Abstract
BACKGROUND CONTEXT
Astronauts experience back pain, particularly low back pain, during and after spaceflight. Recent studies have described histological and biochemical changes in rat intervertebral discs after space travel, but there is still no in vitro model to investigate the effects of microgravity on disc metabolism.
PURPOSE
To study the effects of microgravity on disc degeneration and to establish an in vitro simulated microgravity study model
STUDY DESIGN
Discs were cultured in static and rotating conditions in bioreactor, and the characteristics of disc degeneration were evaluated
METHODS
The mice discs were cultured in a rotating wall vessel bioreactor where the microgravity condition was simulated. Intervertebral discs were cultured in static and microgravity condition. Histology, biochemistry, and immunohistochemical assays were performed to evaluate the characteristics of the discs in microgravity condition.
RESULTS
Intervertebral discs cultured in rotating bioreactors were found to develop changes of disc degeneration manifested by reduced red Safranin-o staining within the annulus fibrosus, downregulated GAG content and GAG/Hypro ratio, increased MMP-3 expression, and upregulated apoptosis.
CONCLUSIONS
We conclude that simulated microgravity induces the molecular changes of disc degeneration. The rotating bioreactor model will provide a foundation to investigate the effects of microgravity on disc metabolism.
Keywords: intervertebral disc, microgravity, disc degeneration, apoptosis, extracellular matrix
Introduction
With advances in space exploration, astronauts are being subjected to hostile physical environments and are faced with severe physiological changes during both short and long duration spaceflight. Risks to spaceflight include bone loss, muscle atrophy, cardiac dysrythmias, cognitive dysfunction, and altered orientation [1–5]. The severity of these risks directly correlates with flight duration. Among the potential physiological hazards of spaceflight, bone loss has been relatively well described. Exposure to weightless environment results in a bone loss of approximately 1% per month [6]. Astronauts also experience greater back pain, particularly low back pain, during and after spaceflight, and increased rates of immediate post-flight herniated nucleus pulposus have been reported [7, 8]. In addition, animals subjected to space flight demonstrate advanced degenerative changes within their intervertebral disc [9].
Intervertebral disc (IVD) degeneration is a major cause of low back pain. The development of disc degeneration is a complex process and has a number of poorly understood determinants that involves both environmental and genetic contributions [10]. The degenerative process begins in the nucleus pulposus with loss of cellularity and proteoglycan breakdown leading to diminished water-binding capacity [11–14]. IVD function is chiefly dependent upon the dynamic balance between matrix synthesis and catabolism. Once this balance is disturbed, the altered matrix composition and organization are unable to bear even physiologic loads, thus resulting in disc degeneration. Meanwhile, the well organized lamellar architecture of the annulus fibrosus begins to deteriorate, and eventually internal fissures develop which spread around the periphery of the annulus. Whether the astronaut’s low back pain is caused by a disturbed balance between IVD matrix synthesis and breakdown, and how the process is initiated are unknown.
Due to payload constraints and flight infrequency, spaceflight experiments are limited. Therefore, in vitro and in vivo systems which mimic microgravity conditions become necessary. NASA at the Johnson Space Center has developed a commercially available Rotatory Cell Culture System (RCCS™) with which to perform simulated microgravity in ground-based experiments. It has been used previously with numerous cell culture systems to simulate the effects of a microgravity environment [13, 14]. It is equipped with High Aspect Ratio Vessels (HARVs; Synthecon, Inc., Houston, TX). During simulated microgravity, the vessel wall and medium containing cells with carrier rotate at the same speed, producing a vector-averaged gravity comparable with that of near-earth free-fall orbit [12].
Using this system, we demonstrate in the current study that microgravity induces disc degeneration by altering extracellular matrix components and stimulating apoptosis within the nucleus pulposus. This disc degeneration organ culture model also provides a foundation with which to test potential therapeutic targets for preventing and treating back pain during or after spaceflight.
Materials and Methods
Rotary wall vessel (RWV) bioreactor
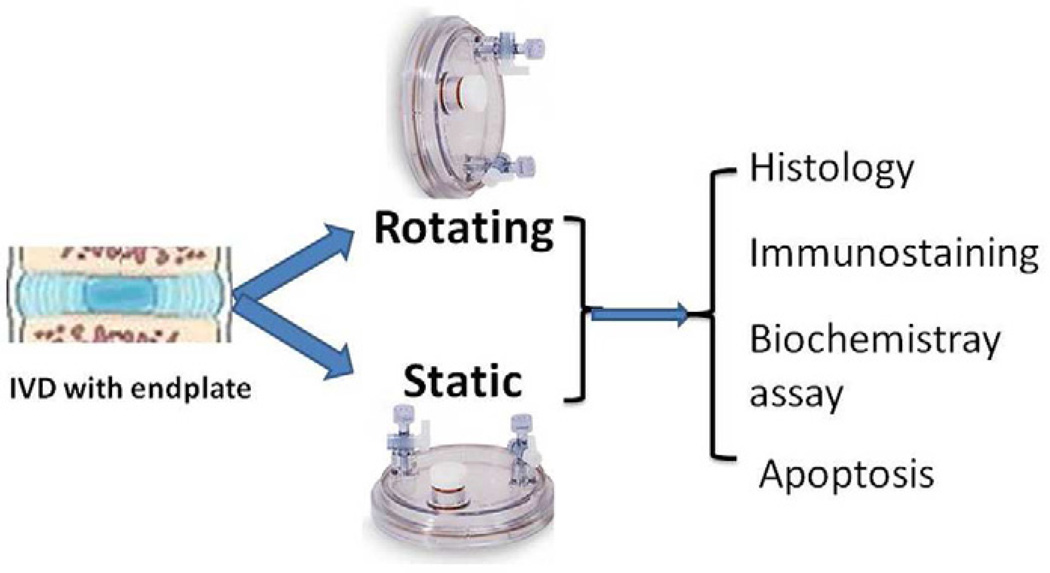
The bioreactor (Model HARV, size 50 ml) was purchased from Synthecon. It consists of a cylindrical growth chamber that contains a flat silicone rubber gas transfer membrane for oxygenation. The two basic principles of this system are: 1) solid body rotation around a horizontal axis, resulting in randomization of the gravitational vector, low fluid shear stress, and three-dimensional spatial freedom; and 2) oxygenation by diffusion of dissolved gasses from the reactor chamber, yielding a vessel devoid of gas bubbles [12]. The fluid dynamic principles of the RWV bioreactor allow oxygenation without turbulence, co-localization of particles with different sedimentation rates, and reduction of fluid shear forces. Fig. 1 schema shows the bioreactor culture apparatus and research design.
Figure 1.
Schema showing the bioreactor culture research design
Disc tissue culture
Lumbar disc tissues were separated from 24 10-week Balb/C mice. The animals were euthanized with CO2. The University of Virginia Animal Care and Handle protocol was followed with all animal procedures. In brief, lumbar IVDs including the superior and inferior endplates, annulus fibrosus, and nucleus pulposus were dissected and cleaned from connective tissues under aseptic conditions. The discs were cultured in DMEM media supplemented with 2% penicillin/streptomycin [15, 16]. After incubation with antibiotic, discs were transferred to 50 ml high aspect ratio vessels and either maintained statically as controls or rotated at 36 rpm in rotating bioreactors. DMEM/F-12 media with 20% fetal bovine serum (Invitrogen), 1% penicillin/streptomycin, insulin-transferrin-selenium (10µg/ml insulin, 5.5 µg/ml transferrin, and 0.5 ng/ml sodium selenium) (Sigma), and 1% ascorbate at 37 °C was used for culture and media was changed every 3 days. Discs were harvested at varying time points.
Biochemistry assay
Six discs from rotating and static culture groups in 2-, 4-, 6-, and 8-week time point were used for biochemistry assay. Amino sugars and hydroxyproline (Hypro), as indicators of glycosaminoglycan (GAG) and collagen, respectively, were measured as previously described [14, 17]. Briefly, the discs cultured in static and rotating conditions were digested with Papain buffer solution at 60°C for 24 h. The amount of GAG was determined by the dimethylmethylene blue (DMMB) colorimetric assay using Chondrotin Sulfate as a standard. For the Hypro measurement, an aliquot of Papain digest was hydrolyzed in 6N HCI for 16 h at 110°C, and quantif ied with dimethylaminobenzaldehyde (DMBA)/chloramine T (Sigma) colorimetric assay using hydroxy-L proline (Sigma) as a standard. The DNA content was measured with Hoechest dye using calf thymus DNA as a standard. The GAG and Hypro concentrations were shown individually and also expressed as GAG to Hypro ratio.
Safranin-O staining
Five discs from rotating and static bioreactor culture were harvested at day 10, 20, and 30 days for histological studies. Discs were washed with PBS and fixed in 10% buffered formalin for 4 h. The samples were demineralized with 250 mM EDTA. After dehydrating with a series of graded ethanol, tissues were embedded in paraffin. Five to seven micron thick sections were deparaffined and hydrated to distilled water, stained with hematoxylin, and destained quickly in acid EtOH. Samples were then stained with fast green solution, rinsed briefly with 1% acetic acid, and stained with 0.1% Safranin-O solution for 5 minutes before being dehydrated and finally mounted. The samples were visualized with an Axioskop 2 Zeiss microscope.
Immunohistochemistry
The detection of collagen II was performed as previously described [14]. The 5–7 µm thick paraffin embedded disc sections were first deparaffinized and rehydrated before being treated with 3% hydrogen peroxide in methanol for 30 min to block the endogenous peroxidase. The sections were boiled in 10 mM citrate buffer (pH 6.0) and treated with 2% bovine testicular hyaluronidase to retrieve antigen. Sections were incubated with collagen II monoclonal antibody (1:400) pre-conjugated with biotin for 1 h at room temperature followed by blocking solution. For the detection of MMP-3, sections were incubated with rabbit anti-MMP3 antibody (1:100, Abcam) and corresponding secondary IgG conjugated with Alexa Fluor 488. The nucleus was stained with Topro-3 (Invitrogen). The signals were visualized with a confocal microscopy and quantitated with FluoView (Olympus). IgG was used as a negative control.
In situ cell-death detection
The apoptotic cells were detected with an in situ cell death detection POD kit (Roche) by following the manufacturer’s instructions [14]. Briefly, the tissue sections (10 µm thick) were deparaffinized and rehydrated before being treated with proteinase K (20µg/ml in 10 mM Tris/HCl ) and permeabilized with 0.1 % Trinton X-100 in PBS. The TUNEL reaction mixture was applied to the tissues for 1h at 37°C. The nucleus was stained with Topro-3 (Invitrogen). The signals were visualized under a fluorescence microscope and quantitated with FluoView. The tissues exposed to the label solution without the TUNEL reaction mixture were used as negative control.
Statistical analysis
Statistical significance among the differences in the means of biochemistry assay data and apoptotic fluorescence signal intensity was analyzed by analysis of variance (ANOVA). All data is expressed as the mean ± standard error. A P value of ≤ 0.05 was considered as statistically significant.
Results
Microgravity induced disc degeneration
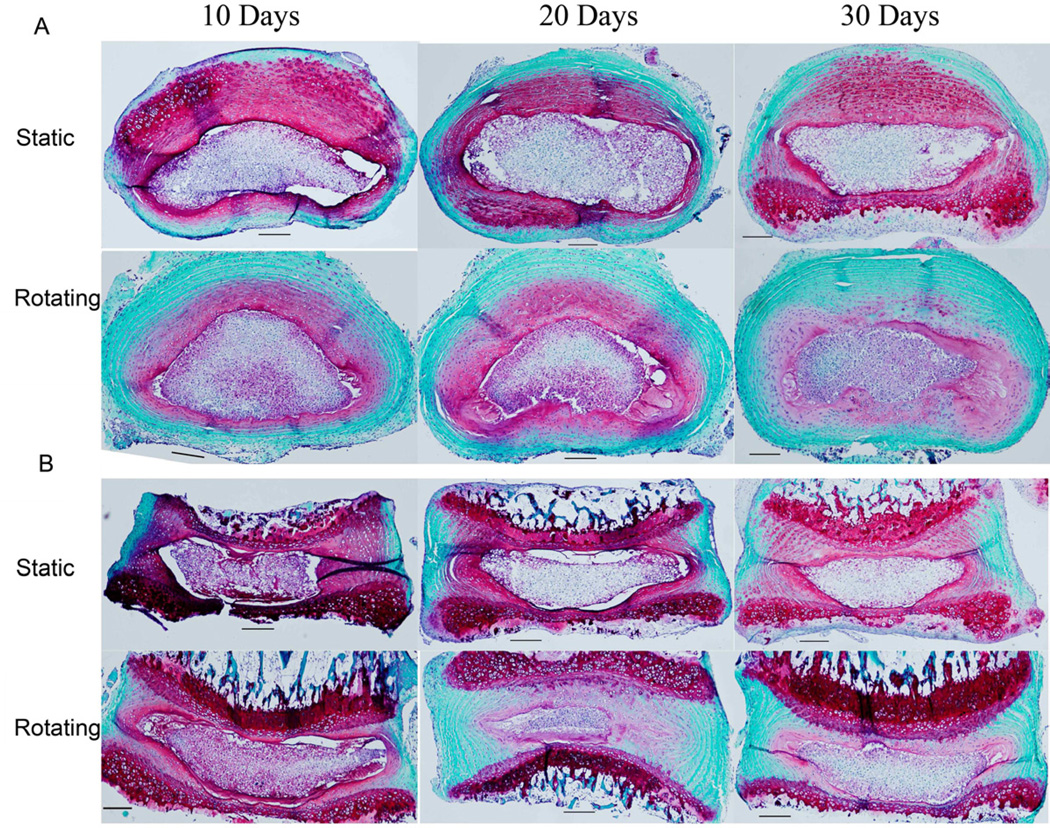
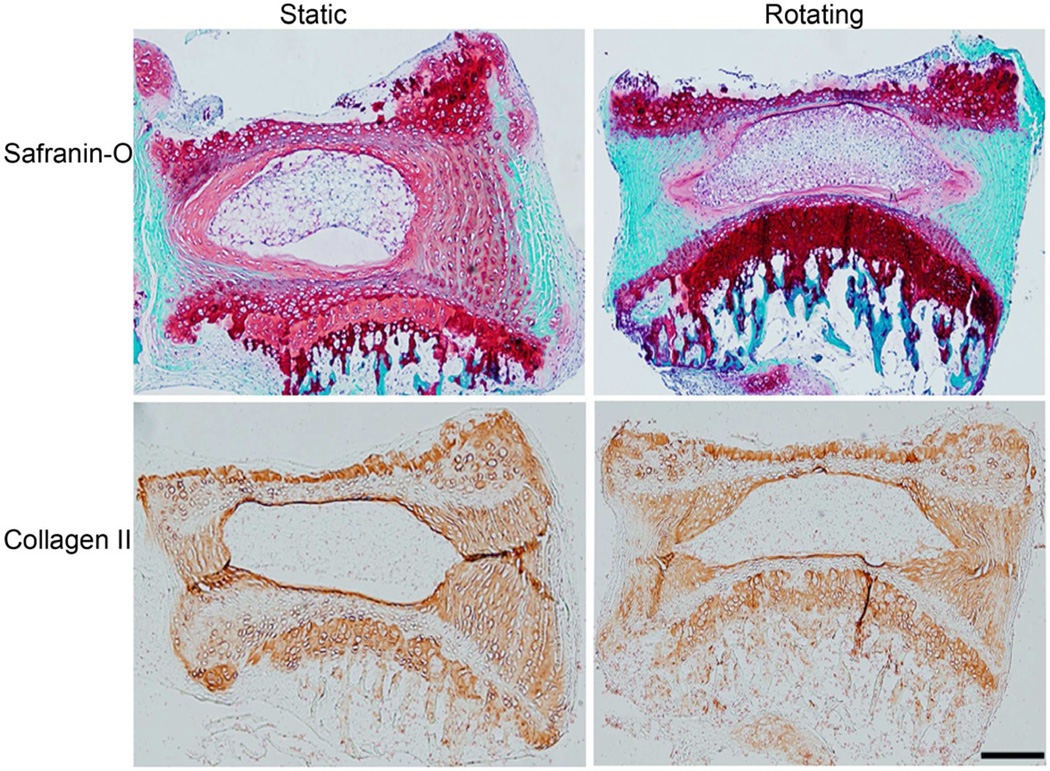
To simulate the space environment, the IVDs were maintained in organ culture either in a rotating or static bioreactor. Discs were harvested at 10, 20, and 30 days, and subjected to Safranin-O staining (proteoglycan in red). As shown in Fig. 2, compared with in static culture, the disc tissues of the rotating culture showed markedly decreased red staining in the annulus fibrosus region. These changes were observed in both horizontal (Fig. 2A) and vertical (Fig. 2B) disc sections and were shown as early as 10 days becoming worse with prolonged culture time up to 30 days. In contrast, in static conditions, the Safranin-O signal decreased slightly over time, but disc structure was unaffected. These results suggest that simulated microgravity was one of contributing factors for disc degeneration and correlate greater degeneration with longer duration of exposure to the simulated microgravity.
Figure 2.
Histological study demonstrating less cellularity and structural disruption of discs in rotating culture conditions. A. Horizontal section; B. Vertical section. Mouse IVDs were cultured in the bioreactor in static or rotating conditions. Discs were harvested at 10, 20, and 30 days. The paraffin embedded sections were stained with Safranin-O, and the morphological changes were photographed on an Axioskop 2 Zeiss microscope. (Scale bar = 50µm)
Proteoglycan was decreased in discs in microgravity condition
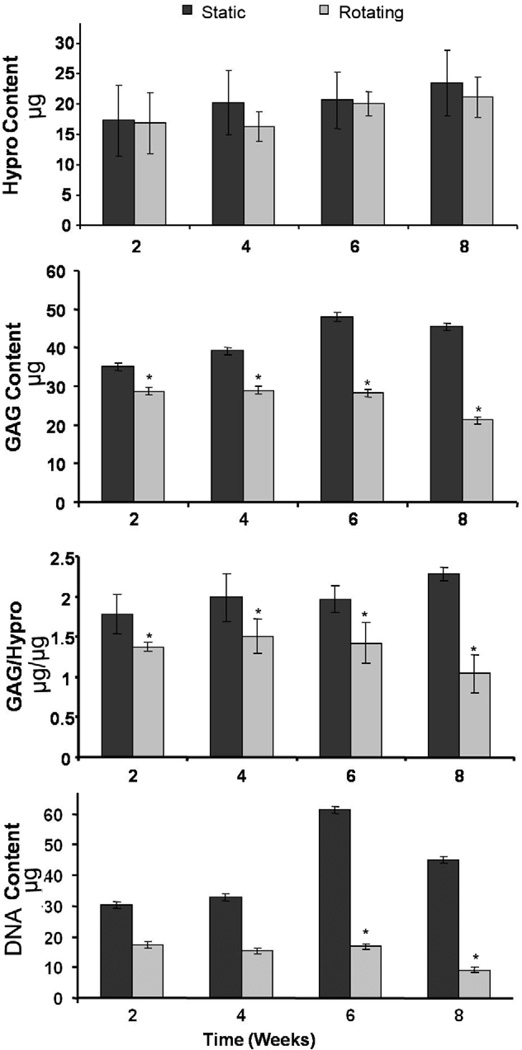
A major feature of disc degeneration is the progressive reduction in proteoglycan expression. To further confirm the changes of disc degeneration seen in the histology study, biochemical assays were performed. Amino sugars and Hypro, as indicators of GAG and collagen, respectively, were measured in the cultured discs. As indicated in Fig. 3, when compared with static culture, rotating conditions demonstrated significantly decreased GAG content within the IVDs. This decrease was evident as early as 2 weeks and progressively decreased at 4, 6 and 8 weeks to 80%, 70%, 40% and approximately 30%, respectively, of that seen in corresponding static culture. However, similar levels of Hypro were maintained in both static and rotating conditions. The DNA content (as a reflection of overall cell number) was significantly decreased in the rotating bioreactor group compared with static culture group (Fig. 3). In addition, the ratio of GAG to Hypro was found to progressively decrease in rotating conditions. These results were consistent with our histological study as well as previously published work [18]
Figure 3.
Biochemical assays showing decreased GAG content of discs in rotating culture conditions, while no significant change in Hypro content was observed. Organ cultured mouse IVDs were harvested at 2, 4, 6, and 8 weeks, and digested with papain solution. The DNA content was measured with Hoechest dye using calf thymus DNA as a standard. The GAG and Hypro contents were measured by dimethylmethylene blue colorimetric assay and dimethylamino-benzaldehyde /chloramine T colorimetric assay, respectively. Values represent mean ±SEM (n=6). *, p<0.05 compared to the static culture condition.
Microgravity increased MMP3 expression but not collagen II in discs
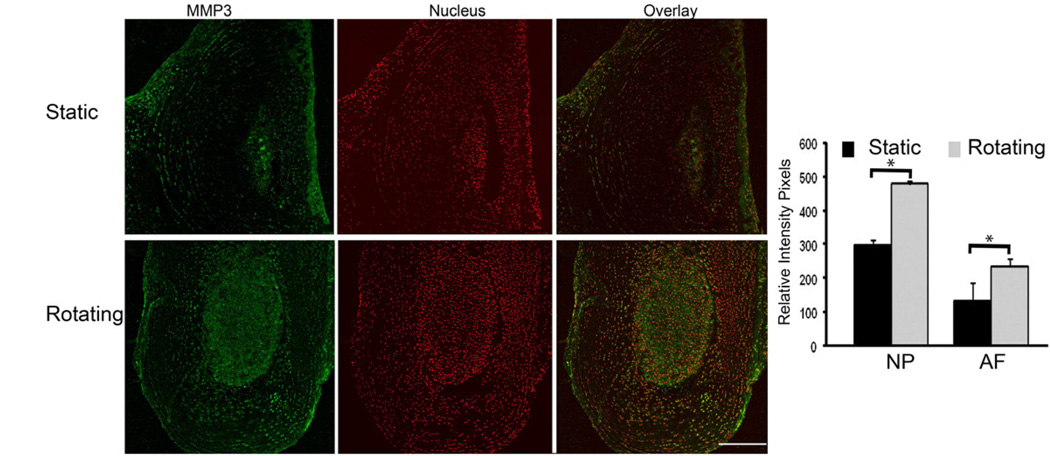
Studies have shown that the expression of matrix metalloproteinases (MMPs) increases in the degenerated disc, and elevated MMPs correlate with progressive disc degeneration. We measured the expression of MMP3 in the discs in simulated microgravity conditions using immunofluorescence. As shown in Fig. 4, the expression of MMP3 was significantly increased in both annulus fibrosus and nucleus pulposus areas of discs cultured in the rotating bioreactor at 30 days compared to that in static condition (p<0.05). Bar graph showed the quantitation of the MMP-3 fluorescence (Green) intensity. In contrast, the expression of type II collagen did not change between rotating and static conditions up to the 30 day time point (Fig. 5). This data is consistent with the biochemical assay (Fig. 3) and Safranin-O staining (Fig. 2), which showed decreased GAG content and stable Hypro content, and less red staining, respectively, in the discs cultured in the rotating bioreactor.
Figure 4.
The expression of MMP3 was increased in discs in rotating conditions. The organ cultured discs were harvested at day 30. The paraffin embedded sections were stained with rabbit anti-MMP3 antibody followed by corresponding secondary antibody conjugated Alexa 488. The signal was visualized with confocal microscopy and quantitated with FluoView software. Bar graph shows the quantitation of fluorescence intensity. * p<0.05, Scale bar=100 µm.
Figure 5.
The expression of collagen II was not changed in discs in rotating conditions. The organ cultured discs were harvested at day 30. The paraffin embedded sections were stained with collagen II antibody conjugated with biotin. The signals were developed with DAB, and photographed on an Axioskop 2 Zeiss microscope.
Microgravity induced apoptosis in IVDs
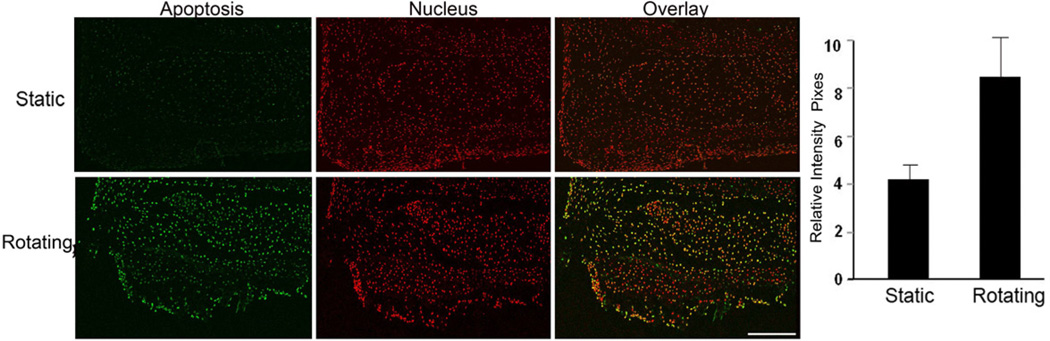
The above results suggested that disc degeneration occurred in microgravity conditions. We further tested the hypothesis that this degeneration was induced by the apoptotic pathway. To address this question, the in situ cell deaths were measured with the TUNEL assay. As shown in Fig. 6, more cells underwent apoptosis (green color) in rotating conditions at 30 days than in static conditions (p<0.05). The nucleus staining was shown in red color. Bar graph shows the quantitation of the apoptotic fluorescence intensity. In addition, the number of apoptotic cells increased as the culture time increased (data not shown) in rotating condition. These results suggest that apoptosis plays a role in microgravity induced disc degeneration.
Figure 6.
The apoptotic cells were increased in discs in rotating conditions. The cultured discs were harvested at day 30. A. Representative sections were stained with an in situ cell death kit (scale bar = 200µm). Green color, apoptotic cells; red color, nucleus staining with topro-3; and yellow color: overlay. B. The signals were captured with a fluorescence microscope and quantitated with FluoView software.
Discussion
It has been reported that 50% of astronauts experience back pain (low back pain in particular) during and after spaceflight [9, 19]. Although the intervertebral disc has not been shown specifically to be the source of the pain in astronauts, it is a well described source of low back pain in those of us earthbound. As opposed to its normal function of resisting essentially constant compressive forces under typical gravity conditions, the intervertebral disc experiences low compressive forces during space flight. This microgravity produces, among other things, a lower hydrostatic pressure effect on the disc cells which is thought to be central to the pathophysiology of disc degeneration experienced by astronauts [20]. However, the detailed mechanisms of back pain experienced by astronauts are still elusive. Using an in vitro organ culture under simulated microgravity system, we demonstrated noticeable disc degeneration as seen by decreased proteoglycan within the annulus fibrosis, increased MMP-3 expression, and the upregulated apoptosis.
Numerous groups have reported different animal models for investigation of disc degeneration. Although in vivo models are possible to a certain extent, understanding the effect of complex environment would be feasible to a greater extent in ex vivo using a whole organ system. Therefore, several intervertebral disc organ culture models have been established in recent years [21, 22, 23, 24]. The large animals have a similar disc structure to humans, but are limited by the long duration and high cost. In addition, the insufficient nutrient supply caused cell deaths. Discs cultured with intact endplates preserved their integrity and shape, but most of the cells were dead after 10 days in culture, To overcome the cell death, a bioreactor has been used to investigate the effects of limiting nutrition to the intervertebral discs under high-frequency loading, and found cells survived for a period of 21 days [21, 25]. Although we did not measure the disc height and volume, we observed little disc shape change between static and rotating conditions, which is consistent with the other study that showed discs maintained integrity with bony endplate in organ culture system [25]. It would be interesting to investigate the compressive properties of the disc in microgravity in response to a controlled axial load, which may provide valuable information about the response of the spine to microgravity.
The extracellular matrix of the IVD is composed largely of proteoglycan and includes a high concentration of water. Proteoglycan plays an important role as a shock absorber within the IVD providing protection against various stresses. The proportion of the extracellular matrix changes across the disc with the nucleus containing a higher concentration of aggrecan and a lower level of type II collagen than the annulus fibrosus [26]. With aging, the proportions of aggrecan and water in the nucleus decreased markedly, while the proportion of collagen rises; a similar change is seen in degenerate discs. The degenerative process starts with cellular loss, proteoglycan breakdown, and decreased proteoglycan/collagen ratio, leading to diminished water-binding capacity and disc dehydration. In simulated microgravity condition, the cultured discs also undergo disc degeneration as observed by progressively decreased red color in Safranin-O staining and reduced GAG/Hypro ratio (Fig. 2 and 3). The collagen II contents did not significantly change in the rotating conditions compared with the static ones. (Fig. 5).These data are consistent with previous findings which characterize intervertebral disc degeneration by progressive loss of proteoglycan [27] and decreased proteoglycan/collagen ratio in rat IVD after a 12.5 days in space flight [9]. In another disc degeneration model: the needle puncture model, we [28] and others [29] have also found that the GAG contents were significantly decreased while no appreciable collagen changes have been observed. The reason for the unchanged level of collagen may due to that the current method was unable to detect the damaged collagen. Collagenase cleaves collagen molecules at a single site within their triple helical region, but because of the extensive cross-linking within the collagen fibrils, proteolysis does not necessarily result in collagen loss. Instead, the damaged collagen molecules persist in the fibril structure. Such damage increases with disc degeneration and becomes more extensive from the outer annulus fibrosus to the nucleus pulposus [13]. The accumulation of such damaged collagen is a reflection of the very slow turnover of the collagen fibrils and would be expected to eventually weaken the mechanical strength of the tissue and ultimately result in tissue loss, as occurs in the later stages of disc degeneration. All of this knowledge suggests that the amount and balance of extracellular matrix exhibit in a temporal and spatial manner. The change of GAG/Hypro may better represent the pathology of disc degeneration.
Matrix metalloproteinases play a major role in extracellular matrix degradation within the disc and an imbalance in their production and activation relative to their inhibition by tissue inhibitors of metalloproteinases can cause disc degeneration. http://gateway.ut.ovid.com/gw1/ovidweb.cgi-108
Evidence exists for the presence of collagenases (MMP1, 8 and 13), gelatinases (MMP2 and 9), and stromelysin (MMP3) within the disc [30–32] and the actions of these various proteins within the IVD account for much of the degradation of collagen, aggrecan, versican, and link protein. In microgravity conditions, the expression of the catabolic gene MMP3 was increased compared to that of the static condition as measured by the immunofluorescence assay. This may account for, at least partially, the proteoglycan degradation observed within the disc.
Another mechanism of disc generation is through induction of the apoptosis pathway. Studies have shown that apoptotic cell surface proteins, including those from the Fas/Fas ligand system, are present in disc cells [33, 34]. Apoptotic cell death in degenerated disc cells occurs through the type-II (mitochondrial) pathway [35, 36]. In the rotating culture condition, we found that the apoptotic cells were increased in the cultured disc at 30 days, which suggests that apoptosis pathway may involve in the microgravity induced disc degeneration. From our observations, we favor that both MMPs and apoptosis contribute to the disc degeneration in microgravity conditions.
A novel aspect of the current study is the development an in vitro disc degeneration model. In vitro disc degeneration models could be divided into two main categories. The first includes biochemically induced models, as achieved through the application of IL-1 [37], fibronectin fragment [38], and hydrogen [39]. The other is biomechanically induced, using mechanical factors such as vibration, compression, and distraction [40, 41]. The biochemically induced disc degeneration model is applied most often to cell culture due to inherent limitations related to infusion of detrimental chemicals into the intact disc. Biomechanically induced degeneration models are limited by the expensive nature of products designed to generate and apply the required variables such as force production at particular frequencies. The microgravity induced disc degeneration model mimics the in vivo degeneration changes as discussed above. It is an organ culture system and does not require expensive equipment. Any therapeutic treatment with attempts to deter, delay, or reverse the process of disc degeneration could be easily adapted.
There are several limitations of the current study. One, due to the small size of the mice disc, we do not separate annulus from nucleus tissue which would be expected to have different responses to early disc degeneration stimulators including microgravity. At very early time points, nucleus tissue has progressively declining extracellular matrix (ECM) production while the annulus fibrosus initially has increased ECM production and then followed by decreased. Second, we only demonstrated the changes of extracellular matrix, but did not analyze changes in cell phenotype. Additional studies are needed for further characterization of the disc cells and organs cultured in a simulated microgravity environment. Third, while GAG in IVDs in the rotating bioreactor was decreased, a measure of GAG content in the corresponding culture media was not performed. A complimentary increase of GAG in this corresponding culture media would be further verification of our finding of decreased IVD GAG content. Fourth, although the rotating bioreactor produces a time-averaged gravitational vector of zero, which is one of the causes of disc degradation changes, other factors that contribute to the changes should not be neglected. These include low flow-induced shear in the rotating bioreactor and differences in nutrition transport between the rotating and static bioreactors. Fifth, extracellular matrix analysis was not performed at mRNA level. The disc is composed of two kinds of tissue: annulus and nucleus. A healthy nucleus is rich in proteoglycans and type II collagen, whereas the annulus is rich in type I collagen. The cells in the two regions are very different. Grouping these two cell populations will somewhat cloud the results and limit the interpretation. The best way is to perform in situ hybridization to detect gene expressions of type II collagen, aggrecan and MMP-3.
In conclusion, using an in vitro simulated microgravity model, we have characterized the deleterious effects of microgravity on the mouse intervertebral disc. This model may be used for the development and testing of future therapeutic strategies of human disc degeneration. Additional studies are needed to determine the mechanisms by which microgravity induces disc degeneration, and what genes may compensate for the disease.
Acknowledgement
The work was supported by AO Foundation (F-07-97L), Scoliosis Research Society, and NIH R03 AR053653.
Abbreviations
- IVD
intervertebral disc
- GAG
glycosaminoglycan
- MMP-3
matrix metalloproteinase 3
- Hypro
hydroxyproline
- ECM
extracellular matrix
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morey ER, Baylink DJ. Inhibition of bone formation during space flight. Science. 1978;201:1138–1141. doi: 10.1126/science.150643. [DOI] [PubMed] [Google Scholar]
- 2.Vico L, Collet P, Guignandon A, Lafage-Proust MH, Thomas T, Rehaillia M, Alexandre C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet. 2000;355:1607–1611. doi: 10.1016/s0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 3.Hargens AR, Watenpaugh DE. Cardiovascular adaptation to spaceflight. Med Sci Sports Exerc. 1996;28:977–982. doi: 10.1097/00005768-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Vico L, Novikov VE, Very JM, Chappard D, Alexandre C. Effects of a 40 day tail-suspension on rat weight-bearing bones. Physiologist. 1990;33:S96–S97. [PubMed] [Google Scholar]
- 5.Globus RK, Bikle DD, Halloran B, Morey-Holton E. Skeletal response to dietary calcium in a rat model simulating weightlessness. J Bone Miner Res. 1986;1:191–197. doi: 10.1002/jbmr.5650010205. [DOI] [PubMed] [Google Scholar]
- 6.Rabin R, Gordon SL, Lymn RW, Todd PW, Frey MA, Sulzman FM. Effects of spaceflight on the musculoskeletal system: NIH and NASA future directions. FASEB J. 1993;7:396–398. doi: 10.1096/fasebj.7.5.8462780. [DOI] [PubMed] [Google Scholar]
- 7.Sayson JV, Hargens AR. Pathophysiology of low back pain during exposure to microgravity. Aviation Space & Environmental Medicine. 2008;79:365–373. doi: 10.3357/asem.1994.2008. [DOI] [PubMed] [Google Scholar]
- 8.Johnston SLC, Mark R, Scheuring Rick Feiveson, Alan H. Risk of herniated nucleus pulposus among U.S. astronauts. Aviation Space & Environmental Medicine. 2010;81:566–574. doi: 10.3357/asem.2427.2010. [DOI] [PubMed] [Google Scholar]
- 9.Foldes I, Kern M, Szilagyi T, Oganov VS. Histology and histochemistry of intervertebral discs of rats participated in spaceflight. Acta Biologica Hungarica. 1996;47:145–156. [PubMed] [Google Scholar]
- 10.Anderson DG, Tannoury C. Molecular pathogenic factors in symptomatic disc degeneration. Spine J. 2005;5:260S–266S. doi: 10.1016/j.spinee.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Anderson DG, Albert TJ, Fraser JK, Risbud M, Wuisman P, Meisel HJ, Tannoury C, Shapiro I, Vaccaro AR. Cellular therapy for disc degeneration. Spine (Phila Pa 1976) 2005;30:S14–S19. doi: 10.1097/01.brs.0000175174.50235.ba. [DOI] [PubMed] [Google Scholar]
- 12.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoniou J, Goudsouzian NM, Heathfield TF, Winterbottom N, Steffen T, Poole AR, Aebi M, Alini M. The human lumbar endplate. Evidence of changes in biosynthesis and denaturation of the extracellular matrix with growth, maturation, aging, and degeneration. Spine (Phila Pa 1976) 1996;21:1153–1161. doi: 10.1097/00007632-199605150-00006. [DOI] [PubMed] [Google Scholar]
- 14.Feng G, Wan Y, Shen FH, Li X. Nucleus pulposus explant culture model. J Orthop Res. 2009;27:814–819. doi: 10.1002/jor.20803. [DOI] [PubMed] [Google Scholar]
- 15.Risbud MV, Di Martino A, Guttapalli A, Seghatoleslami R, Denaro V, Vaccaro AR, Albert TJ, Shapiro IM. Toward an optimum system for intervertebral disc organ culture: TGF-beta 3 enhances nucleus pulposus and anulus fibrosus survival and function through modulation of TGF-beta-R expression and ERK signaling. Spine (Phila Pa 1976) 2006;31:884–890. doi: 10.1097/01.brs.0000209335.57767.b5. [DOI] [PubMed] [Google Scholar]
- 16.Risbud MV, Izzo MW, Adams CS, Arnold WW, Hillibrand AS, Vresilovic EJ, Vaccaro AR, Albert TJ, Shapiro IM. An organ culture system for the study of the nucleus pulposus: description of the system and evaluation of the cells. Spine (Phila Pa 1976) 2003;28:2652–2658. doi: 10.1097/01.BRS.0000099384.58981.C6. discussion 2658-9. [DOI] [PubMed] [Google Scholar]
- 17.Cui M, Wan Y, Anderson DG, Shen FH, Leo BM, Laurencin CT, Balian G, Li X. Mouse growth and differentiation factor-5 protein and DNA therapy potentiates intervertebral disc cell aggregation and chondrogenic gene expression. Spine J. 2008;8:287–295. doi: 10.1016/j.spinee.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Maynard JA. The effects of space flight on the composition of the intervertebral disc. Iowa Orthop J. 1994;14:125–133. [PMC free article] [PubMed] [Google Scholar]
- 19.Wing PC, Tsang IK, Susak L, Gagnon F, Gagnon R, Potts JE. Back pain and spinal changes in microgravity. Orthop Clin North Am. 1991;22:255–262. [PubMed] [Google Scholar]
- 20.Hutton WC, Elmer WA, Boden SD, Hyon S, Toribatake Y, Tomita K, Hair GA. The effect of hydrostatic pressure on intervertebral disc metabolism. Spine (Phila Pa 1976) 1999;24:1507–1515. doi: 10.1097/00007632-199908010-00002. [DOI] [PubMed] [Google Scholar]
- 21.Haglund LA, Moir J, Beckman L, Mulligan KR, Jim B, Ouellet J, Roughly P, Steffen T. Development of a Bioreactor for Axially Loaded Intervertebral Disc Organ Culture. Tissue Eng Part C Methods. 2011 doi: 10.1089/ten.TEC.2011.0025. [DOI] [PubMed] [Google Scholar]
- 22.Jim B, Steffen T, Moir J, Roughley P, Haglund L. Development of an intact intervertebral disc organ culture system in which degeneration can be induced as a prelude to studying repair potential. Eur Spine J. 2011 doi: 10.1007/s00586-011-1721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gantenbein B, Grunhagen T, Lee CR, van Donkelaar CC, Alini M, Ito K. An in vitro organ culturing system for intervertebral disc explants with vertebral endplates: a feasibility study with ovine caudal discs. Spine (Phila Pa 1976) 2006;31:2665–2673. doi: 10.1097/01.brs.0000244620.15386.df. [DOI] [PubMed] [Google Scholar]
- 24.Lee CR, Iatridis JC, Poveda L, Alini M. In vitro organ culture of the bovine intervertebral disc: effects of vertebral endplate and potential for mechanobiology studies. Spine (Phila Pa 1976) 2006;31:515–522. doi: 10.1097/01.brs.0000201302.59050.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Illien-Junger S, Gantenbein-Ritter B, Grad S, Lezuo P, Ferguson SJ, Alini M, Ito K. The combined effects of limited nutrition and high-frequency loading on intervertebral discs with endplates. Spine (Phila Pa 1976) 2010;35:1744–1752. doi: 10.1097/BRS.0b013e3181c48019. [DOI] [PubMed] [Google Scholar]
- 26.Eyre DR, Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977;492:29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- 27.Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35:652–655. doi: 10.1042/BST0350652. [DOI] [PubMed] [Google Scholar]
- 28.Greg Anderson D, Li X, Tannoury T, Beck G, Balian G. A fibronectin fragment stimulates intervertebral disc degeneration in vivo. Spine (Phila Pa 1976) 2003;28:2338–2345. doi: 10.1097/01.BRS.0000096943.27853.BC. [DOI] [PubMed] [Google Scholar]
- 29.Han B, Zhu K, Li FC, Xiao YX, Feng J, Shi ZL, Lin M, Wang J, Chen QX. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine (Phila Pa 1976) 2008;33:1925–1934. doi: 10.1097/BRS.0b013e31817c64a9. [DOI] [PubMed] [Google Scholar]
- 30.Crean JK, Roberts S, Jaffray DC, Eisenstein SM, Duance VC. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine (Phila Pa 1976) 1997;22:2877–2884. doi: 10.1097/00007632-199712150-00010. [DOI] [PubMed] [Google Scholar]
- 31.Johnson WE, Evans H, Menage J, Eisenstein SM, El Haj A, Roberts S. Immunohistochemical detection of Schwann cells in innervated and vascularized human intervertebral discs. Spine (Phila Pa 1976) 2001;26:2550–2557. doi: 10.1097/00007632-200112010-00007. [DOI] [PubMed] [Google Scholar]
- 32.Roberts S, Johnson WE. Analysis of aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1999;24:500–501. doi: 10.1097/00007632-199903010-00026. [DOI] [PubMed] [Google Scholar]
- 33.Kaneyama S, Nishida K, Takada T, Suzuki T, Shimomura T, Maeno K, Kurosaka M, Doita M. Fas ligand expression on human nucleus pulposus cells decreases with disc degeneration processes. J Orthop Sci. 2008;13:130–135. doi: 10.1007/s00776-007-1204-4. [DOI] [PubMed] [Google Scholar]
- 34.Yang H, Wu J, Liu J, Ebraheim M, Castillo S, Liu X, Tang T, Ebraheim NA. Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta1 decrease rabbit intervertebral disc degeneration. Spine J. 2010;10:802–810. doi: 10.1016/j.spinee.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Kim HJ, Park JB, Lee JK, Park EY, Park EA, Riew KD, Rhee SK. Transplanted xenogenic bone marrow stem cells survive and generate new bone formation in the posterolateral lumbar spine of non-immunosuppressed rabbits. Eur Spine J. 2008;17:1515–1521. doi: 10.1007/s00586-008-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JB, Lee JK, Park EY, Riew KD. Fas/FasL interaction of nucleus pulposus and cancer cells with the activation of caspases. Int Orthop. 2008;32:835–840. doi: 10.1007/s00264-007-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, An HS, Toofanfard M, Li Z, Andersson GB, Thonar EJ. Low-dose interleukin-1 partially counteracts osteogenic protein-1-induced proteoglycan synthesis by adult bovine intervertebral disk cells. Am J Phys Med Rehabil. 2005;84:322–329. doi: 10.1097/01.phm.0000159972.85053.7e. [DOI] [PubMed] [Google Scholar]
- 38.Anderson DG, Li X, Balian G. A fibronectin fragment alters the metabolism by rabbit intervertebral disc cells in vitro. Spine (Phila Pa 1976) 2005;30:1242–1246. doi: 10.1097/01.brs.0000164097.47091.4c. [DOI] [PubMed] [Google Scholar]
- 39.Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Hanley EN. IGF-1 rescues human intervertebral annulus cells from in vitro stress-induced premature senescence. Growth Factors. 2008;26:220–225. doi: 10.1080/08977190802273814. [DOI] [PubMed] [Google Scholar]
- 40.Illien-Junger S, Gantenbein-Ritter B, Grad S, Lezuo P, Ferguson SJ, Alini M, Ito K. The combined effects of limited nutrition and high-frequency loading on intervertebral discs with endplates. Spine (Phila Pa 1976) 2011;35:1744–1752. doi: 10.1097/BRS.0b013e3181c48019. [DOI] [PubMed] [Google Scholar]
- 41.Junger S, Gantenbein-Ritter B, Lezuo P, Alini M, Ferguson SJ, Ito K. Effect of limited nutrition on in situ intervertebral disc cells under simulated-physiological loading. Spine (Phila Pa 1976) 2009;34:1264–1271. doi: 10.1097/BRS.0b013e3181a0193d. [DOI] [PubMed] [Google Scholar]