SYNOPSIS
Sepsis remains an important challenge in pediatric critical care medicine. The current review intends to provide an appraisal of adjunctive therapies for sepsis and to highlight opportunities for meeting selected challenges in the field. Future clinical studies should address long-term and functional outcomes, as well as acute outcomes. Potential adjunctive therapies such as corticosteroids, hemofiltration, hemoadsorption, and plasmapheresis may have important roles, but still require formal and more rigorous testing by way of clinical trials. Finally, the design of future clinical trials should consider novel approaches for stratifying outcome risks as a means of improving the risk to benefit ratio of experimental therapies.
Keywords: epidemiology, outcomes, antibiotics, quality improvement, corticosteroids, hemofiltration, hemoadsorption, plasmapheresis, stratification
INTRODUCTION
Sepsis remains a major challenge in the field of pediatric critical care medicine. Several recent publications cover the general principles of sepsis management, as well as pathophysiology in a developmental context [1–5]. The current review intends to provide an appraisal of adjunctive therapies for sepsis and to highlight opportunities for meeting selected challenges in the field.
EPIDEMIOLOGIC AND QUALITY IMPROVEMENT CHALLENGES
Sepsis is estimated to be the leading cause of death in infants and children worldwide, with an annual mortality of approximately 1.6 million per year. In the United States, approximately 42,000 cases of severe sepsis occur annually and in-hospital mortality is estimated at 10.3% [6, 7]. The mean length of stay and cost for a child with severe sepsis in the United States are estimated to be 31 days and over $40,000, respectively. Clearly, sepsis remains an important public health issue in both underdeveloped and developed countries, and consequently brings many opportunities for translational research and quality improvement efforts.
The ability to benchmark outcomes, based on a reliable outcome metric (i.e. reliable outcome prediction), is fundamental to quality improvement efforts and improvement science [8]. Unfortunately, there is no quality metric or outcome benchmark specific to pediatric sepsis. Scoring systems based on physiological and clinical variables, such as the Pediatric Risk of Mortality (PRISM) score and the Pediatric Index of Mortality (PIM), are very robust for predicting outcomes of general pediatric intensive care unit populations, but begin to perform less well when applied to specific diseases, such as sepsis [9]. Recently, a multi-biomarker-based outcome risk model was developed and validated that reliably predicts outcome in children with severe sepsis and septic shock [10, 11]. While the model requires further prospective testing, it is hoped that this model will enhance currently available scoring systems and therefore provide a sepsis-specific quality metric to better assess short-term outcomes of pediatric sepsis.
While short-term outcomes (i.e. acute mortality or survival) will continue to be important considerations in translational research efforts and clinical trials, increasingly greater attention is now focused on sepsis-related morbidity and mortality beyond the acute phase. Quartin et al. reported that adults who initially recover from the acute stage of sepsis have an increased risk of death for up 5 years after discharge, even after accounting for the effects of co-morbidities [12]. Karlsson et al. documented a two year mortality rate of 45% for adults after severe sepsis and decreased quality of life in sepsis survivors at a median of 17 months after the acute episode of severe sepsis [13].
Similar data are now being reported in pediatric survivors of severe sepsis. Czaja et al. retrospectively studied over 7,000 pediatric severe sepsis cases [14]. Almost one-half of the patients that were discharged after the initial admission were re-admitted at least once, at a median of 3 months after discharge. Respiratory infection was the most common indication for readmission and >30% of these readmissions were in children without co-morbidities. An additional 6.5% of patients died during these readmissions. Thus, sepsis has important long-term consequences and there is a need to more robustly assess long-term outcomes, as well as functional outcomes beyond the acute dichotomy of “alive” or “dead”.
The Functional Status Scale (FSS) was recently developed to specifically meet the need of assessing functional outcomes of critically ill children [15]. The FSS incorporates several relevant functional assessments including mental status, sensory functioning, communication, motor functioning, feeding, and respiratory status, and is designed to be applied in diverse and time limited environments. The FSS appears to have very good inter-rater reliability, and its performance compares favorably with more complex and labor-intensive functional outcome tools. A major challenge moving forward, as stated by the FSS investigators, is the development of subgroup-specific versions of FSS (e.g. sepsis specific) [15].
APPROPRIATENESS AND TIMING OF ANTIBIOTICS
Along with aggressive fluid resuscitation, prompt and appropriate administration of antibiotics continues to be a cornerstone of therapy for patients with severe sepsis and septic shock. The 2008 Surviving Sepsis Campaign Guidelines, utilizing a grading scheme involving both strength of recommendation and quality of evidence, assigned a 1B level recommendation for the use of broad spectrum intravenous antibiotics within the first hour of septic shock [16]. There are two major components to this recommendation: appropriateness and timing of antibiotics.
While it seems intuitive that inappropriate antibiotic usage in septic shock is likely to be associated with poorer outcome, corroboration with existing proof of concept literature provides further support. Notable studies include a prospective cohort study involving 492 critically ill adults with documented bloodstream infections, 147 (29.9%) of whom received inadequate antimicrobial treatment, defined as infection with an organism that was not susceptible to the antimicrobials being used at the time of positive culture results [17]. Patients not adequately treated had a mortality rate of 61.9%, whereas patients who were adequately treated had a mortality rate of 28.4%. Similarly, a retrospective review of 5,175 adult patients with septic shock, in three countries, reported 5-fold increased hospital mortality for those receiving inappropriate antibiotics. In this study, inappropriate antibiotics were defined based on sensitivity testing, or in the case of culture negative septic shock, expert clinical opinion and existing guidelines (6). These and other related studies strongly support the importance of appropriate antimicrobial therapy in severe sepsis and septic shock.
Kumar et al have provided compelling experimental evidence supporting the importance of early antibiotic treatment of sepsis (i.e. timing of antibiotics) in animal models [18]. In mice undergoing intraperitoneal implantation of an E. coli-laced, gelatin capsule-encased fibrinogen clot, treatment with antibiotics at ≤ 12 hours after implantation resulted in ≤ 20% mortality. In comparison, antibiotic administration at ≥ 15 hours after implantation resulted in > 85% mortality. Of note, significant hypotension was noted by 12 hours after implantation in untreated mice, leading the authors to propose the existence of a “critical inflection point” for the impact of antibiotics on survival.
A large, retrospective, multicenter study of adults with septic shock followed this laboratory-based study [19]. The overall in hospital mortality for this cohort was 56.2%, however, for patients that received appropriate antibiotics within the first hour of hypotension, in hospital mortality was 20.1%. Interestingly, following onset of hypotension, mortality was found to increase by an average of 7.6% for every hour untreated over the subsequent 6 hours. Similarly, a study involving 291 adults with sepsis in the emergency department reported that while longer times between initial triage and antibiotic administration were not independently associated with increased mortality, delay in treatment following recognition of shock was independently associated with increased mortality [20].
Similar scale studies do not exist in the pediatric sepsis literature. One small retrospective study involving children with community acquired pneumonia and the need for mechanical ventilation (n = 45) assessed the impact of antibiotic timeliness and appropriateness on surrogate outcomes [21]. Delays in antibiotic administration as short as 2 to 4 hours were independently associated with longer duration of mechanical ventilation, and longer intensive care unit and hospital lengths of stay.
Collectively, these data provide substantial support that the readily tangible concept of “timeliness of appropriate antibiotics” is an important area of focus for quality improvement efforts in the field of clinical sepsis. In this regard, Cruz et al. recently reported the implementation of a protocol to optimize antibiotic administration in children with signs of sepsis and presenting to the emergency department of a large, tertiary children’s hospital [22]. Prior to protocol implementation, the median time from initial triage to antibiotic administration was 130 minutes. The median time from initial triage to antibiotic administration decreased to 38 minutes after protocol implementation.
FLUID RESUSCITATION
Over 20 years ago, Carcillo, Davis, and Zaritsky reported that in children with septic shock, fluid resuscitation in excess of 40 ml/kg within the first hour of presentation was associated with improved survival, without an increase in the risk of cardiogenic pulmonary edema or acute respiratory distress syndrome [23]. Since that time, aggressive fluid resuscitation has been a mainstay of both adult and pediatric guidelines for the management of septic shock [5, 16]. In addition, subsequent observational and interventional studies further support and corroborate the importance of aggressive fluid resuscitation early in septic shock [24–27].
While seemingly one of the most fundamental principles in the field of critical care medicine, aggressive fluid resuscitation in septic shock has been recently questioned and critiqued as being weakly supported [28]. Indeed, cohort studies have reported an association between positive fluid balance and mortality [29–32]. Most recently, The Fluid Expansion as Supportive Therapy (FEAST) study compared 20 to 40 ml/kg fluid boluses versus no bolus in over 3,000 acutely ill African children [33]. The FEAST study reported a significantly increased mortality risk in the group randomized to the fluid bolus arm.
These data should not lead one directly to the premature and erroneous conclusion that fluid resuscitation is intrinsically deleterious for patients with septic shock. The concepts of “fluid overload” and “aggressive volume resuscitation” are certainly related, but are also distinct temporally and mechanistically. None of the observational studies linking fluid overload and mortality have clearly proven a cause and effect relationship. Positive fluid balance could simply be a marker of increased illness severity leading to increased vascular leak and increased third spacing of fluid, rather than a direct cause of increased mortality. Finally, the results of the FEAST study need to be carefully considered contextually [34]. For example, the definition of shock in the FEAST study has been called into question, and 57% of the patients in the FEAST study had a positive blood smear for malaria. In addition, 32% of the participants had a hemoglobin concentration <5 gm/dL. Thus, the results of the FEAST study could reflect the inclusion of a large number of participants for whom fluid administration may indeed be intrinsically deleterious, particularly in a low resource setting, rather than leading to the conclusion that fluid administration is intrinsically deleterious in septic shock [34].
Fundamental aspects of sepsis biology and physiology, including vascular leak, increased fluid loss, and increased vascular capacitance, well support the need for aggressive volume resuscitation, particularly early in the course of septic shock [1]. Since the 1991 study by Carcillo and colleagues [23], the concept of aggressive fluid resuscitation in septic shock has remained well supported [24–27]. The important clinical question that needs to be addressed is not “should” patients with septic shock receive fluid resuscitation, but rather “how much” fluid resuscitation is most optimal [35].
CORTICOSTEROIDS AND SEPSIS
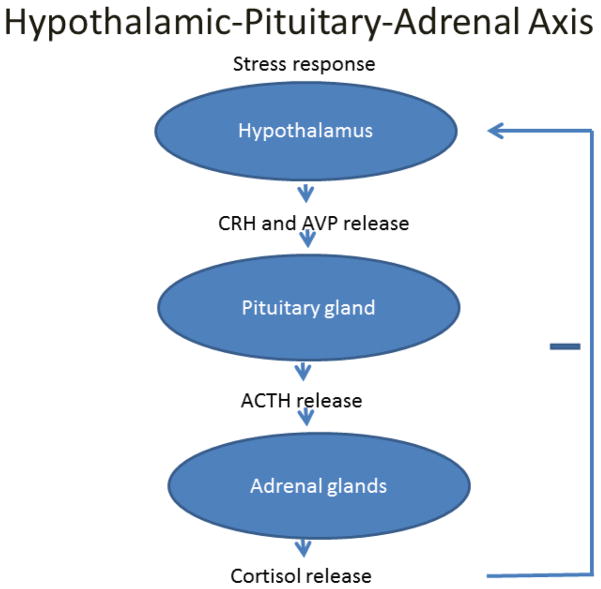
The hypothalamic-pituitary-adrenal (HPA) axis is a neurohormonal feedback mechanism well described in human physiology (Figure 1). In addition to the sympathoadrenal axis, the HPA axis serves a vital role in homeostatic adaptation to physiological and biological stress. Under normal circumstances, pituitary adrenocorticotropic hormone (ACTH) release is stimulated by hypothalamic corticotropin-releasing hormone (CRH) and arginine vasopressin production (AVP), and in turn stimulates cortisol production from the adrenal cortex. Cortisol acts through a myriad of mechanisms to maintain homeostasis, including modulating inflammation, glucose availability, and vascular reactivity [36].
Figure 1.
Schematic illustrating the hypothalamic-pituitary-adrenal axis.
Both adult and pediatric studies suggest a form of HPA axis impairment in critical illness and sepsis [37–43]. “Critical illness related corticosteroid insufficiency” (CIRCI) is a recently coined term intended to describe a group of critically ill patients who seemingly have an inadequate cortisol response relative to their degree of illness. The mechanisms of this “relative adrenal insufficiency” are thought to include both deficiency of cortisol production and tissue resistance to cortisol [44].
Corticosteroids have been considered as potential adjunctive therapy in sepsis for decades. Initial efforts, centered around the concept of sepsis as an excessive and dysregulated proinflammatory condition, primarily studied short courses of high dose corticosteroids intended for inflammatory suppression, often using single bolus doses of up to 30 mg/kg of methylprednisolone or equivalent steroid dosing. However, results were inconsistent, and with meta-analyses arising in the 1980–90s suggesting either no survival benefit or a trend towards increased mortality, the use of steroids in sepsis and septic shock began to fall out of favor [45–47].
The pediatric literature during the same time largely consisted of case studies and a number of trials investigating high dose steroid therapy in dengue shock syndrome. The first of these involved a randomized, double-blinded, placebo controlled trial of 98 children with dengue shock syndrome and showed a significant mortality improvement (19% vs. 44% case fatality rate) with corticosteroid administration [48]. However, subsequent trials failed to replicate this mortality benefit [49, 50]. In addition, a randomized trial of dexamethasone therapy, before antibiotic administration, failed to improve outcome in a cohort of African children with sepsis from causes other than dengue [51].
More recently, the CIRCI and relative adrenal insufficiency concepts described above have renewed interest in adjunctive corticosteroid therapy in sepsis. Annane et al. conducted the initial study supporting the concept of relative adrenal insufficiency. These investigators described three classes of adults with septic shock based on random cortisol levels and cortisol levels resulting from a corticotropin stimulation test [52]. In a cohort of 189 adult patients with septic shock, a class of patients with “high” baseline cortisol levels (≥ 34 μg/dl) and a “poor” response to a high dose corticotropin stimulation test (change in serum cortisol concentration of ≤ 9 μg/dl) had the highest mortality rate (82%) among the three classes. Thus, this study initiated the concept that a class of identifiable patients with septic shock and relative adrenal insufficiency may exist, having the potential to benefit from corticosteroid replacement. The pediatric literature suggests a similar phenomenon in children with septic shock, but it is challenging to interpret the data because of the small cohort sizes, and the high degree of heterogeneity in study design, definitions of relative adrenal insufficiency, and approaches to corticotropin stimulation [37, 39–42].
Menon et al. conducted the most comprehensive study of relative adrenal insufficiency in critically ill children, to date [38]. This prospective study included 381 critically ill children across seven, tertiary-care, pediatric intensive care units in Canada. The primary goal of the study was to determine the prevalence of relative adrenal insufficiency in a general population of critically ill children (i.e. not just in children with septic shock). The study examined various definitions of adrenal insufficiency, but the primary analysis for prevalence was based on a low dose corticotropin stimulation test (1 μg) and the resulting increment of serum cortisol concentration; a cortisol increment of ≤ 9 μg/dl after corticotropin stimulation was considered diagnostic of relative adrenal insufficiency.
Menon et al. reported a 30.2% overall prevalence of relative adrenal insufficiency in this cohort. Subgroup analyses revealed that patients suffering from trauma had the highest prevalence (62.5%), whereas the prevalence in patients with sepsis was 32.8%. Increasing age was associated with a higher prevalence of relative adrenal insufficiency, with each additional year of age increasing the odds of relative adrenal insufficiency by 11%. Finally, the study also demonstrated that relative adrenal insufficiency was associated with a greater need for the number of catecholamines, a greater duration of catecholamine requirement, and a greater need for volume resuscitation. This study by Menon et al. well supports the concept of relative adrenal insufficiency, including functional consequences, in pediatric critical illness.
Given the potential functional implications of relative adrenal insufficiency, the more recent interventional trials have focused on lower doses of corticosteroids, with a primary intention of replacing a putative defective cortisol response, rather than globally inhibiting the inflammatory response of sepsis. Annane et al. conducted a randomized, placebo-controlled trial of simultaneous hydrocortisone (50 mg every 6 hours) and fludrocortisone (50 μg once per day) administration for seven days, in 300 adults with septic shock [43]. Relative adrenal insufficiency (i.e. “nonresponders”) was defined as a serum cortisol concentration increment of ≤ 9 μg/dl after corticotropin stimulation, and the outcome analysis was stratified based on the response to corticotropin stimulation. There was a significant mortality benefit in nonresponders treated with hydrocortisone and fludrocortisone (hazard ratio, 0.67; 95% confidence interval, 0.47 – 0.95; p = 0.02), whereas there was no benefit in the responders. In addition, the duration of vasopressor therapy was shorter in the nonresponders treated with hydrocortisone and fludrocortisone. Finally, there was no difference in adverse events between the treatment and placebo groups, irrespective of the response to corticotropin stimulation.
This study by Annane et al. led to much enthusiasm for the use of cortisol measurements, corticotropin stimulation, and hydrocortisone replacement therapy in both adults and children with septic shock. However, the study by Annane et al. was followed by the Corticosteroid Therapy of Septic Shock (CORTICUS) study, which also evaluated the efficacy of hydrocortisone replacement in patients with septic shock, based on “responder” and “nonresponder” classifications after corticotropin stimulation [53]. The study involved 499 patients across multiple European intensive care units. This study showed no mortality difference between hydrocortisone-treated patients and placebo-treated patients, irrespective of the responder/nonresponder status. The duration of time until reversal of shock was shorter across all patients treated with hydrocortisone, but there was also a higher rate of shock relapse in the patients treated with hydrocortisone, possibly related to new infections.
There are some important differences worth mentioning between the initial study by Annane et al. and the subsequent CORTICUS study. The patients in the study by Annane et al. had an overall higher level of illness severity and possibly more profound shock compared to the patients in the CORTICUS study. In addition, enrollment in the Annane et al. study was restricted to a relatively narrow window of within 8 hours of meeting study entry criteria, whereas the CORTICUS study had a 72-hour window for enrollment. While these differences may account for the divergence in the results between the two studies, the aftermath of CORTICUS study has nonetheless profoundly influenced the enthusiasm for hydrocortisone replacement as an adjunctive therapy in septic shock.
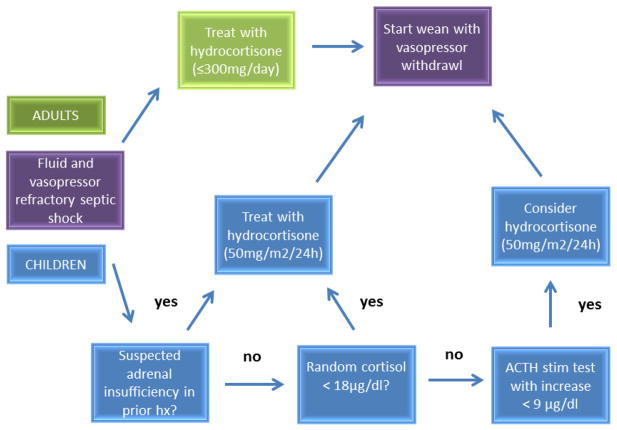
In the context of the above studies, the 2008 Surviving Sepsis Guidelines recommend against corticotropin stimulation testing to identify subsets of patients who may benefit from hydrocortisone, and that the decision to initiate hydrocortisone therapy should be predicated on confirmation that blood pressure is poorly responsive to fluid resuscitation and vasopressor therapy (Figure 2) [16]. In direct contrast to this recommendation, a recently published, single author, evidence-based guide for physicians, provides a decision tree for the use of hydrocortisone in adults with septic shock, which is based on both specific vasopressor requirements and results from corticotropin stimulation testing [54].
Figure 2.
Schematic illustrating the recommendations for adjunctive corticosteroid administration in septic shock. The adult recommendations do not include the use of serum cortisol concentrations or corticotropin stimulation testing [16]. The pediatric recommendations, however, recommend measurement of serum cortisol concentrations and corticotropin stimulation testing [5].
The most recent pediatric specific guidelines differ somewhat from the adult guidelines, reflecting the relative lack of pediatric data [5]. The pediatric guidelines recommend the use of corticosteroids for patients with fluid and catecholamine resistant septic shock, and evidence of adrenal insufficiency (Figure 2). The definition of catecholamine resistance is somewhat subjective, and the recommendation implies the need to conduct corticotropin stimulation testing, in contrast to the adult-specific recommendations.
There are retrospective, pediatric-specific data that should raise some doubts in the mind of the practitioner concerning the efficacy of corticosteroids in pediatric septic shock. Markovitz et al. analyzed over 6,000 cases of pediatric severe sepsis in the Pediatric Health Information System (PHIS) administrative database, with the goal of determining correlates of outcome, including the use of corticosteroids [55]. The use of corticosteroids was an independent predictor of mortality (RR 1.7, 95% CI 1.7 – 2.2). While the lack of illness severity data leaves open the possibility that more severely ill children received steroids, at the very least, this retrospective study could not find evidence for a benefit of corticosteroids in pediatric severe sepsis. In agreement with this observation, Zimmerman and Williams conducted a post hoc analysis of the RESOLVE database (n = 477), which is derived from the largest interventional clinical trial in pediatric severe sepsis conducted to date [56, 57]. Their analysis of the RESOLVE data could not find any evidence to support the efficacy of adjunctive corticosteroids in pediatric severe sepsis.
For children with septic shock who are at risk for “classical” adrenal insufficiency (e.g. patients receiving chronic steroids, or patients with hypothalamic, pituitary, or adrenal disease), adjunctive corticosteroids are clearly indicated. For the general pediatric patient with septic shock, however, the current evidence does not definitively support the use of adjunctive corticosteroids, and it should be kept in mind that the risk profile of adjunctive corticosteroids may not be benign [58]. Thus, it is imperative that we unite as a critical care community and organize a robust, multi-center, prospective, randomized trial to objectively test the efficacy of adjunctive corticosteroids in pediatric septic shock [58].
Several fundamental issues must be resolved in the design of such a trial. These include standardized, consensus definitions of refractory shock and relative adrenal insufficiency, a standardized approach to corticotropin stimulation testing, and strong consideration for pre-enrollment outcome risk stratification. An important issue that has emerged surrounds the measurement of free cortisol, as opposed to the more commonly used laboratory test that measures total cortisol [59]. Finally, consideration must be given to the issue of tissue resistance to corticosteroid stimulation, which has not been directly addressed in any clinical trial to date.
Genome-wide expression studies in children with septic shock recently highlighted the issue of tissue resistance to corticosteroid stimulation [60–62]. These studies revealed and validated the existence of at least three pediatric septic shock classes based on differential patterns of gene expression. Importantly, one of the three subclasses has a higher level of illness severity and mortality rate, compared to the other two gene expression-based subclasses. The gene signature that defines the subclasses includes a large number of genes corresponding to the glucocorticoid receptor signaling pathway, and these genes are repressed in the subclass of patients with the highest illness severity and mortality. These observations support the concept that there may be a subset of patients with septic shock who are relatively unresponsive to conventional doses of corticosteroids, and if this were true, it would represent an enormous confounding factor for any trial seeking to establish the efficacy of adjunctive corticosteroids in pediatric septic shock.
BLOOD PURIFICATION STRATEGIES
Whitehouse one of them much heated and fortiegued on his arrivall drank a very hearty draught of water and was taken almost instantly extreemly ill. His pulse were full and I therefore bled him plentifully from which he felt great relief. I had no other instrument with which to perform this operation but my penknife, however it answered very well.
--Captain Meriwether Lewis, during the portage of the Great Falls of the Missouri River, June 26, 1805.
The concept of blood purification can be traced at least to medieval times in which barbers would “bleed” patients for a variety of ailments, with the goal of removing “evil humors”. Ample evidence for this concept can also be found in the journals of the Lewis and Clark expedition, which contains multiple entries documenting the deliberate “bleeding” of expedition members for a variety of ailments. While not a randomized trial, it is important to note that 59 individuals took part in the Lewis and Clark expedition, and all but one individual survived the over 2 year long journey across the North American continent (98.3% survival; 95% CI 91 – 100).
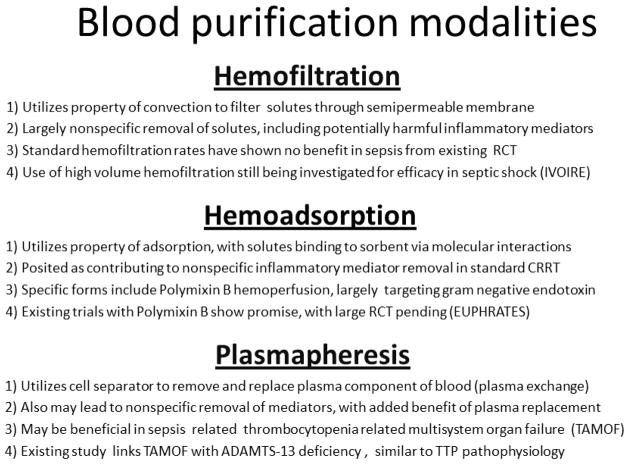
The concept of blood purification persists today in the modern intensive care unit [63]. The “evil humors” are now a plethora of soluble mediators and toxins thought to be involved in the pathobiology of sepsis. Bloodletting is now replaced by a variety of extracorporeal techniques that remove blood, purify the blood in some manner, and return the blood to the patient (Figure 3).
Figure 3.
Schematic highlighting the major differences between hemofiltration, hemoadsorption, and plasmapheresis as blood purification strategies for sepsis.
Hemofiltration
Given the size (5 – 60 kDa) and water-soluble nature of putative sepsis mediators (e.g. cytokines), hemofiltration through continuous renal replacement therapy (CRRT) has been studied with increasing interest as a nonspecific approach to mediator removal. Randomized controlled trials to date focusing on standard filtration rates in adults, however, have largely failed to support CRRT use for the purpose of blood purification in sepsis [64–66]. For example, Payen et al. studied 76 ICU patients with severe sepsis/septic shock in the absence of renal dysfunction, with the primary study endpoint being the degree of organ failure [64]. A hemofiltration rate of 25 ml/kg/hr, initiated within the first 24 hours of the first organ failure, failed to reduce plasma concentrations of inflammatory mediators, and did not reduce the degree of organ failure. In fact, the patients assigned to the hemofiltration arm had a significantly higher degree of organ failure.
A common criticism of these trials is that the hemofiltration rate is not sufficient to clear inflammatory mediators from the blood compartment. Accordingly, the use of high volume hemofiltration (HVHF; often defined as > 35 ml/kg/hr) has been increasingly studied as a plausible solution, and this approach showed some initial promise in terms of reduction of inflammatory mediators and improvements in clinical outcomes [67–69]. One very large trial involved 1,505 critically ill adults with acute kidney injury that were randomized to standard CRRT or HVHF, with the primary measure being death within 90 days after randomization [70]. There was no difference in mortality for the overall study population, nor for the predefined subgroup of patients with sepsis. Thus, the efficacy of HVHF as a blood purification approach in general critical illness and in sepsis remains to be demonstrated [71].
In the pediatric population, estimates suggest that roughly 40% of those with multi-organ dysfunction syndrome and receiving CRRT concomitantly have sepsis. The advent of the Prospective Pediatric Continuous Replacement Therapy Registry Group (ppCRRT) in 2000 has led to a comprehensive characterization of critically ill children receiving CRRT [32, 72–77]. However, despite provocative findings such as associations between increased volume status at initiation of therapy and mortality, few data exist with sufficient granularity to evaluate specifically the efficacy of CRRT as a blood purification strategy in pediatric sepsis. One recent retrospective study analyzed 22 pediatric patients undergoing CRRT and having concomitant systemic inflammatory response syndrome and multiple organ dysfunction syndrome [78]. The study reported no improvement in hemodynamic and respiratory parameters while accounting for lack of weight change during 48 hours of therapy. In addition to the lack of a control group, the study allowed for large patient heterogeneity, and no analyses of ultrafiltration rates were performed.
An international consensus statement published in 2010 recommends against the use of HVHF in patients with sepsis in the absence of acute kidney injury, citing both a lack of “strong, scientific rationale” and “reproducible, proof-of-principle efficacy” in existing clinical trials [79]. Although this strong consensus statement is based primarily on adult data, there is no reason to believe that the recommendation should be any different for the pediatric population, at this time. The highly anticipated IVOIRE (High Volume in Intensive Care) trial (clinicaltrials.gov identifier: NCT00241228), a large randomized multicenter trial comparing ultrafiltration rates of 35 vs. 70 ml/kg/hr in adult patients with septic shock and acute kidney injury, with 28 day mortality as the primary outcome measure, may help clarify the current value of CRRT in sepsis and concomitant acute kidney injury.
Hemoadsorption
The concepts reviewed in the previous section are centered on the convective filtration properties of CRRT. There has also been interest in the adsorptive properties of various hemofilters as a potential mechanism for mediator removal. Studies have shown decreases in mediator concentrations approximately 1 hour after CCRT initiation and with frequent filter changes, suggesting initial efficacy through adsorption, with subsequent membrane saturation [67]. A similar principal has led to the use of polymyxin B coated filters as a means to remove endotoxin in patients with sepsis [80]. It is estimated that over 80,000 patients have been treated with extracorporeal removal of endotoxin via polymyxin B coated filters in Japan since 1994, and the procedure is covered by the Japanese national health insurance system [81].
The EUPHAS (Early Use of Polymyxin B Hemoperfusion in Abdominal Sepsis) trial randomized 64 adults, with septic shock and requiring emergency surgery for intra-abdominal infection, to conventional therapy or conventional therapy plus 2 sessions of polymyxin G hemoperfusion [82]. The group undergoing polymyxin B hemoperfusion showed significant improvements in hemodynamics, improvements in organ dysfunction, and decreased mortality. Although this trial is thought to be underpowered, a systematic review of 28 publications including 9 randomized trials, lends further support for the potential efficacy of this approach [83].
Similar to the EUPHAS trial in design and outcome measures, the ongoing EUPHRATES trial (clinicaltrials.gov identifier: NCT01046669) anticipates enrollment of 360 adult patients in 15 centers, with an estimated completion date of January 2015. The primary endpoint measure for the EUPHRATES trial is all cause 28-day mortality. Importantly, the entry criteria include a requirement for an increased blood concentration of endotoxin, as measured by a rapid assay platform. Thus, this trial is employing the concept of pre-enrollment stratification based on a biological mechanism, a refreshing approach in the field of human septic shock trials.
There is one report in the literature describing the successful application of polymyxin B hemoperfusion in a child with sepsis [84]. One could surmise that the quintessential pediatric critical illness, meningococcemia, which is characterized by a profound systemic load of endotoxin, would be potentially amenable to this approach. However, experimental data indicate that polymyxin B does not bind endotoxin from N. meningitidis as effectively as endotoxin from E. coli [85].
Plasmapheresis
Plasmapheresis is another extracorporeal approach to blood purification, but is based on separation, removal, and replacement of the plasma component of blood [86]. Plasmapheresis is thought to derive its efficacy from two basic principles: 1) removal of pathologic molecules contributing to the disease process, and 2) replacement of molecules vital to homeostasis and found to be endogenously deficient due to the disease. Much like the use of hemofiltration, the removal of such molecules is often of a non-specific nature, and may not have a clear therapeutic objective in mind. An added component, however, is the replacement of plasma for that removed, more accurately termed plasma exchange, which may account for potential differences in clinical results.
Setgmayr et al. reported a large case series of 76 adult patients with disseminated intravascular coagulation (DIC) and multiple organ dysfunction syndrome, including acute renal failure. These patients underwent “rescue therapy” with plasmapheresis until DIC was reversed (median of 2 treatments, with a range of 1 to 14 treatments). Eighty two percent of these patients survived, compared to a historical rate of 20% with similar clinical characteristics [87]. Busund et al. randomized 106 adults with severe sepsis to conventional therapy vs. conventional therapy plus plasmapheresis [88]. This study reported an absolute 28-day mortality risk reduction of 20% and relative risk of death in the plasmapheresis group of 0.6. Other reports focused on meningococcemia and plasmapheresis in children and young adults, reported improved survival rates of 70–80% when compared to historical controls and projected mortality based on clinical scores [89, 90].
The most recent iteration of the use of plasmapheresis in sepsis is centered on the concept of “thrombocytopenia associated multi organ failure” (TAMOF) as a complication of sepsis [86]. TAMOF has been compared to thrombotic thrombocytopenic purpura (TTP). TTP is an acute thrombotic microangiopathy thought to be primarily caused by deficiency or inhibition of the enzyme ADAMTS13, which is responsible for cleaving procoagulant multimers of von Willebrand factor into small, less procoagulant units. Plasmapheresis has become the standard of care for TTP in that it can eliminate the inhibitory ADAMTS13 factor and replaces ADAMTS13 [91, 92].
Nguyen et al. described 37 children with a least two organ failures and suspected sepsis, of whom 76% had thrombocytopenia, defined as platelet counts <100,000/mm3 [93]. The subset of patients with thrombocytopenia (i.e. TAMOF, n = 28) had decreased ADAMTS13 activity compared to patients with organ failure, but no thrombocytopenia (n = 9). All of the nonsurvivors in this cohort (n = 7) met clinical criteria for TAMOF and demonstrated von Willebrand factor-rich microvascular thrombosis at autopsy. The same investigators then randomized 10 children with TAMOF to plasmapheresis or conventional therapy, and showed improvements in 28-day organ dysfunction scores and mortality in the patients treated with plasmapheresis.
Current American Society of Apheresis guidelines list the use of plasmapheresis in “sepsis with multiorgan failure” as a category III recommendation (optimum role of plasmapheresis is not established and decision-making should be individualized), with a 2B grade (weak recommendation, moderate-quality evidence) [94]. In summary, the use of plasmapheresis in both adult and pediatric sepsis may have value, but remains to be demonstrated. Similarly, it could be argued that TAMOF is a construct, rather than a genuine clinical entity. These issues can only be resolved through equipoise and the conduct of rigorous, prospective clinical trials, which are currently being planned [95].
RISK STRATIFICATION
The adjunctive therapies for sepsis discussed above, as well as therapies not discussed (e.g. extracorporeal life support), all have biological and physiological plausibility in terms of efficacy, but require more rigorous testing. Moving forward with testing, consideration should be given to objective and effective outcome risk stratification as means of optimizing clinical trial design. The therapeutic successes of our oncology colleagues are, in large part, predicated on effective outcome risk stratification. In an analogous manner, we must develop tools that allow early assessment of outcome risk, and thus provide a means for excluding patients with low mortality risks, while simultaneously selecting patients with higher mortality risks for trial inclusion. Experts in the field of critical care medicine have strongly stated that currently available, physiology-based scoring systems should not be used for risk stratification in clinical trials [9].
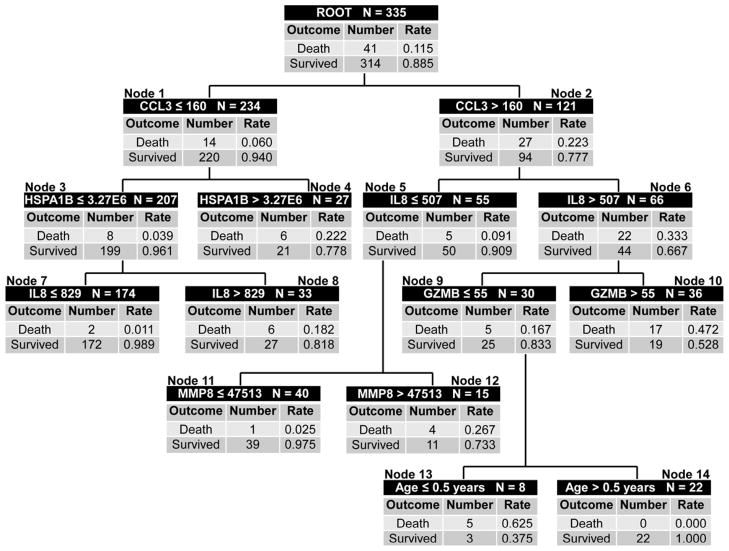
Very recently, a multi-biomarker-based risk model was derived and successfully tested, which robustly predicts outcome (i.e. 28 day mortality) in children with septic shock [10, 11]. The biomarkers for the risk model are readily measured serum proteins, and were selected objectively based on a series of extensive, exploratory, genome-wide expression studies in children with septic shock [96, 97]. Importantly, the biomarker measurements are conducted within the first 24 hours of meeting criteria for septic shock, an optimal time point for clinically relevant outcome risk stratification. The risk model is based on a classification and regression tree (CART) approach, which has the potential to discover predictor variable interactions that may not be apparent by more traditional modeling approaches [98].
The model is called PERSEVERE (PEdiatRic SEpsis biomarkEr Risk modEl) and has the following test characteristics for predicting death in a cohort of 355 children with septic shock from 17 different institutions in the United States: sensitivity of 93%, specificity of 74%, positive predictive value of 32%, negative predictive value of 99%, and an area under the receiver operating curve of 0.883 [10]. Importantly, the model out performs a physiology-based scoring system, and the false positives generated by the model (i.e. patients predicted to die, but actually survive) have a higher degree of illness severity than the true negatives generated by the model (i.e. patients predicted to survive and actually survive), as measured by persistence of organ failure and intensive care unit length of stay. The PERSEVERE classification tree is provided in Figure 4.
Figure 4.
The classification tree for PERSEVERE based on 355 subjects [10]. The classification tree consists of 6 biomarker-based decision rules, 1 age-based decision rule, and 14 daughter nodes. The classification tree includes 5 stratification biomarkers: C-C chemokine ligand 3 (CCL3), heat shock protein 70 kDa 1B (HSPA1B), interleukin-8 (IL8), granzyme B (GZMB), and matrix metalloproteinase-8 (MMP8). Each node provides the total number of subjects in the node, the biomarker serum concentration- or age-based decision rule, and the number of survivors and non-survivors with the respective rates. The serum concentrations of all stratification biomarkers are provided in pg/ml. Terminal nodes 7, 11, and 14 are low risk nodes, with mortality probabilities ranging from 0.0 to 2.5%. Terminal nodes 4, 8, 10, 12, and 13 are high-risk terminal nodes, with mortality probabilities ranging from 18.2 to 62.5%.
It has been proposed that PERSEVERE can enhance our current tools to select patients for interventional trials by providing a means to exclude and include patients with low and high mortality risks, respectively. This type of approach would have the potential to optimize the risk to benefit ratio of an experimental therapy that carries more than minimal risk, such as the experimental therapies described in the previous sections.
Key Points.
Sepsis continues to be a major challenge in the field of pediatric critical care medicine.
Studies of sepsis outcomes should include both short-term and long-term outcomes, and should also focus on functional outcomes.
The issue of timeliness and appropriateness of antibiotics is a timely and important area for quality improvement in the field of pediatric sepsis.
The issue of corticosteroids as an adjunctive therapy in pediatric septic shock remains largely unresolved and requires formal testing by way of clinical trials.
Hemofiltration, hemoperfusion, and plasmapheresis are potential adjunctive therapies for pediatric sepsis, but require formal testing by way of clinical trials.
A new multi-biomarker-based model has been developed to reliably stratify outcome risk in children with septic shock.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wong HR, Nowak JE, Standage S, de Oliveira CF. Sepsis and Septic Shock. In: Fuhrman BP, Zimmerman JJ, editors. Pediatric Critical Care Medicine. 4. St. Louis: Mosby; 2011. pp. 1413–1429. [Google Scholar]
- 2.Cornell TT, Wynn J, Shanley TP, Wheeler DS, Wong HR. Mechanisms and regulation of the gene-expression response to sepsis. Pediatrics. 2010;125:1248–1258. doi: 10.1542/peds.2009-3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin Perinatol. 2010;37:439–479. doi: 10.1016/j.clp.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics. 2010;125:1031–1041. doi: 10.1542/peds.2009-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–688. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Resp Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 7.Watson RS, Carcillo JA. Scope and epidemiology of pediatric sepsis. Pediatr Crit Care Med. 2005;6:S3–5. doi: 10.1097/01.PCC.0000161289.22464.C3. [DOI] [PubMed] [Google Scholar]
- 8.Marcin JP, Pollack MM. Review of the acuity scoring systems for the pediatric intensive care unit and their use in quality improvement. J Inten Care Med. 2007;22:131–140. doi: 10.1177/0885066607299492. [DOI] [PubMed] [Google Scholar]
- 9.Vincent JL, Opal SM, Marshall JC. Ten reasons why we should NOT use severity scores as entry criteria for clinical trials or in our treatment decisions. Crit Care Med. 2010;38:283–287. doi: 10.1097/CCM.0b013e3181b785a2. [DOI] [PubMed] [Google Scholar]
- 10.Wong HR, Salisbury S, Xiao Q, et al. The pediatric sepsis biomarker risk model. Crit Care. 2012;16:R174. doi: 10.1186/cc11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan JM, Wong HR. Biomarker discovery and development in pediatric critical care medicine. Pediatr Crit Care Med. 2011;12:165–173. doi: 10.1097/PCC.0b013e3181e28876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quartin AA, Schein RM, Kett DH, Peduzzi PN Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. Magnitude and duration of the effect of sepsis on survival. JAMA. 1997;277:1058–1063. [PubMed] [Google Scholar]
- 13.Karlsson S, Ruokonen E, Varpula T, Ala-Kokko TI, Pettila V. Long-term outcome and quality-adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274. doi: 10.1097/CCM.0b013e31819c13ac. [DOI] [PubMed] [Google Scholar]
- 14.Czaja AS, Zimmerman JJ, Nathens AB. Readmission and late mortality after pediatric severe sepsis. Pediatrics. 2009;123:849–857. doi: 10.1542/peds.2008-0856. [DOI] [PubMed] [Google Scholar]
- 15.Pollack MM, Holubkov R, Glass P, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009;124:e18–28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367–374. doi: 10.1097/CCM.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Haery C, Paladugu B, Symeoneides S, Taiberg L, Osman J, Trenholme G, Opal SM, Goldfarb R, Parrillo JE. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis. 2006;193:251–258. doi: 10.1086/498909. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 20.Puskarich MA, Trzeciak S, Shapiro NI, et al. Association between timing of antibiotic administration and mortality from septic shock in patients treated with a quantitative resuscitation protocol. Crit Care Med. 2011;39:2066–2071. doi: 10.1097/CCM.0b013e31821e87ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muszynski JA, Knatz NL, Sargel CL, Fernandez SA, Marquardt DJ, Hall MW. Timing of correct parenteral antibiotic initiation and outcomes from severe bacterial community-acquired pneumonia in children. Ped Infect Dis J. 2011;30:295–301. doi: 10.1097/INF.0b013e3181ff64ec. [DOI] [PubMed] [Google Scholar]
- 22.Cruz AT, Perry AM, Williams EA, Graf JM, Wuestner ER, Patel B. Implementation of goal-directed therapy for children with suspected sepsis in the emergency department. Pediatrics. 2011;127:e758–766. doi: 10.1542/peds.2010-2895. [DOI] [PubMed] [Google Scholar]
- 23.Carcillo JA, Davis AL, Zaritsky A. Role of early fluid resuscitation in pediatric septic shock. JAMA. 1991;266:1242–1245. [PubMed] [Google Scholar]
- 24.Han YY, Carcillo JA, Dragotta MA, et al. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112:793–799. doi: 10.1542/peds.112.4.793. [DOI] [PubMed] [Google Scholar]
- 25.de Oliveira CF, de Oliveira DS, Gottschald AF, et al. ACCM/PALS haemodynamic support guidelines for paediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med. 2008;34:1065–1075. doi: 10.1007/s00134-008-1085-9. [DOI] [PubMed] [Google Scholar]
- 26.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 27.Smith SH, Perner A. Higher vs. lower fluid volume for septic shock: clinical characteristics and outcome in unselected patients in a prospective, multicenter cohort. Crit Care. 2012;16:R76. doi: 10.1186/cc11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilton AK, Bellomo R. A critique of fluid bolus resuscitation in severe sepsis. Crit Care. 2012;16:302. doi: 10.1186/cc11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 30.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 31.Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136:102–109. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67:653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 33.Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 34.Duke T. What the African fluid-bolus trial means. Lancet. 2011;378:1685–1687. doi: 10.1016/S0140-6736(11)60881-7. [DOI] [PubMed] [Google Scholar]
- 35.Russell JA. How much fluid resuscitation is optimal in septic shock? Crit Care. 2012;16:146. doi: 10.1186/cc11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prigent H, Maxime V, Annane D. Science review: mechanisms of impaired adrenal function in sepsis and molecular actions of glucocorticoids. Crit Care. 2004;8:243–252. doi: 10.1186/cc2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hebbar K, Rigby MR, Felner EI, Easley KA, Fortenberry JD. Neuroendocrine dysfunction in pediatric critical illness. Pediatr Crit Care Med. 2009;10:35–40. doi: 10.1097/PCC.0b013e3181936ef3. [DOI] [PubMed] [Google Scholar]
- 38.Menon K, Ward RE, Lawson ML, Gaboury I, Hutchison JS, Hebert PC. A prospective multicenter study of adrenal function in critically ill children. Am J Resp Crit Care Med. 2010;182:246–251. doi: 10.1164/rccm.200911-1738OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casartelli CH, Garcia PC, Branco RG, Piva JP, Einloft PR, Tasker RC. Adrenal response in children with septic shock. Intensive care Med. 2007;33:1609–1613. doi: 10.1007/s00134-007-0699-7. [DOI] [PubMed] [Google Scholar]
- 40.Hebbar KB, Stockwell JA, Leong T, Fortenberry JD. Incidence of adrenal insufficiency and impact of corticosteroid supplementation in critically ill children with systemic inflammatory syndrome and vasopressor-dependent shock. Crit Care Med. 2011;39:1145–1150. doi: 10.1097/CCM.0b013e31820eb4e4. [DOI] [PubMed] [Google Scholar]
- 41.Pizarro CF, Troster EJ, Damiani D, Carcillo JA. Absolute and relative adrenal insufficiency in children with septic shock. Crit Care Med. 2005;33:855–859. doi: 10.1097/01.ccm.0000159854.23324.84. [DOI] [PubMed] [Google Scholar]
- 42.Sarthi M, Lodha R, Vivekanandhan S, Arora NK. Adrenal status in children with septic shock using low-dose stimulation test. Pediatr Crit Care Med. 2007;8:23–28. doi: 10.1097/01.pcc.0000256622.63135.90. [DOI] [PubMed] [Google Scholar]
- 43.Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 44.Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937–1949. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 45.Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995;23:1294–1303. doi: 10.1097/00003246-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 46.Cronin L, Cook DJ, Carlet J, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23:1430–1439. doi: 10.1097/00003246-199508000-00019. [DOI] [PubMed] [Google Scholar]
- 47.The Veterans Administration Systemic Sepsis Cooperative Study Group. Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med. 1987;317:659–665. doi: 10.1056/NEJM198709103171102. [DOI] [PubMed] [Google Scholar]
- 48.Min M, UT, Aye M, Shwe TN, Swe T. Hydrocortisone in the management of dengue shock syndrome. Southeast Asian J Trop Med Public Health. 1975;6:573–579. [PubMed] [Google Scholar]
- 49.Tassniyom S, Vasanawathana S, Chirawatkul A, Rojanasuphot S. Failure of high-dose methylprednisolone in established dengue shock syndrome: a placebo-controlled, double-blind study. Pediatrics. 1993;92:111–115. [PubMed] [Google Scholar]
- 50.Sumarmo, Talogo W, Asrin A, Isnuhandojo B, Sahudi A. Failure of hydrocortisone to affect outcome in dengue shock syndrome. Pediatrics. 1982;69:45–49. [PubMed] [Google Scholar]
- 51.Slusher T, Gbadero D, Howard C, et al. Randomized, placebo-controlled, double blinded trial of dexamethasone in African children with sepsis. Pediatr Infect Dis J. 1996;15:579–583. doi: 10.1097/00006454-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283:1038–1045. doi: 10.1001/jama.283.8.1038. [DOI] [PubMed] [Google Scholar]
- 53.Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 54.Annane D. Corticosteroids for severe sepsis: an evidence-based guide for physicians. Ann Intensive Care. 2011;1:7. doi: 10.1186/2110-5820-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Markovitz BP, Goodman DM, Watson RS, Bertoch D, Zimmerman J. A retrospective cohort study of prognostic factors associated with outcome in pediatric severe sepsis: what is the role of steroids? Pediatr Crit Care Med. 2005;6:270–274. doi: 10.1097/01.PCC.0000160596.31238.72. [DOI] [PubMed] [Google Scholar]
- 56.Zimmerman JJ, Williams MD. Adjunctive corticosteroid therapy in pediatric severe sepsis: observations from the RESOLVE study. Pediatr Crit Care Med. 2011;12:2–8. doi: 10.1097/PCC.0b013e3181d903f6. [DOI] [PubMed] [Google Scholar]
- 57.Nadel S, Goldstein B, Williams MD, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007;369:836–843. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 58.Zimmerman JJ. A history of adjunctive glucocorticoid treatment for pediatric sepsis: moving beyond steroid pulp fiction toward evidence-based medicine. Pediatr Crit Care. 2007;8:530–539. doi: 10.1097/01.PCC.0000288710.11834.E6. [DOI] [PubMed] [Google Scholar]
- 59.Zimmerman JJ, Donaldson A, Barker RM, et al. Real-time free cortisol quantification among critically ill children. Pediatr Crit Care Med. 2011;12:525–531. doi: 10.1097/PCC.0b013e3181fe4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong HR, Cvijanovich NZ, Allen GL, et al. Validation of a gene expression-based subclassification strategy for pediatric septic shock. Crit Care Med. 2011;39:2511–2517. doi: 10.1097/CCM.0b013e3182257675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong HR, Wheeler DS, Tegtmeyer K, et al. Toward a clinically feasible gene expression-based subclassification strategy for septic shock: proof of concept. Crit Care Med. 2010;38:1955–1961. doi: 10.1097/CCM.0b013e3181eb924f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong HR, Cvijanovich N, Lin R, et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med. 2009;7:34. doi: 10.1186/1741-7015-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rimmele T, Kellum JA. Clinical review: blood purification for sepsis. Crit Care. 2011;15:205. doi: 10.1186/cc9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Payen D, Mateo J, Cavaillon JM, Fraisse F, Floriot C, Vicaut E. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit Care Med. 2009;37:803–810. doi: 10.1097/CCM.0b013e3181962316. [DOI] [PubMed] [Google Scholar]
- 65.Sander A, Armbruster W, Sander B, Daul AE, Lange R, Peters J. Hemofiltration increases IL-6 clearance in early systemic inflammatory response syndrome but does not alter IL-6 and TNF alpha plasma concentrations. Intensive Care Med. 1997;23:878–884. doi: 10.1007/s001340050425. [DOI] [PubMed] [Google Scholar]
- 66.Cole L, Bellomo R, Hart G, et al. A phase II randomized, controlled trial of continuous hemofiltration in sepsis. Crit Care Med. 2002;30:100–106. doi: 10.1097/00003246-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 67.De Vriese AS, Colardyn FA, Philippe JJ, Vanholder RC, De Sutter JH, Lameire NH. Cytokine removal during continuous hemofiltration in septic patients. J Am Soc Nephrol. 1999;10:846–853. doi: 10.1681/ASN.V104846. [DOI] [PubMed] [Google Scholar]
- 68.Cole L, Bellomo R, Journois D, Davenport P, Baldwin I, Tipping P. High-volume haemofiltration in human septic shock. Intensive Care Med. 2001;27:978–986. doi: 10.1007/s001340100963. [DOI] [PubMed] [Google Scholar]
- 69.Honore PM, Jacobs R, Boer W, et al. New insights regarding rationale, therapeutic target and dose of hemofiltration and hybrid therapies in septic acute kidney injury. Blood Purif. 2012;33:44–51. doi: 10.1159/000333837. [DOI] [PubMed] [Google Scholar]
- 70.Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 71.Rimmele T, Kellum JA. High-volume hemofiltration in the intensive care unit: a blood purification therapy. Anesthesiology. 2012;116:1377–1387. doi: 10.1097/ALN.0b013e318256f0c0. [DOI] [PubMed] [Google Scholar]
- 72.Fleming GM, Walters S, Goldstein SL, et al. Nonrenal indications for continuous renal replacement therapy: A report from the Prospective Pediatric Continuous Renal Replacement Therapy Registry Group. Pediatr Crit Care Med. 2012;13:e299–e304. doi: 10.1097/PCC.0b013e31824fbd76. [DOI] [PubMed] [Google Scholar]
- 73.Goldstein SL. Advances in pediatric renal replacement therapy for acute kidney injury. Semin Dial. 2011;24:187–191. doi: 10.1111/j.1525-139X.2011.00834.x. [DOI] [PubMed] [Google Scholar]
- 74.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55:316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 75.Zappitelli M, Goldstein SL, Symons JM, et al. Protein and calorie prescription for children and young adults receiving continuous renal replacement therapy: a report from the Prospective Pediatric Continuous Renal Replacement Therapy Registry Group. Crit Care Med. 2008;36:3239–3245. doi: 10.1097/CCM.0b013e31818f3f40. [DOI] [PubMed] [Google Scholar]
- 76.Flores FX, Brophy PD, Symons JM, et al. Continuous renal replacement therapy (CRRT) after stem cell transplantation. A report from the prospective pediatric CRRT Registry Group. Pediatr Nephrol. 2008;23:625–630. doi: 10.1007/s00467-007-0672-2. [DOI] [PubMed] [Google Scholar]
- 77.Hackbarth R, Bunchman TE, Chua AN, et al. The effect of vascular access location and size on circuit survival in pediatric continuous renal replacement therapy: a report from the PPCRRT registry. Int J Artif Organs. 2007;30:1116–1121. doi: 10.1177/039139880703001212. [DOI] [PubMed] [Google Scholar]
- 78.Naran N, Sagy M, Bock KR. Continuous renal replacement therapy results in respiratory and hemodynamic beneficial effects in pediatric patients with severe systemic inflammatory response syndrome and multiorgan system dysfunction. Pediatr Crit Care Med. 2010;11:737–740. doi: 10.1097/PCC.0b013e3181ce7593. [DOI] [PubMed] [Google Scholar]
- 79.Brochard L, Abroug F, Brenner M, et al. An Official ATS/ERS/ESICM/SCCM/SRLF Statement: Prevention and Management of Acute Renal Failure in the ICU Patient: an international consensus conference in intensive care medicine. Am J Respir Crit Care Med. 2010;181:1128–1155. doi: 10.1164/rccm.200711-1664ST. [DOI] [PubMed] [Google Scholar]
- 80.Shoji H, Tani T, Hanasawa K, Kodama M. Extracorporeal endotoxin removal by polymyxin B immobilized fiber cartridge: designing and antiendotoxin efficacy in the clinical application. Ther Apher. 1998;2:3–12. doi: 10.1111/j.1744-9987.1998.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 81.Tani T, Shoji H, Guadagni G, Perego A. Extracorporeal removal of endotoxin: the polymyxin B-immobilized fiber cartridge. Contrib Nephrol. 2010;167:35–44. doi: 10.1159/000315917. [DOI] [PubMed] [Google Scholar]
- 82.Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445–2452. doi: 10.1001/jama.2009.856. [DOI] [PubMed] [Google Scholar]
- 83.Cruz DN, Perazella MA, Bellomo R, et al. Effectiveness of polymyxin B-immobilized fiber column in sepsis: a systematic review. Crit Care. 2007;11:R47. doi: 10.1186/cc5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morishita Y, Kita Y, Ohtake K, et al. Successful treatment of sepsis with polymyxin b-immobilized fiber hemoperfusion in a child after living donor liver transplantation. Dig Dis Sci. 2005;50:757. doi: 10.1007/s10620-005-2569-x. [DOI] [PubMed] [Google Scholar]
- 85.Baldwin G, Alpert G, Caputo GL, et al. Effect of polymyxin B on experimental shock from meningococcal and Escherichia coli endotoxins. J Infect Dis. 1991;164:542–549. doi: 10.1093/infdis/164.3.542. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen TC, Kiss JE, Goldman JR, Carcillo JA. The role of plasmapheresis in critical illness. Crit Care Clin. 2012;28:453–468. doi: 10.1016/j.ccc.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stegmayr BG, Banga R, Berggren L, Norda R, Rydvall A, Vikerfors T. Plasma exchange as rescue therapy in multiple organ failure including acute renal failure. Crit Care Med. 2003;31:1730–1736. doi: 10.1097/01.CCM.0000064742.00981.14. [DOI] [PubMed] [Google Scholar]
- 88.Busund R, Koukline V, Utrobin U, Nedashkovsky E. Plasmapheresis in severe sepsis and septic shock: a prospective, randomised, controlled trial. Intensive Care Med. 2002;28:1434–1439. doi: 10.1007/s00134-002-1410-7. [DOI] [PubMed] [Google Scholar]
- 89.van Deuren M, Santman FW, van Dalen R, Sauerwein RW, Span LF, van der Meer JW. Plasma and whole blood exchange in meningococcal sepsis. Clin Infect Dis. 1992;15:424–430. doi: 10.1093/clind/15.3.424. [DOI] [PubMed] [Google Scholar]
- 90.Gardlund B, Sjolin J, Nilsson A, et al. Plasmapheresis in the treatment of primary septic shock in humans. Scand J Infect Dis. 1993;25:757–761. doi: 10.3109/00365549309008575. [DOI] [PubMed] [Google Scholar]
- 91.Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393–397. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 92.Zheng XL, Kaufman RM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood. 2004;103:4043–4049. doi: 10.1182/blood-2003-11-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nguyen TC, Han YY, Kiss JE, et al. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit Care Med. 2008;36:2878–2887. doi: 10.1097/ccm.0b013e318186aa49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Szczepiorkowski ZM, Winters JL, Bandarenko N, et al. Guidelines on the use of therapeutic apheresis in clinical practice--evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apher. 2010;25:83–177. doi: 10.1002/jca.20240. [DOI] [PubMed] [Google Scholar]
- 95.Fortenberry JD, Paden ML. Extracorporeal therapies in the treatment of sepsis: experience and promise. Semin Pediatr Infect Dis. 2006;17:72–79. doi: 10.1053/j.spid.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 96.Wong HR. Genetics and genomics in pediatric septic shock. Crit Care Med. 2012;40:1618–1626. doi: 10.1097/CCM.0b013e318246b546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wong HR. Clinical review: sepsis and septic shock--the potential of gene arrays. Crit Care. 2012;16:204. doi: 10.1186/cc10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Che D, Liu Q, Rasheed K, Tao X. Decision tree and ensemble learning algorithms with their applications in bioinformatics. Adv Exp Med Biol. 2011;696:191–199. doi: 10.1007/978-1-4419-7046-6_19. [DOI] [PubMed] [Google Scholar]