resta O, Bonfitto P, Sabato R, De Pergola G, Barbaro MP. Prevalence of obstructive sleep apnoea in a sample of obese women: effect of meno-pause. Diabetes Nutr Metab. 2004;17:296–303. [PubMed] [Google Scholar]
- 45.Johansson P, Alehagen U, Svanborg E, Dahlstrom U, Brostrom A. Sleep disordered breathing in an elderly community-living population: Relationship to cardiac function, insomnia symptoms and daytime sleepiness. Sleep Med. 2009;10:1005–11. doi: 10.1016/j.sleep.2009.01.011. [DOI] [PubMed] [Google Scholar]


