Abstract
Polycystic ovary syndrome (PCOS) is a common fertility disorder with metabolic sequelae. Our lab previously characterized reproductive phenotypes in a prenatally androgenized (PNA) mouse model for PCOS. PNA mice exhibited elevated testosterone and luteinising hormone (LH) levels, irregular oestrous cycles, and neuroendocrine abnormalities suggesting increased central drive to the reproductive system. In this study we examined metabolic characteristics of female PNA mice. PNA mice exhibited increased fasting glucose and impaired glucose tolerance (IGT) that were independent of age and were not associated with changes in body composition or peripheral insulin sensitivity. IGT was associated with defects in pancreatic islet function leading to an impaired response to high glucose, consistent with impaired insulin secretion. Exposure of isolated pancreatic islets to androgen in vitro demonstrated an impaired response to glucose stimulation similar to that in PNA mice, suggesting androgens may have activational in addition to organizational effects on pancreatic islet function. PNA mice also exhibited increased size of visceral adipocytes, suggesting androgens programmed differences in adipocyte differentiation and/or function. These studies demonstrate that in addition to causing reproductive axis abnormalities, in utero androgen exposure can induce long-term metabolic alterations in female mice.
Introduction
Developmental programming by steroid hormones is important to establish sex differences in the reproductive tract and in other physiological systems. Androgen levels surge during gestation and postnatally in the male (Tapanainen et al., 1981; Quigley, 2002), while in females androgens typically remain low during embryonic development, except in rare instances of pathologic exposure from intrinsic (fetal) or extrinsic (maternal or environmental) sources. Recent work on endocrine disruptors has demonstrated the existence of environmental substances with androgenic actions, such as 17-β-trenbolone and triclocarban, which are a potential cause of abnormal fetal androgenisation (Gray et al., 2006; Hotchkiss et al., 2007; Chen et al., 2008). Because hormonal perturbations during this critical time may have adverse effects that persist into adulthood, it is important to study the consequences of androgen exposure in utero.
Polycystic ovary syndrome (PCOS), the most common cause of infertility in women, is one disorder that may originate in prenatal androgen excess. PCOS is characterized by hyperandrogenemia, elevated central drive to the reproductive system, and irregular or absent menstrual cycles due to oligo- or anovulation (Dunaif, 1997). PCOS also predisposes women to metabolic dysfunction characterized by impaired glucose homeostasis and abdominal adiposity (Sam and Dunaif, 2003). Animal models have exhibited reproductive and metabolic abnormalities similar to PCOS following prenatal androgenisation (Abbott et al., 2005; Dumesic et al., 2007; Demissie et al., 2008). The finding that prenatal androgen can lead to defects in both reproduction and metabolism suggests it may play a major role in the etiology of at least some cases of PCOS. Consistent with this idea, women with PCOS have elevated circulating androgens during late gestation (Sir-Petermann et al., 2002), potentially exposing their offspring, who are at increased risk for PCOS (Sir-Petermann et al., 2009). Alternatively, it has been proposed that the fetal ovary itself is the source of androgen due to abnormalities in genes controlling steroidogenesis (Legro et al., 1998).
To mimic the gestational androgen excess associated with PCOS and other inappropriate androgen exposures, our lab developed a mouse model treated with dihydrotestosterone (DHT) late in gestation. Previously, we described reproductive neuroendocrine abnormalities in this model (Sullivan, 2004), including elevated androgen and LH levels, irregular oestrous cycles, and increased excitatory neurotransmission to GnRH neurons. Here we investigate metabolic phenotypes in PNA mice.
Materials and Methods
Generation of PNA mice
Adult (2–4 mo) female GnRH-GFP (descended from CBB6/F1 founder, currently ~75% C57Bl/6J by speed congenics) transgenic mice were used to generate PNA mice. Mice were housed under a 14 h light:10 h dark cycle with chow (2916, Harlan, Indianapolis, IN) and water available ad libitum. Females were paired with males and checked for copulatory plugs. The date of plug was considered day 1 of gestation. Pregnant mice were injected daily subcutaneously with 50 μl sesame oil containing 250 μg of dihydrotestosterone (DHT) on day 16–18 of gestation. DHT was used to eliminate the possibility of aromatisation to oestradiol, thereby permitting the study of primarily androgen receptor-mediated effects. Of note, DHT can be metabolized to 5α-androstane-3β,17β-diol, which can bind ERβ (Handa et al., 2008); however, the levels of this metabolite attained in the fetal compartment are unknown. Female offspring were subjected to glucose tolerance tests beginning at one month of age; all other studies were performed at 3–6 months. Control mice (CON) were offspring of either oil-injected dams or untreated mice; no differences were observed between these groups, and they were combined for analysis. Three rounds of PNA mice were generated for use in this study: 9 CON and 7 PNA mice for repeated glucose tolerance testing; 22 CON and 21 PNA mice for DEXA, adipocyte studies, islet studies, and hormone measurements; and 10 CON and 10 PNA mice for insulin tolerance testing. For in vitro examination of steroid effects on pancreatic islets, C57BL/6J female mice 8–12 weeks old were purchased from Jackson Laboratories (Bar Harbor, ME). These mice were ovariectomized (OVX) three days before islet isolation under isoflurane anesthesia (Burns Veterinary Supply, Westbury, NY). Long-acting postoperative local analgesia was provided by 0.25% bupivacaine (Abbott Laboratories, North Chicago, IL). All procedures were approved by the University of Virginia Animal Care and Use Committee and conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Glucose tolerance tests (GTT)
Mice were singly housed on Sani-Chip bedding (Harlan, Indianapolis, IN) and fasted overnight for sixteen hours (1600h to 0800h) prior to the test. The tail was anesthetized with the skin refrigerant ethyl chloride (Gebauer, Cleveland, OH) and the tip removed with a sterile scalpel blade. Tail blood (~1 μl/ sample) was collected for glucose measurement with a OneTouch Ultra glucometer (Lifescan, Milpitas, CA). Following a fasting glucose measurement, mice were injected intraperitoneally with a bolus of 1g/kg glucose in 0.9% NaCl. Blood glucose was assessed at 10, 20, 30, 45, 60, 75, 90, and 120 minutes post injection. Glucose tolerance tests were performed monthly from 1 to 6 months.
Insulin tolerance tests (ITT)
Studies were performed 10 hours after lights-on in singly-housed fed mice. Although mice had free access to food prior to testing, their active (feeding) period normally ends at the time of lights-on. Following an initial glucose measurement, mice were injected intraperitoneally with a bolus of 0.75 U/kg of insulin in sterile 0.9% NaCl. Blood glucose was determined at 10, 20, 30, 45, 60, and 75 minutes post injection as described above.
Fasting insulin measurements
Fasting insulin was measured following an overnight fast (1600h to 0800h). Five- to 6-month-old mice were restrained, and ~50 μl blood was collected from the tail vein using a heparinized capillary tube. Plasma insulin was determined by radioimmunoassay (Millipore, cat# SRI-13K). All samples were determined in a single assay with a sensitivity of 0.1 ng/mL and intra-assay coefficient of variation (CV) of <10%.
Dual Energy X-ray Absorptiometry (DEXA)
Mice were anesthetized by intraperitoneal injection of 100–150 μl of a ketamine/xylazine mix in saline (ketamine at 20 mg/ml and xylazine at 2 mg/ml). Anesthetized mice were introduced into the DEXA machine (GE Lunar Piximus II) and subjected to total body imaging. Lean body mass and fat mass were determined using the Lunar Piximus II software and percent fat mass was calculated. Abdominal fat percentage was calculated by selecting a region of interest (ROI) in the scanned image. Mice were scanned at age 3, 4, and 5 months.
Measurement of adipocyte size
Mice were euthanized with CO2. The left parametrial fat pad and adjacent uterus were fixed in 10% formalin for 48 hours and embedded in paraffin. Sections (5 μm) were cut and stained with hematoxylin-eosin. Pictures of stained sections were taken at 10× magnification with a Zeiss Axioplan Universal Microscope (Thornwood, NY); the uterus was used for photographic orientation and the adipocytes sized were in the same frame as the uterus to minimize bias due to regional differences. Cell areas (in μm2) of 50 adipocytes were determined for each mouse using Scion Image Corporation software (Frederick, MD). Only 40 cells were used for one animal in which tissue damage precluded analysis of 50 cells.
Glucose uptake assays in adipocytes
The right parametrial adipose tissues were dissected, and adipocytes were isolated and subjected to glucose uptake assays as previously described in detail (Liu et al., 1999). In brief, isolated adipocytes were pre-incubated without (basal) or with different concentrations of insulin (0–10 nM) for 30 min. [U-14C] D-glucose was then added. Thirty min after the addition of radiolabeled glucose, the cell suspension was harvested and adipocytes separated from the medium. Cell-associated radioactivity was determined and glucose uptake expressed in amol/min/cell. Cell numbers were determined by measuring lipid content of aliquots of cell suspension and measuring sizes of the adipocytes in aliquots of the cell suspension as described above. For each condition, the measurements were done in quadruplicate.
Islet isolation
Islets were harvested from 5-month-old PNA and CON mice on dioestrus (determined by vaginal lavage). Although non-cycling animals likely have a very different steroid milieu despite similar vaginal cytology, this is a consequence of the considerable reproductive disruption of the model, and dioestrous vs. long-term dioestrous animals is the most practical comparison to make. Mice were euthanized with CO2 and cardiac puncture was performed for blood collection. The pancreas was dissected and islets were isolated by collagenase digestion and Histopaque centrifugation using previously detailed methods (Carter et al., 2009). Isolated islets were transferred to a Petri dish containing standard RPMI 1640 (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum and penicillin/streptomycin. Islets were incubated in this medium overnight to allow recovery from digestion prior to experiments.
Intracellular calcium imaging of islets
The day following isolation, imaging recordings of intracellular calcium ([Ca2+]i) were made from groups of 10–20 islets from a single mouse approximately 18–26 hours post-isolation; no differences were noted based on time of recording. [Ca2+]i was measured using the ratiometric dye fura-2 AM and previously described methods (Jahanshahi et al., 2009). Briefly, islets were incubated with 1 μM fura-2 AM for 30 minutes in a modified KRB solution containing 3 mM glucose, washed, then transferred to a small volume chamber (Warner Instruments, Hamden, CT) mounted on the stage of an upright Olympus BX51WI fluorescence microscope (Olympus, Tokyo, Japan). Islets were perifused using a peristaltic pump (Minipuls 2, Gilson, France) and maintained at 35 C with an in-line heater (model SF-28 with automatic temperature controller model TC-324B, Warner Instruments, Hamden, CT). A Hamamatsu ORCA-ER camera (Hamamatsu Photonics, Japan) was used to take sequential images during 340 and 380 nm excitation, and the ratio of emitted light at 510 nm used to determine the [Ca2+]i. Excitation from a xenon burner was accomplished using a light pipe and filter wheel (Sutter Instrument Company, Novato, CA). Paired images were recorded every 5 seconds for 15 minutes. After 5 minutes in 3 mM glucose, 11 mM glucose was applied for 10 minutes, during which fluorescence levels were recorded continuously.
Insulin release in vitro
A subset of islets isolated from PNA and CON mice were used for studies of in vitro insulin release. Following the overnight culture, islets were incubated at 37 C and 5% CO2 for one hour in standard KRB solution. Islets were then washed and treated for one hour in KRB supplemented with 3 mM glucose, followed by one hour in KRB containing 11 mM glucose. Supernatants were collected after each treatment. Insulin concentration in the supernatant was measured by an ELISA insulin assay kit (Mercodia Inc., Winston Salem, NC) according to manufacturer instructions. This assay differed from that used for mouse serum because insulin levels in media differ from those in vivo. Intra-assay variation was <10% and inter-assay variation was <5%. In vitro insulin release was also assessed in islets isolated from 3-month-old female C57BL/6J mice three days post ovariectomy. Islets were harvested and cultured as described above. During the overnight culture, 50 islets per mouse were incubated in DHT, DHT+oestradiol (DHT+OE), or ethanol vehicle (0.0001%). All steroids were used at a final concentration of 10 nM. The following day, islets from each steroid treatment group were incubated in standard KRB solution with steroids omitted to preclude acute effects. Insulin release in 3 and 11 mM glucose was quantified by ELISA as above.
Endocrine measures
Testosterone was measured in serum using a radioimmunoassay kit according to the manufacturer’s instructions (Siemens Medical Solutions, cat# TKTT2, Los Angeles, CA). Sensitivity averaged 10 ng/dl, and the intra- and inter-assay CVs were 4.4 and 8.1%, respectively. An adipokine panel was used to assess insulin, leptin, IL-6, TNF-α, PAI-1, and resistin (mouse serum adipokine kit, Millipore, cat# MADPK-71K-07). Sensitivity was 12 pg/mL for insulin, leptin, PAI-1, and resistin, 2 pg/mL for TNF-α, and 5 pg/mL for IL-6. Intra- and inter-assay CV were <10% for all analytes. Adiponectin was measured via radioimmunoassay (Millipore, cat# MADP-60K); assay sensitivity was 1.3 ng/ml and inter- and intra-assay CVs were <9% and <5%, respectively.
Oestrous cycle monitoring
Oestrous cycles were monitored by vaginal lavage. Cycle stage was classified as oestrus (primarily cornified cells), dioestrus (primarily leukocytes), or prooestrus (primarily nucleated cells).
Analyses and statistics
GraphPad Prism software (La Jolla, CA) was used for all analyses unless otherwise indicated. For glucose and insulin tolerance tests, glucose values from PNA and CON mice were compared at each time point using a two-tailed Student’s t-test. Area under the curve (AUC) for the GTT was calculated using Igor Pro software (Wavemetrics, Lake Oswego, OR); AUC across age was compared in PNA vs CON mice using a repeated-measures analysis of variance (ANOVA) and Fisher’s protected LSD post-hoc test. Two-tailed Student’s t-test or Mann-Whitney test was used to compare body mass and fat pad mass, fat percentages, fat cell sizes, adipokines, HOMA indices, and glucose uptake in adipocytes. For islet calcium imaging studies, calcium measurements from 10–20 islets per mouse were averaged, and AUC was calculated using Igor Pro. Insulin secretion in 3 mM and 11 mM glucose from control, DHT, and DHT+OE groups were compared by two-way ANOVA. For all statistical tests, significance was set at p<0.05. Parametric or non-parametric comparisons were used as dictated by data distribution.
Results
PNA mice do not have altered body composition but do have enlarged visceral adipocytes
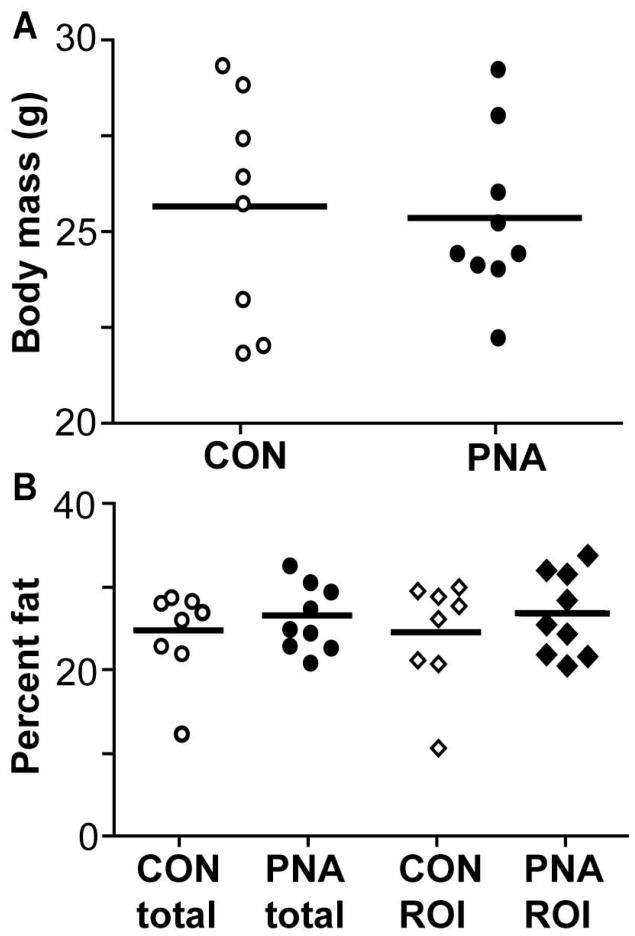
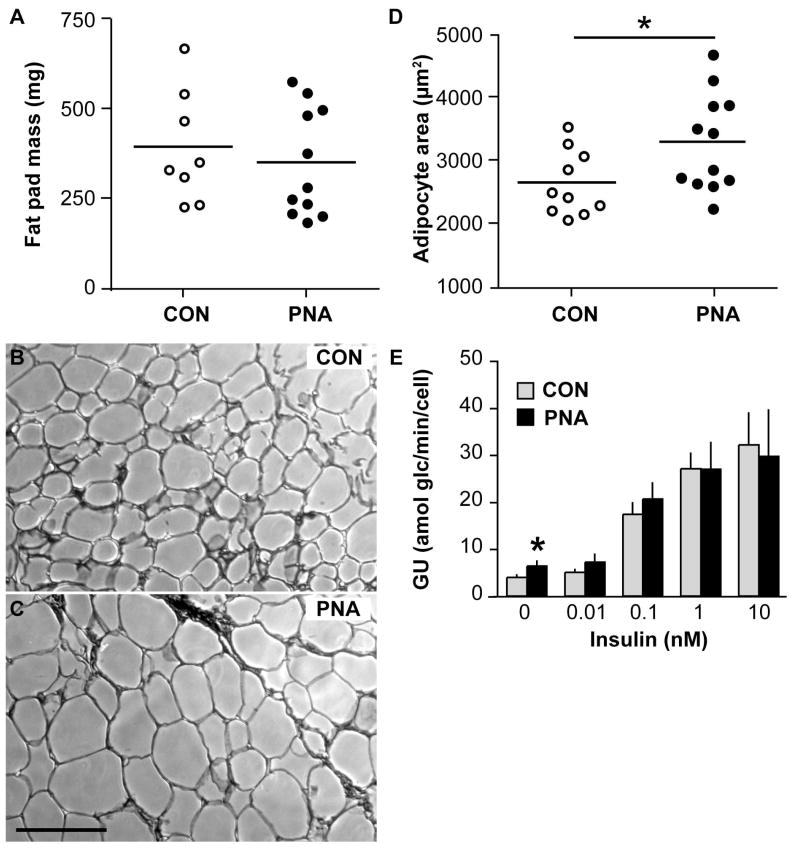
Because adiposity contributes to metabolic disease, we assessed body mass and composition in PNA (n=8) and CON (n=9) mice. Body mass at age 3, 4, and 5 months did not differ between groups (all comparisons p>0.8); representative data from age 5 months are shown (Fig 1A). Body fat percentages as measured by DEXA also showed no difference in total body fat or abdominal fat at these ages (p>0.4, Fig 1B). Since DEXA cannot differentiate visceral and subcutaneous fat compartments, we could not exclude the possibility that PNA mice have changes in fat distribution. To better assess visceral adiposity, parametrial fat pads were weighed in a subsequent group of PNA mice and further analyzed for adipocyte size. Fat pad weights were similar (n=8 CON, n=11 PNA, p>0.5, Fig 2A), indicating no increase in visceral adiposity in PNA mice, consistent with the DEXA measurements. However, PNA mice had larger visceral adipocytes (2592±150 μm2 CON, n=10, 3230±211 μm2 PNA, n=12, p<0.05, Fig 2B–D), indicating that despite similar total amounts of parametrial fat in these mice, prenatal androgenisation induced changes in adipocyte differentiation and/or function leading to larger fat cells. Adipokine assays showed no differences between PNA and CON mice in the fed or fasted state, except for a strong trend (two-tailed p=0.08) for reduced adiponectin in fed PNA mice (Table 1). Fasting decreased insulin, leptin, PAI-1, IL-6, and resistin in CON mice (p<0.05). In PNA mice, insulin was reduced and IL-6 and resistin showed a tendency (p<0.06) to be lower in fasted animals. Of note, however, leptin was not reduced by fasting in PNA mice. Glucose uptake assays in isolated adipocytes showed no change in insulin sensitivity or maximal insulin-stimulated glucose uptake, but higher basal glucose uptake in PNA adipocytes (basal: 4.0±0.5 amol/glc/min/cell CON, 6.4±1.0 PNA, n=5 each, p<0.05; at 0.1 nM insulin: 17.4±2.4 CON, 20.6±3.3 PNA, n=5 each, p>0.4; at 1 nM insulin: 27.1±3.3 CON, n=5, 26.9±5.7 PNA, n=4, p>0.9; Fig 2E).
Figure 1.
PNA does not alter body mass or composition in PNA mice at 5 months of age. A. Body mass in CON (open circles, n=8) and PNA mice (closed circles, n=9) (p>0.8). B. Total body fat or abdominal fat (region of interest, ROI, subcutaneous and visceral fat combined) in CON and PNA (p>0.4).
Figure 2.
PNA does not alter fat pad mass but increases adipocyte size. A. Fat pad mass in CON (open circles) and PNA mice (closed circles) (n=8 CON, n=11 PNA, p>0.5). B, C. Representative photomicrographs of adipose tissue from control (B) and PNA (C) mice. Scale bar represents 200 μM. D. Mean adipocyte size (n=10 CON, n=12 PNA). E. Mean ± SEM glucose uptake (GU) into CON (gray bars) and PNA (black bars) adipocytes in response to varying concentrations of insulin (n=2 to 5 assays per insulin concentration). Basal uptake was higher in PNA adipocytes, but insulin sensitivity was similar. *p<0.05.
Table 1.
Adipokine levels in plasma from control (CON) and prenatally androgenized (PNA) mice under fed and fasted conditions (n=10–16 per group).
| Analyte | CON fed | PNA fed | CON fasted | PNA fasted |
|---|---|---|---|---|
| insulin (pg/ml) | 1071 ± 178* | 1331 ± 306* | 146 ± 22.5 | 107 ± 16.5 |
| leptin (pg/ml) | 2476 ± 350* | 1849 ± 435 | 926 ± 192 | 1468 ± 366 |
| TNF-α (pg/ml) | 4.7 ± 0.4 | 5.3 ± 0.5 | 6.4 ± 2.0 | 4.1 ± 0.5 |
| PAI-1 (pg/ml) | 8361 ± 1049* | 6858 ± 1377 | 3635 ± 635 | 4246 ± 664 |
| IL-6 (pg/ml) | 17.7 ± 2.2* | 24.6 ± 5.4† | 9.2 ± 1.7 | 5.3 ± 0.8 |
| resistin (pg/ml) | 1758 ± 160* | 1497 ± 144† | 891 ± 95 | 1073 ± 159 |
| adiponectin (mg/ml) | 16.2 ± 0.7‡ | 14.0 ± 1.0 | -- | -- |
p<0.05 vs fasted.
p<0.06 vs fasted.
p=0.08 vs PNA.
PNA mice exhibit impaired glucose tolerance
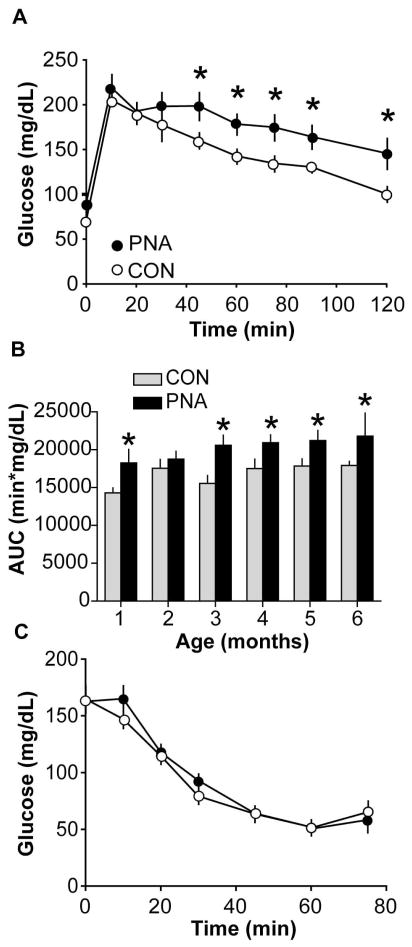
To test if prenatal androgenisation alters glucose disposal in PNA mice, glucose tolerance tests were performed. PNA mice exhibited impaired glucose tolerance at all ages studied, with the exception of 2 months, when glucose tolerance transiently worsened in controls (n=9 CON, n=7 PNA, p<0.05 at age 1, 3–6 months at 45–120 min time points). Figure 3A shows representative average glucose curves at age 5 months; Figure 3B shows the average area under the glucose curve at each age studied (repeated measures ANOVA, p<0.05). Fasting glucose was significantly higher in PNA mice (glucose: CON 68.7±4.2 mg/dL, n=9, PNA 86.7±±4.9 mg/dL, n=7, p<0.02).
Figure 3.
PNA mice exhibit impaired glucose tolerance but not insulin resistance. A. Glucose tolerance was impaired in 5 month-old PNA (closed circles) compared to CON (open circles) mice. B. Area under the curve (AUC) in CON (gray bars) and PNA (black bars) mice examined at different ages illustrates that glucose intolerance develops by 1 month of age. C. PNA mice are not insulin-resistant based on insulin tolerance testing (n=10 per group, p>0.2). *p<0.05.
PNA mice exhibit normal peripheral insulin sensitivity
Glucose intolerance occurs due to the failure of insulin target tissues to adequately dispose of circulating glucose. This can be a consequence of impaired insulin secretion and/or impaired insulin action. To assess the latter, we performed an insulin tolerance test in a group of three-month-old mice. No differences were found between PNA and CON mice (n=10 per group, all comparisons p>0.2, Fig 3C). Fed basal glucose levels were also not different (CON 164.8±11.8 mg/dl, PNA 162.7±10.1 mg/dl, n=10 per group, p>0.8). Additionally, fasting insulin and glucose values were used to determine HOMA-IR as a surrogate measure of insulin resistance. This index was developed using human data but subsequently has been validated in rodents (Cacho et al., 2008; Mather, 2009). No difference was found between groups (0.78±0.10 CON vs 0.86±0.18 PNA, p>0.6).
PNA mice have an early form of islet dysfunction
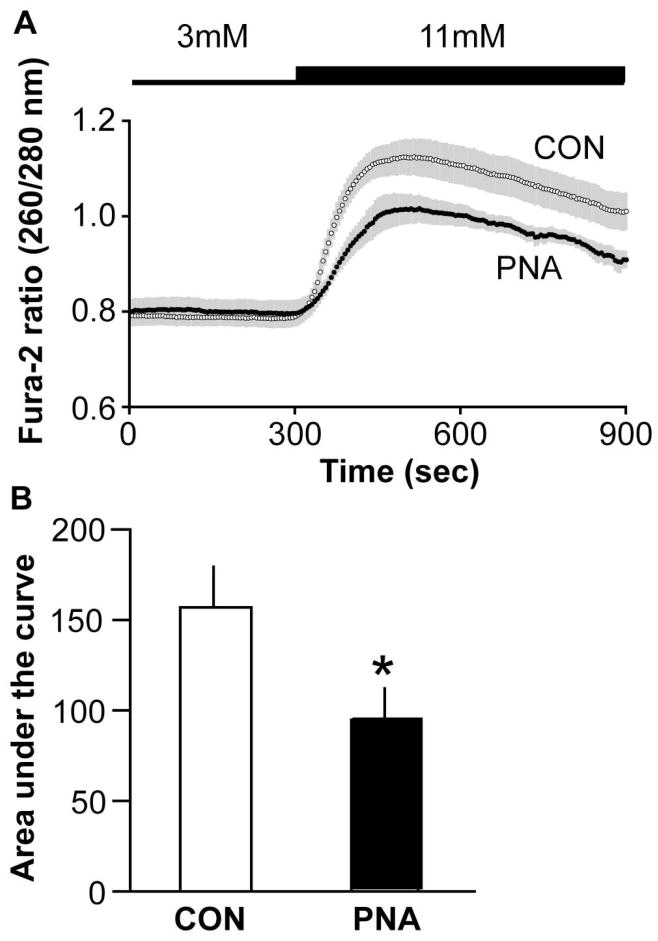
The presence of apparently normal insulin sensitivity in PNA mice suggested glucose intolerance may have originated in an insulin secretion defect at the level of the pancreatic beta cell. To assess pancreatic islet function, we used the ratiometric fluorescent probe fura-2 AM to measure glucose-stimulated calcium (GSCa) in islets from PNA and CON mice. GSCa is a measure of islet glucose sensitivity that allows high frequency sampling, which captures the dynamics of the biphasic response (Guest et al., 1989; Jahanshahi et al., 2009) and approximates that of glucose-stimulated insulin secretion (Henquin et al., 2006). [Ca2+]i was monitored in islets during perifusion with 3 mM glucose and following a switch to 11 mM glucose. Islets from PNA (n=6) and CON (n=8) mice had similar calcium levels in 3 mM glucose (p>0.4), but the rise in intracellular calcium following a switch to 11 mM glucose was blunted in islets from PNA mice (p<0.05, Fig 4).
Figure 4.
PNA mice have impaired pancreatic response to elevated glucose. A. Mean±SEM ratios (n=8 CON, n=6 PNA) of fura-2 fluorescence at 260 and 280 nm excitation, demonstrating the impaired response of islets from PNA mice upon an increase in glucose concentration from 3 mM to 11 mM. Shaded area indicates SEM. B. Area under the curve was lower in PNA mice. *p<0.05.
The limited number of islets precluded performing parallel insulin secretion studies in islets from all mice. Insulin secretion was measured in islets from 2 mice per group. While this sample size was insufficient to perform statistical comparisons, there was good agreement between calcium responses and insulin release in high glucose, consistent with observations in the literature (Deering et al., 2009; Evans-Molina et al., 2009) (3 mM: CON 2.1±0.06 pg/mL, PNA 5.7±1.5 pg/ml; 11 mM: CON 33.0±2.1 pg/mL, PNA 20.8±4.2 pg/ml).
Insulin release from isolated islets
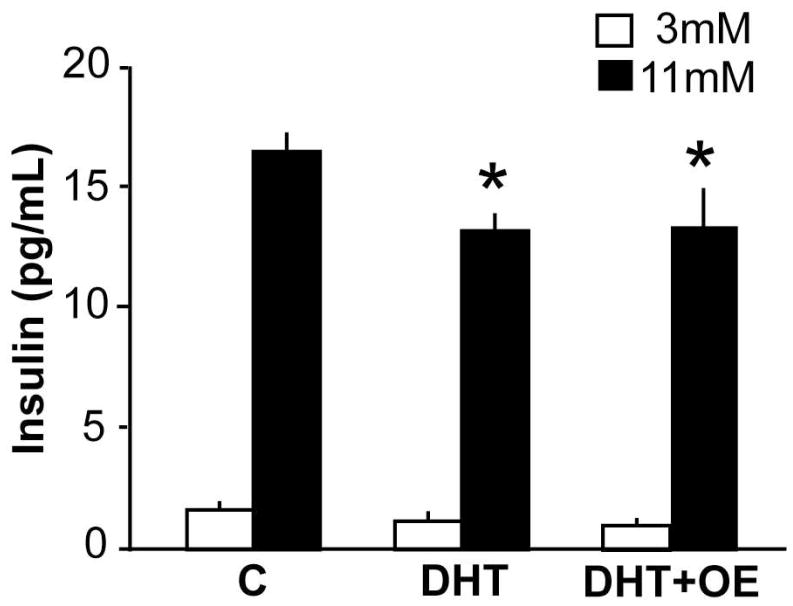
Steroids can exert organizational effects, which are mediated by developmental programming and persist in the absence of hormone, as well as activational effects, which require the immediate presence of hormone (Arnold and Breedlove, 1985). To probe the activational role of androgens in adult islet function, we performed an in vitro study of islet insulin secretion after incubation with different steroids. Pancreatic islets express steroid receptors, including receptors for androgen (Winborn et al., 1987; Díaz-Sánchez et al., 1995), but few studies have examined the direct roles of androgens in the islet. Islets were harvested from ovariectomized mice three days post surgery. Ovariectomized mice were used to control for effects of intrinsic steroids and oestrous cycles. Isolated islets were incubated overnight in 10 nM DHT, DHT+OE, or ethanol vehicle. Insulin secretion was measured in 3 mM and 11 mM glucose. Neither DHT nor the combination of DHT+OE had an effect on insulin secretion in 3 mM glucose (p>0.05, n=12 per group, Fig 5). However, DHT and the combination of DHT+OE significantly inhibited insulin secretion in 11 mM glucose (p<0.01 and p<0.05, respectively). This blunting of the islet response to high glucose was similar to that observed in PNA mice.
Figure 5.
Steroids can act directly at the islet to alter insulin release in response to a glucose challenge. DHT or DHT in combination with oestradiol (DHT+OE) impair the release of insulin in response to a high glucose challenge (n=12 mice) *p<0.05 versus control (C, ethanol vehicle) in 11 mM glucose.
Reproductive measures
The lack of a difference in glucose tolerance at 2 months was due to an increase in glucose levels in control mice (p<0.05 by paired t-test), rather than an improvement in glucose tolerance in PNA mice. Interestingly, two months is around the age of final sexual maturation in mice (Gore et al., 1999). Given that puberty is a period of relative insulin resistance (Amiel et al., 1986), we speculated that the increase in glucose levels in CON mice may be due to pubertal changes, and PNA mice may have experienced puberty earlier or later than control mice. To address this question, vaginal opening was monitored in a subsequent group of mice and found to occur earlier in PNA mice (CON 34.4±1.3 d, n=18, PNA 29.5±1.7 d, n=16, p<0.05). Body mass at the time of vaginal opening was significantly lower in PNA mice (CON 14.9±0.3 g, PNA 12.7±0.5 g, p<0.001), suggesting that increased body mass was not the cause of early vaginal opening.
Serum testosterone was assayed in blood samples taken at euthanasia on the day of islet harvest. In contrast to our previous report (Sullivan, 2004), PNA mice in this study did not exhibit elevated testosterone levels at 5 months of age (CON 10.5±3.6 ng/dL, n=9, PNA 11.9±3.0 ng/dL, n=8, p>0.7). However, testosterone levels measured in a different group of mice from this cohort of PNA animals at 8 months of age revealed significantly higher levels than CON mice (CON 17.7±2.7 ng/dL, n=7, PNA 28.9±1.9 ng/dL, n=5, p<0.01). Thus, as the mice continue to age, differences in androgen levels may become apparent. In this report, metabolic studies were performed up to age 6 months.
Despite the absence of elevated testosterone, the primary reproductive phenotype of disrupted oestrous cycles was apparent in PNA compared to CON mice. Cycle duration, defined as the oestrus-to-oestrus interval, was significantly lengthened (6.6+0.4 d CON, 15.7+2.6 d PNA, n=10 each, p=0.002) with the percent of time in oestrus significantly decreased (15.2+1.6% CON, 4.8+1.6% PNA, p=0.0002). PNA mice exhibited prolonged periods in which leukocytes were the primary cell in the vaginal lavage. This finding differs somewhat from the original report of this model, in which similar disruptions were observed in cyclicity but prolonged periods of cornified cells were observed. We believe the difference may be attributed to a switch from phytoestrogen-containing to reduced-phytoestrogen chow. The observations of cyclicity for the three rounds of prenatal androgenization used for this study, as well as ongoing studies in the lab, are consistent with prolonged dioestrus.
PNA mice exhibit normal birth weight
Some of the metabolic effects observed in this model coincide with those induced by intrauterine growth restriction, which has been demonstrated in sheep prenatally androgenized with testosterone (Steckler et al., 2005). Birth weight was assessed in subsequent litters of PNA mice but was not altered by prenatal treatment (CON 1.4±0.03 g, n=33, PNA 1.4±0.03 g, n=29, p>0.7).
Discussion
Prenatal androgenisation of female mammals has profound lasting effects on reproductive function in adulthood, and may underlie the fertility and metabolic disorder polycystic ovary syndrome. In this study we assessed whether the same prenatal DHT treatment that caused reproductive abnormalities in female mice (Sullivan, 2004) could also induce metabolic dysfunction. PNA mice exhibited glucose intolerance that was present before puberty and persisted into adulthood. Impaired glucose tolerance was not associated with increased adiposity or peripheral insulin resistance; however, pancreatic islet function was altered in PNA mice and may be a causative factor in glucose intolerance.
Glucose tolerance was studied across postnatal development since increasing adiposity with age, or changes in circulating hormones following puberty, might influence the phenotype. Of interest, PNA mice exhibited glucose intolerance at the earliest age studied, four weeks, and the difference relative to controls remained stable throughout the study, except at the two-month time point. A difference in the timing of puberty may account for the disparity at two months. PNA mice underwent vaginal opening earlier than control mice; thus, we may have missed the window of pubertal insulin resistance in this group. Alternatively, the already impaired glucose handling in PNA mice may have masked the effects of pubertal insulin resistance. The finding of earlier puberty in PNA mice is supported by work in sheep showing pubertal advancement following prenatal androgen exposure in females (Wood et al., 1991; Jackson et al., 2008), and recent studies implicating androgens in the timing of puberty (Brill and Moenter, 2009). Further, the observation of impaired glucose tolerance in PNA mice at only one month of age corresponds with the appearance of some aspects of PCOS in adolescents (McCartney et al., 2006).
PNA mice exhibited elevated fasting glucose levels in the presence of normal fasting insulin. Impaired fasting glucose is associated with hepatic insulin resistance (Bock et al., 2007), which is characterized by a failure of insulin to suppress gluconeogenesis under fasting conditions. Typically insulin would also be elevated in this situation, but this assumes normal pancreatic beta cell compensation. Another possibility is that PNA mice have a higher stress response to fasting and handling, leading to acutely elevated glucose. However, studies of steroid programming of the hypothalamic-pituitary-adrenal axis indicate that adult stress responses are blunted by developmental androgen exposure (McCormick et al., 1998). DHT administered close to parturition as in our study could potentially change maternal nurturing behaviour, which could also affect offspring stress responses (Francis et al., 1999).
Isolated islet studies were performed because the limited blood volume of mice makes sequential measurements of insulin secretion in vivo difficult to perform. [Ca2+]i is closely coupled to insulin release in the beta cell, permitting [Ca2+]i changes to be monitored as a surrogate for insulin secretion amid changing glucose concentrations (Jahanshahi et al., 2009). Islets are a heterogeneous tissue comprised of alpha, beta, delta, PP, and epsilon cells (Brissova and Powers, 2008), but beta cells comprise 65–80% of the islet mass; thus glucose-stimulated [Ca2+]i variations primarily reflect changes in this cell type. Impaired glucose tolerance in PNA mice did not progress to frank diabetes. Nevertheless, the observed defects in islet function are similar to the early islet dysfunction in type 2 diabetes mellitus (Jahanshahi et al., 2009). Hallmarks of pending islet failure include elevated basal calcium, loss of oscillatory activity, and failure to generate an appropriate rise in calcium (and thus insulin secretion) upon high glucose stimulation (Evans-Molina et al., 2009; Jahanshahi et al., 2009). Type 2 diabetes occurs in the context of peripheral insulin resistance, when pancreatic compensation to increase insulin production is no longer adequate (Kahn, 2001). Thus, impaired pancreatic islet function in PNA mice may predispose them to develop type 2 diabetes in the presence of other risk factors, such as obesity. Similarly, women with PCOS have impaired beta cell function and are at increased risk for diabetes (Dunaif and Finegood, 1996; Legro et al., 1999).
An additional tissue-specific abnormality was identified, with PNA mice exhibiting increased visceral adipocyte size. Although enlarged adipocytes are often observed with increased fat mass, total fat pad mass was unchanged in PNA mice, suggesting that adipocyte number may be reduced. This idea is speculative; however, reports in the literature suggest that androgens can indeed alter adipocyte differentiation and size. DHT reduces omental adipocyte differentiation in tissue culture, and both DHT and testosterone inhibit differentiation of pluripotent cells into the adipogenic lineage (Singh et al., 2003; Blouin et al., 2009). Androgen receptor knockout mice have smaller adipocytes than wildtype controls, suggesting androgen receptor activation increases adipocyte size (Yeh et al., 2002). It must be emphasized that the change in adipocyte size observed here was small and did not result in associated changes in adipocyte insulin sensitivity (Lundgren et al., 2007) or changes in circulating levels of the majority of adipokines measured (Scherer and Trujillo, 2006; Skurk et al., 2007). In PNA mice, there was a strong trend for decreased adiponectin in the fed state, which is in agreement with reduced adiponectin levels in women with PCOS (Wickham et al., 2010). Further, leptin levels were not reduced by fasting in PNA mice, suggesting altered regulation of this hormone, which is permissive for fertility (Moschos et al., 2002). Differences in other adipokines, or in adipocyte insulin sensitivity, could appear under conditions of metabolic stress, such as diet-induced obesity. This is an interesting area for future studies.
Activational effects of androgens in islets were assessed because PNA mice previously exhibited elevated androgens (Sullivan, 2004), a cardinal feature of PCOS. The androgen receptor (Li et al., 2008) and synthesis enzyme cytochrome P450(17alpha) (Ogishima et al., 2008) are expressed in beta cells, but their roles in this tissue are unclear. One study showed that micromolar concentrations of testosterone increase insulin transcription and secretion in the rat in vivo and in islets in vitro (Morimoto et al., 2001), but the possibility that effects were mediated by aromatisation to oestradiol or by non-genomic pathways were not examined. We found that overnight treatment with nanomolar concentrations of DHT impaired high-glucose-stimulated insulin release in islets from ovariectomized mice. This effect was most likely genomic, as steroids were not present when insulin release was measured. Oestradiol had no added effect. The reduction in glucose-stimulated insulin secretion following islet exposure to DHT in vitro was similar to the decrement in glucose-stimulated calcium flux in islets from PNA mice. Since we did not observe elevated circulating androgens at five months of age when islets were isolated, islet dysfunction could not be attributed to activational effects of androgens. However, the effects of elevated androgen in adult life may be additive to those induced by prenatal androgen. PCOS is thought to involve elevated androgen prenatally and in adulthood, potentially generating a double insult on pancreatic function.
The absence of peripheral insulin resistance in PNA mice differs from previously published studies of prenatally androgenized monkeys, sheep, and rats (Bruns et al., 2004; Recabarren et al., 2005; Demissie et al., 2008). In these models, insulin resistance was accompanied by an increase in total and/or visceral adiposity; the association between these conditions is well recognized (Kahn et al., 2006). PNA mice did not exhibit changes in body mass or composition, which may correlate with the absence of insulin resistance. An important distinction that may account for these differences is the androgen used. This study employed DHT to examine primarily androgen receptor-mediated effects, whereas other studies used testosterone, which can be aromatised to oestradiol. This suggests a possible role of excess prenatal oestrogen, or oestrogen in combination with androgen, in programming obesity and peripheral insulin resistance. Prenatal exposure to oestrogenic substances such as bisphenol A and diethylstilbestrol increases postnatal weight and adiposity (Miyawaki et al., 2007; Newbold et al., 2009). Oestrogens may also potentiate the effects of androgens by upregulating the androgen receptor in specific tissues, including adipocytes and brain (McAbee and Doncarlos, 1999; Shao R, 2007). Further, intrauterine growth restriction and its metabolic sequelae have been shown to be an effect of prenatal testosterone, but not DHT exposure (Steckler et al., 2005; Carlsen et al., 2006). In addition to the type of steroid treatment, other differences that may account for phenotypic discrepancies include the difference in species and variations in the timing of androgen administration. Sexual differentiation occurs during gestation in primates and sheep, whereas in rodents, it is incomplete at birth (MacLusky and Naftolin, 1981; Jackson et al., 2009). Thus, androgen exposure late in gestation in mice may be comparable to an early treatment in other species. Nevertheless, the developmental timeline in rats is similar to that in mice, and the treatment period in the prenatally androgenized rat (d16–19) overlaps ours. Hence the steroid may be most important in establishing differences among models.
In this study, we have shown that prenatal androgens program long-term alterations in metabolic function in female mice. These findings have implications for gestational androgen exposure that originates from endogenous or exogenous sources. The absence of changes in circulating glucose, insulin, or the majority of adipokines assessed under fed conditions suggests that the previously observed reproductive dysfunction in this model was likely caused by androgen programming of the reproductive axis, and was not secondary to metabolic changes. Further, as the previously reported effects of prenatal androgenisation to induce adiposity and insulin resistance were absent from PNA mice, it appears that metabolic programming by testosterone may be dependent on aromatisation to oestradiol in addition to androgen receptor-mediated effects. The impairments observed in glucose tolerance and pancreatic islet function, as well as increased adipocyte size, may predispose PNA mice to develop diabetes in the presence of aggravating factors. This suggests a two-hit hypothesis, in which prenatal androgen programming sets the stage for metabolic dysfunction, and weight gain and insulin resistance secondary to prenatal oestrogens or postnatal weight management drive the progression to diabetes.
Acknowledgments
Funding
This research was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54 HD28934 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, National Institute of Diabetes and Digestive and Kidney Disease DK063609, and National Institute of Neurological Disorders and Stroke National Research Service Award F31 NS062646 (A.V.R).
We thank the following for their contributions to this paper: Animal Characterization Core and Cell and Islet Isolation Core at the UVA DERC (DK063609), Ligand Assay and Analysis Core and Histology Core at the UVA Center for Research in Reproduction, Melissa Horal, Hongxia Chao, Toni Barbera, Runpei Wu, Jeff Carter, and Dan Haisenleder. We thank Debra Fisher for excellent technical assistance, and Justyna Pielecka, Jessica Kennett, and Jianli Sun for editorial comments on the manuscript.
Footnotes
Declarations of interest: A.V.R, C.S.N, S.R.K, and S.M.M. have nothing to disclose.
Author contributions
Craig Nunemaker performed islet calcium imaging and insulin secretion studies. Susanna Keller performed glucose uptake assays in adipocytes. All other experiments were designed and carried out by Alison Roland under the supervision of her graduate advisor Suzanne Moenter. The manuscript was written by Alison Roland.
References
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315:215–219. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Blouin K, Veilleux A, Luu-The V, Tchernof A. Androgen metabolism in adipose tissue: recent advances. Mol Cell Endocrinol. 2009;301:97–103. doi: 10.1016/j.mce.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, Landau BR, Rizza R. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes. 2007;56:1703–1711. doi: 10.2337/db06-1776. [DOI] [PubMed] [Google Scholar]
- Brill DS, Moenter SM. Androgen Receptor Antagonism and an Insulin Sensitizer Block the Advancement of Vaginal Opening by High-Fat Diet in Mice. Biol Reprod. 2009 doi: 10.1095/biolreprod.109.079301. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova M, Powers A. Chapter 1. Architecture of Pancreatic Islets. Pancreatic Beta Cell in Health and Disease. 2008:3–11. [Google Scholar]
- Bruns CM, Baum ST, Colman RJ, Eisner JR, Kemnitz JW, Weindruch R, Abbott DH. Insulin resistance and impaired insulin secretion in prenatally androgenized male rhesus monkeys. J Clin Endocrinol Metab. 2004;89:6218–6223. doi: 10.1210/jc.2004-0918. [DOI] [PubMed] [Google Scholar]
- Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab. 2008;295:E2169–2176. doi: 10.1152/ajpendo.90207.2008. [DOI] [PubMed] [Google Scholar]
- Carlsen SM, Jacobsen G, Romundstad P. Maternal testosterone levels during pregnancy are associated with offspring size at birth. Eur J Endocrinol. 2006;155:365–370. doi: 10.1530/eje.1.02200. [DOI] [PubMed] [Google Scholar]
- Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A Practical Guide to Rodent Islet Isolation and Assessment. Biol Proced Online. 2009 doi: 10.1007/s12575-009-9021-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ahn K, Gee N, Ahmed M, Duleba A, Zhao L, Gee S, Hammock B, Lasley B. Triclocarban enhances testosterone action: a new type of endocrine disruptor? Endocrinology. 2008;149:1173–1179. doi: 10.1210/en.2007-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deering TG, Ogihara T, Trace AP, Maier B, Mirmira RG. Methyltransferase Set7/9 maintains transcription and euchromatin structure at islet-enriched genes. Diabetes. 2009;58:185–193. doi: 10.2337/db08-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demissie M, Lazic M, Foecking EM, Aird F, Dunaif A, Levine JE. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. Am J Physiol Endocrinol Metab. 2008;295:E262–268. doi: 10.1152/ajpendo.90208.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Sánchez V, Morimoto S, Morales A, Robles-Díaz G, Cerbón M. Androgen receptor in the rat pancreas: genetic expression and steroid regulation. Pancreas. 1995;11:241–245. doi: 10.1097/00006676-199510000-00005. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8:127–141. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocrine Reviews. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Finegood DT. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 1996;81:942–947. doi: 10.1210/jcem.81.3.8772555. [DOI] [PubMed] [Google Scholar]
- Evans-Molina C, Robbins RD, Kono T, Tersey SA, Vestermark GL, Nunemaker CS, Garmey JC, Deering TG, Keller SR, Maier B, Mirmira RG. Peroxisome proliferator-activated receptor gamma activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure. Mol Cell Biol. 2009;29:2053–2067. doi: 10.1128/MCB.01179-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Gore AC, Roberts JL, Gibson MJ. Mechanisms for the regulation of gonadotropin-releasing hormone gene expression in the developing mouse. Endocrinology. 1999;140:2280–2287. doi: 10.1210/endo.140.5.6711. [DOI] [PubMed] [Google Scholar]
- Gray LE, Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, Howdeshell K, Ankley GT, Guillette L. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int J Androl. 2006;29:96–104. doi: 10.1111/j.1365-2605.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- Guest PC, Rhodes CJ, Hutton JC. Regulation of the biosynthesis of insulin-secretory-granule proteins. Co-ordinate translational control is exerted on some, but not all, granule matrix constituents. Biochem J. 1989;257:431–437. doi: 10.1042/bj2570431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa R, Pak T, Kudwa A, Lund T, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin JC, Nenquin M, Stiernet P, Ahren B. In vivo and in vitro glucose-induced biphasic insulin secretion in the mouse: pattern and role of cytoplasmic Ca2+ and amplification signals in beta-cells. Diabetes. 2006;55:441–451. doi: 10.2337/diabetes.55.02.06.db05-1051. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Furr J, Makynen EA, Ankley GT, Gray LE. In utero exposure to the environmental androgen trenbolone masculinizes female Sprague-Dawley rats. Toxicol Lett. 2007;174:31–41. doi: 10.1016/j.toxlet.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Jackson LM, Timmer KM, Foster DL. Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology. 2008;149:4200–4208. doi: 10.1210/en.2007-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LM, Timmer KM, Foster DL. Organizational actions of postnatal estradiol in female sheep treated prenatally with testosterone: programming of prepubertal neuroendocrine function and the onset of puberty. Endocrinology. 2009;150:2317–2324. doi: 10.1210/en.2008-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi P, Wu R, Carter JD, Nunemaker CS. Evidence of diminished glucose stimulation and endoplasmic reticulum function in nonoscillatory pancreatic islets. Endocrinology. 2009;150:607–615. doi: 10.1210/en.2008-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE. Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:4047–4058. doi: 10.1210/jcem.86.9.7713. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. Journal of Clinical Endocrinology & Metabolism. 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- Legro RS, Driscoll D, Strauss JF, 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RJ, Qiu SD, Wang HX, Tian H, Wang LR, Huo YW. Androgen receptor: a new player associated with apoptosis and proliferation of pancreatic beta-cell in type 1 diabetes mellitus. Apoptosis. 2008;13:959–971. doi: 10.1007/s10495-008-0230-9. [DOI] [PubMed] [Google Scholar]
- Liu SCH, Wang Q, Lienhard GE, Keller SR. Insulin receptor substrate 3 is not essential for growth or glucose homeostasis. J Biol Chem. 1999;275:18093–18099. doi: 10.1074/jbc.274.25.18093. [DOI] [PubMed] [Google Scholar]
- Lundgren M, Svensson M, Lindmark S, Rensrom F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia. 2007;50:625–633. doi: 10.1007/s00125-006-0572-1. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Mather K. Surrogate measures of insulin resistance: of rats, mice, and men. Am J Physiol Endocrinol Metab. 2009;296:E398–399. doi: 10.1152/ajpendo.90889.2008. [DOI] [PubMed] [Google Scholar]
- McAbee MD, Doncarlos LL. Estrogen, but not androgens, regulates androgen receptor messenger ribonucleic acid expression in the developing male rat forebrain. Endocrinology. 1999;140:3674–3681. doi: 10.1210/endo.140.8.6901. [DOI] [PubMed] [Google Scholar]
- McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, Foster CM, Marshall JC. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab. 2006;91:1714–1722. doi: 10.1210/jc.2005-1852. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM. Neonatal sex hormones have ‘organizational’ effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Res Dev Brain Res. 1998;105:295–307. doi: 10.1016/s0165-3806(97)00155-7. [DOI] [PubMed] [Google Scholar]
- Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb. 2007;14:245–252. doi: 10.5551/jat.e486. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Fernandez-Mejia C, Romero-Navarro G, Morales-Peza N, Díaz-Sánchez V. Testosterone effect on insulin content, messenger ribonucleic acid levels, promoter activity, and secretion in the rat. Endocrinology. 2001;142:1442–1447. doi: 10.1210/endo.142.4.8069. [DOI] [PubMed] [Google Scholar]
- Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertil Steril. 2002;77:433–444. doi: 10.1016/s0015-0282(01)03010-2. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Jefferson WN. Environmental estrogens and obesity. Mol Cell Endocrinol. 2009;304:84–89. doi: 10.1016/j.mce.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogishima T, Mitani F, Suematsu M. Cytochrome P-450(17alpha) in beta-cells of rat pancreas and its local steroidogenesis. J Steroid Biochem Mol Biol. 2008;111:80–86. doi: 10.1016/j.jsbmb.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Quigley CA. Editorial: The postnatal gonadotropin and sex steroid surge-insights from the androgen insensitivity syndrome. J Clin Endocrinol Metab. 2002;87:24–28. doi: 10.1210/jcem.87.1.8265. [DOI] [PubMed] [Google Scholar]
- Recabarren SE, Padmanabhan V, Codner E, Lobos A, Durán C, Vidal M, Foster DL, Sir-Petermann T. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endocrinol Metab. 2005;289:E801–806. doi: 10.1152/ajpendo.00107.2005. [DOI] [PubMed] [Google Scholar]
- Sam S, Dunaif A. Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab. 2003;14:365–370. doi: 10.1016/j.tem.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Trujillo ME. Adipose Tissue-Derived Factors: Impact on Health and Disease. Endocr Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- Shao RLK, Weijdegård B, Egecioglu E, Fernandez-Rodriguez J, Zhang FP, Thurin-Kjellberg A, Bergh C, Billig H. Estrogen-induced upregulation of AR expression and enhancement of AR nuclear translocation in mouse fallopian tubes in vivo. Am J Physiol Endocrinol Metab. 2007;292:E604–614. doi: 10.1152/ajpendo.00350.2006. [DOI] [PubMed] [Google Scholar]
- Singh R, Artaza J, Taylor W, Gonzalez-Cadavid N, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Human Reproduction. 2002;17:2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Codner E, Perez V, Echiburu B, Maliqueo M, Ladron de Guevara A, Preisler J, Crisosto N, Sanchez F, Cassorla F, Bhasin S. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:1923–1930. doi: 10.1210/jc.2008-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–3193. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci U S A. 2004;101:7129–7134. doi: 10.1073/pnas.0308058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapanainen J, Kellokumpu-Lehtinen P, Pelliniemi L, Huhtaniemi I. Age-related changes in endogenous steroids of human fetal testis during early and midpregnancy. J Clin Endocrinol Metab. 1981;52:98–102. doi: 10.1210/jcem-52-1-98. [DOI] [PubMed] [Google Scholar]
- Wickham EPr, Cheang KI, Clore JN, Baillargeon JP, Nestler JE. Total and high-molecular weight adiponectin in women with polycystic ovary syndrome. Metabolism. 2010 doi: 10.1016/j.metabol.2010.02.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winborn WB, Sheridan PJ, McGill HCJ. Sex steroid receptors in the stomach, liver, pancreas, and gastrointestinal tract of the baboon. Gastroenterology. 1987;92:23. doi: 10.1016/0016-5085(87)90835-3. [DOI] [PubMed] [Google Scholar]
- Wood RI, Ebling FJ, I’Anson H, Bucholtz DC, Yellon SM, Foster DL. Prenatal androgens time neuroendocrine sexual maturation. Endocrinology. 1991;128:2457–2468. doi: 10.1210/endo-128-5-2457. [DOI] [PubMed] [Google Scholar]
- Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]