Abstract
Background
There are no evidence-based recommendations for statin continuation or discontinuation near the end of life. However, some expert opinion recommends continuing statins prescribed for secondary versus primary prevention of cardiovascular disease.
Objectives
Our aim was to explore statin prescribing patterns in a longitudinal cohort of individuals with life-limiting illness, and to evaluate differences in these patterns based on secondary versus primary prevention of cardiovascular disease.
Design and setting
This study was a retrospective cohort analysis of 539 persons in an integrated, not-for-profit health maintenance organization (HMO) setting who were receiving statins at diagnosis of a cancer with 0% to 25% predicted 5-year survival. Of the cohort patients, 343 were taking statins for secondary prevention and 196 for primary prevention of cardiovascular disease. Measurements included number and timing of statin refills between diagnosis and date of death, disenrollment, or the end of the observation period.
Results
Four hundred and ninety-six cohort members died within the observation period. Fifty-eight percent of the secondary prevention and 62% of the primary prevention group had at least one statin refill after diagnosis. There were no significant differences between groups for number of days between diagnosis and last refill, or between last refill and death. Two deaths were attributable to cardiovascular causes in each group.
Conclusions
Our retrospective cohort analysis of persons with incident poor-prognosis cancer describes diminished, but persistent statin refills after diagnosis. Neither timing of statin discontinuation nor cardiovascular mortality differed by prescribing indication. There may be an opportunity to reevaluate medication burden in persons taking statins for primary prevention, and it is unclear whether continuing statins prescribed for secondary prevention affects cardiovascular outcomes.
Introduction
Patient and caregiver burden near the end of life can be reduced by discontinuing chronic medications that are no longer likely to be beneficial. In particular, the role of medications that are used for prevention or management of comorbidities may be reconsidered in the face of revised care goals.1,2 HMG Co-A reductase inhibitors (“statins”) are commonly prescribed for both secondary and primary prevention of cardiovascular disease in persons with a range of comorbidities. However, there are no evidence-based recommendations for statin continuation or discontinuation near the end of life.
For persons with high cardiovascular risk, but no clinical evidence of cardiovascular disease, the preventive benefits of statins resulting from lipid lowering are manifested over 2 or more years.3,4 In the presence of known cardiovascular disease, secondary prevention with statins likely decreases adverse cardiovascular outcomes through long-term lipid lowering as well as short-term effects on inflammation and on platelet and endothelial function.5 Thus, possible benefits of statin use at the end of life include prevention of acute cardiovascular events, but these benefits may be limited to persons at high risk.5–7 Further, patients and families may have personal preferences to either continue or discontinue treatment. Risks of statin continuation include medication side effects arising from changes in drug metabolism, medication burden for patients and caregivers, and misallocation of health care resources.7,8 Previous investigations have described persistent rather than attenuated statin use at the end of life; however, optimal statin management is unclear.9–12
We explored statin prescribing patterns in a cohort of 539 persons with an incident cancer diagnosis with a relatively poor prognosis (0% to 25% predicted 5-year survival) who were receiving statins at the time of diagnosis. Our investigation differs from previous studies by using a retrospective cohort design, assessing timing of medication refills, identifying the clinical indication for statin use (secondary versus primary prevention), and incorporating information on cause of death.
We hypothesized that a) most patients would continue receiving statins despite the poor prognosis of their cancer; and b) patients would be more likely to continue statins taken for secondary versus primary prevention of cardiovascular disease.
Methods
The retrospective cohort consisted of 539 members of Kaiser Permanente Colorado (KPCO), an integrated, not-for-profit health maintenance organization (HMO), who received an initial cancer diagnosis between 2001 and 2008. We obtained relative 5-year survival from 18 National Cancer Institute Surveillance and Epidemiology End Results (SEER) geographic areas (http://seer.cancer.gov/statfacts/); these rates are based on cancer type and stage at diagnosis. All cohort members had an estimated SEER 5-year survival of 0% to 25% and an active statin prescription at the time of diagnosis. We included all sites of cancer based on our interest in exploring statin use as a function of overall prognosis. Within the cohort, we defined subcohorts of members taking statins for secondary prevention (precancer diagnoses of coronary artery disease, history of stroke, peripheral vascular disease, and/or abdominal aortic aneurism) and for primary prevention (hyperlipidemia only) based on ICD-9 codes (2010 ICD-9-CM. Volumes 1, 2, and 3. Hospital Professional Edition. 2009 MAG Mutual Healthcare Solutions, Inc. Atlanta, GA.) (see Table 1). All data were extracted from claims databases, the electronic medical record, and the KPCO tumor registry (which is populated from manual record review).
Table 1.
Characteristics of Overall Cohort and Prevention and Treatment Groups
| Cohort on statins N=539 | Statin for secondary preventiona N=343 | Statin for primary prevention1 N=196 | P valueb | |
|---|---|---|---|---|
| Age at diagnosis | <0.001 | |||
| Median | 72.0 | 73.0 | 70.0 | − |
| IQR (25%, 75%) | (67.0, 76.0) | (68.0, 78.0) | (63.0, 75.0) | − |
| Gender | 0.002 | |||
| Male | 295 (54.7%) | 205 (59.8%) | 90 (45.9%) | − |
| Female | 244 (45.3%) | 138 (40.2%) | 106 (54.1%) | − |
| Survival | 0.019 | |||
| Alive at end of study | 43 (8.0%) | 28 (8.2%) | 15 (7.7%) | − |
| <90 days | 178 (33.0%) | 126 (36.7%) | 52 (26.5%) | − |
| 91–180 days | 84 (15.6%) | 57 (16.6%) | 27 (13.8%) | − |
| >180 days | 234 (43.4%) | 132 (38.5%) | 102 (52.0%) | − |
| Race | 0.865 | |||
| White | 379 (70.3%) | 240 (70.0%) | 139 (70.9%) | − |
| Black | 9 (1.7%) | 5 (1.5%) | 4 (2.0%) | − |
| Asian | 9 (1.7%) | 5 (1.5%) | 4 (2.0%) | − |
| Other/Unknown | 142 (26.3%) | 93 (27.1%) | 49 (25.0%) | − |
| Hispanic ethnicity | 0.185 | |||
| Hispanic | 40 (7.4%) | 21 (6.1%) | 19 (9.7%) | − |
| Low SESc | 0.745 | |||
| Yes | 100 (18.6%) | 65 (19.0%) | 35 (17.9%) | − |
| Smoking status | 0.062 | |||
| Nonsmoker | 201 (37.3%) | 118 (34.4%) | 83 (42.3%) | − |
| Former smoker | 196 (36.4%) | 134 (39.1%) | 62 (31.6%) | − |
| Smoker | 117 (21.7%) | 71 (20.7%) | 46 (23.5%) | − |
| Body-mass indexd | 0.004 | |||
| Median | 27.3 | 26.8 | 28.3 | − |
| IQR (25%, 75%) | (24.3, 30.6) | (23.9, 30.1) | (25.0, 31.2) | − |
| Statin indicationsa | ||||
| History of CAD | 269 (49.9%) | 269 (78.4%) | NA | |
| History of PVD | 109 (20.2%) | 109 (20.2%) | NA | |
| History of AAA | 36 (6.7%) | 36 (6.7%) | NA | |
| History of stroke | 137 (25.4%) | 137 (25.4%) | NA | |
| Hyperlipidemia | 486 (90.2%) | 314 (91.5%) | 196 (100.0%) | 0.1552 |
| Cancer sites | ||||
| Breast | 9 (1.7%) | 5 (1.5%) | 4 (2.0%) | |
| Colon | 52 (9.6%) | 33 (9.6%) | 19 (9.7%) | |
| Lung | 312 (57.9%) | 207 (60.3%) | 105 (53.6%) | |
| Other | 166 (30.8%) | 98 (28.6%) | 68 (34.7%) | |
We determined statin indications as follows: secondary prevention: history of coronary artery disease (CAD), peripheral vascular disease (PVD), abdominal aortic aneurism (AAA), or stroke. (ICD-9 codes as follows: CAD: 410.x, 411.x, 412.x, V45.81, V45.82, 996.03, 414.x. AAA: 441.3, 441.4. PVD: 441.x, 443.9. Stroke: 430.x–435.x, 438.x, 852.0, 852.2, 852.4, 853.0, V12.54.) Categories for secondary prevention group are not mutually exclusive. Primary prevention: documented diagnosis of hyperlipidemia (ICD-9 codes 272.0, 272.1, 272.2, 272.3, 272.4 or on a statin and low-density lipoprotein [LDL] not at goal).
P values represent eitherχ2 test results for categorical variables or Kruskal-Wallis test results for continuous variables.
Socioeconomic status (SES) estimated based on census data for educational level and federal poverty level.
Body mass index available for 490 cohort members (308 treatment and 182 prevention).
IQR, interquartile range.
The time during which a patient could refill statins was defined as the time from cancer diagnosis to either date of death or disenrollment from the health plan; patients alive at the end of the observation period (12/31/2010) were censored. We calculated the number of days from diagnosis to last refill or censorship. We used the date of last refill as a proxy for clinician intent and patient behavior, because we could not assess the date on which the last pill was actually taken. In calculating Kaplan-Meier estimates of last refill and death, if a patient did not have a refill on or after their date of diagnosis, then they were given a zero value. We also conducted separate analyses limited to those who had refills following diagnosis. We applied all analyses to the cohort overall and to secondary and primary prevention subcohorts.
To explore whether individuals were more likely to continue statins taken for secondary versus primary prevention, we compared the proportion of refills between groups (Cochran-Mantel Haenszel statistic) and median days between diagnosis and last refill and last refill and death using the Wilcoxon rank sum test. Finally, we examined underlying cause of death as determined from state vital records.
The investigation was approved by the institutional review boards of KPCO and of the University of Colorado.
Results
The demographic characteristics of the overall cohort and subcohorts are listed in Table 1. Median age for cohort members was 72 years. Three hundred and forty-three (64%) were taking statins for secondary prevention and, 196 (36%) were taking them for primary prevention (Table 1).
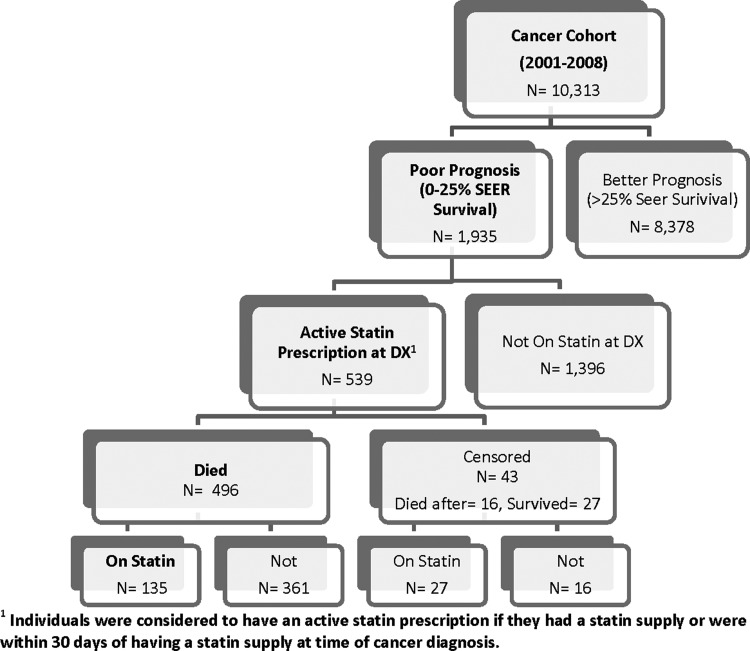
Of 539 cohort members, 496 (92%) died during the observation period—181 in the primary prevention group and 315 in the secondary prevention group (Fig. 1). Median survival time was 231 days for the primary prevention group and 131 days for the secondary prevention group.
FIG. 1.
Flow diagram of generation of cohort of poor prognosis cancer patients.
We did not find significant differences between primary and secondary prevention groups for proportion of refills after diagnosis, for median days between diagnosis and last refill, or for median days between last refill and death. Of the decedents, 62% of the primary prevention group and 58% of the secondary prevention group had at least one statin refill after diagnosis (χ2=1.0326, p=0.31). Median number of days between diagnosis and last refill was 135 for the secondary and 177 for the primary prevention group (Kruskal-Wallis χ2=0.5738, p=0.45). Median number of days between last refill and death was 63 for the secondary and 65 for the primary prevention group (Kruskal-Wallis χ2=0.1704, p=0.68) (Table 2).
Table 2.
Median Days between Cancer Diagnosis and Last Refill, and between Last Refill and Death
| Overall cohorta | Secondary prevention | Primary prevention | |
|---|---|---|---|
| N | 496 | 315 | 181 |
| N (%) with refill after diagnosisb | 295 (59) | 182 (58) | 113 (62) |
| Median (IQR) days between cancer diagnosis and last refillc | 162 (44–460) | 135 (35–464) | 177 (60–447) |
| Median (IQR) days between last refill and deathc | 63 (33–125) | 63 (36–105) | 65 (33–159) |
Limited to cohort members who died during the observation period.
There were no significant differences between treatment and prevention groups for proportion with a refill after diagnosis (χ2=1.0326, p=0.31).
There were no significant differences for median days between diagnosis and last refill (Kruskal-Wallis χ2=0.5738, p=0.45), or between last refill and death (Kruskal-Wallis χ2=0.1704, p=0.68).
IQR, interquartile range.
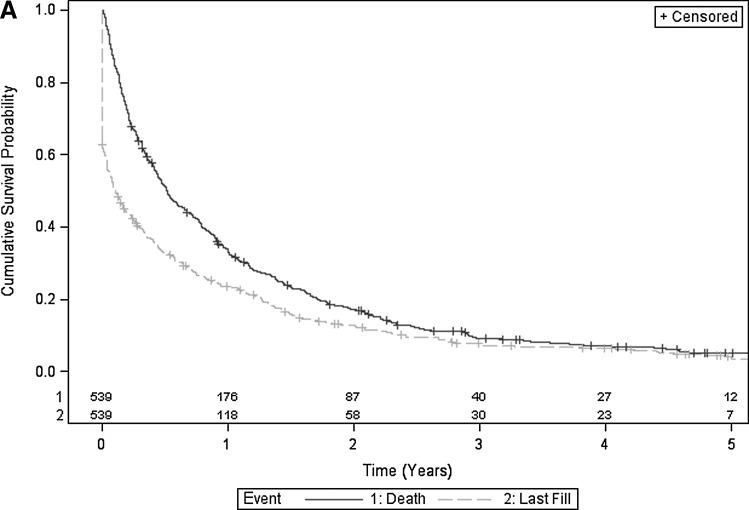
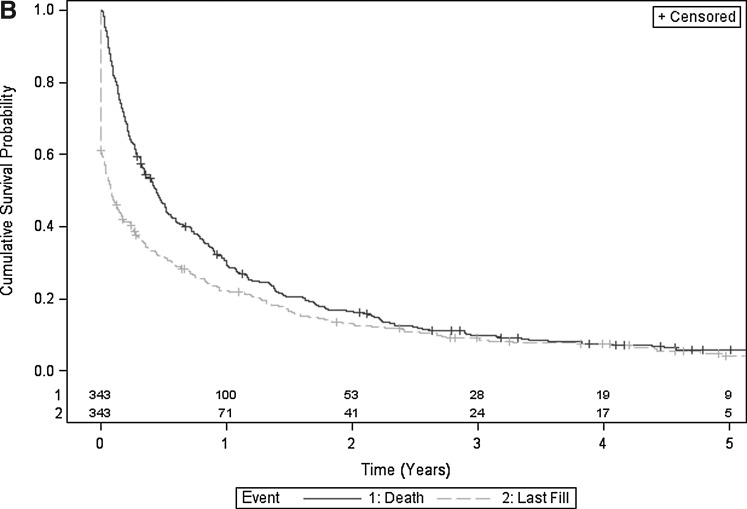
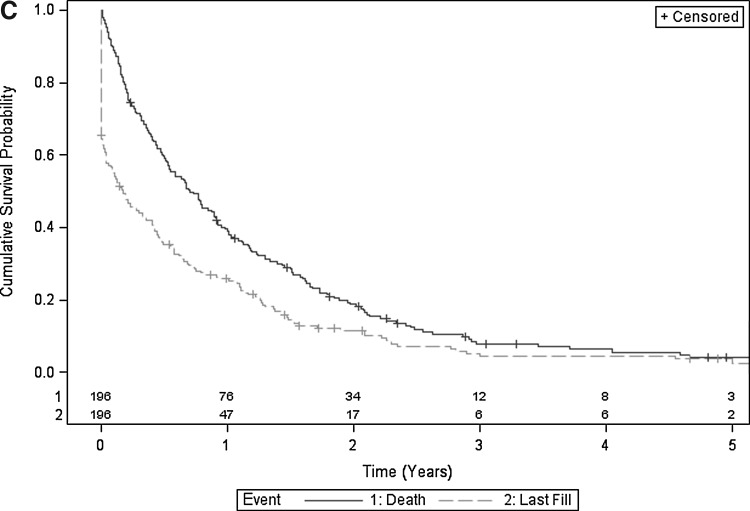
Figures 2A, 2B, and 2C illustrate Kaplan-Meier estimates of time from cancer diagnosis to last fill, and time from cancer diagnosis to death. More than 60% of surviving individuals in the overall cohort, 61% to 79% in the secondary prevention cohort, and 50% to 62% in the primary prevention cohort remained on statins during the 2 years after cancer diagnosis (Table 3). Twenty-five percent (135) of individuals in the overall cohort were on statins at the time of death (Fig. 1).
FIG. 2A.
Superimposed Kaplan-Meier estimates of cumulative survival probability and cumulative time to last statin fill of overall cohort. Includes number of subjects at risk.
FIG. 2B.
Superimposed Kaplan-Meier estimates of cumulative survival probability and cumulative time to last statin fill of secondary prevention cohort. Includes number of subjects at risk.
FIG. 2C.
Superimposed Kaplan-Meier estimates of cumulative survival probability and cumulative time to last statin fill of primary prevention cohort. Includes number of subjects at risk.
Table 3.
Proportion of Surviving Cohort Remaining on Statins
| Time after cancer diagnosis: | 3 months | 6 months | 12 months | 18 months | 24 months |
| Overall cohort (Fig. 2A) | |||||
| Alive | 360 | 271 | 176 | 119 | 87 |
| On statin | 220 | 171 | 118 | 77 | 57 |
| Proportion eligible remaining on statina | 0.61 | 0.63 | 0.67 | 0.65 | 0.67 |
| Secondary prevention cohort (Fig. 2B) | |||||
| Alive | 217 | 156 | 100 | 66 | 53 |
| On statin | 132 | 103 | 71 | 52 | 41 |
| Proportion eligible remaining on statina | 0.61 | 0.66 | 0.71 | 0.79 | 0.77 |
| Primary prevention cohort (Fig. 2C) | |||||
| Alive | 143 | 115 | 76 | 53 | 34 |
| On statin | 88 | 68 | 47 | 25 | 17 |
| Proportion eligible remaining on statina | 0.62 | 0.59 | 0.62 | 0.47 | 0.5 |
Of cohort members alive at time point.
Underlying cause of death was available for 479 individuals. This was due to malignancy in 297 (86.7%) of the secondary prevention group and in 165 (84%) of the primary prevention group. For two members of each group, death was attributable to cardiovascular causes.
Discussion
In this cohort of individuals diagnosed with cancer with a poor prognosis, more than 60% of survivors continued to refill statins during the 2 years following diagnosis. This suggests that an initial diagnosis of a life-limiting illness does not prompt immediate discontinuation of these routine medications. Refilling routine medications in the face of shifting health priorities can persist for multiple reasons—ranging from active clinical recommendations to patient preferences to routinized adherence behavior to benign clinical inattention. Our investigation was designed to explore refill patterns and thus does not explain specific behaviors or rationales behind our findings. Further, medication refills are only a rough proxy for medication prescribing and use—especially in this study population.
We hypothesized that there would be more persistent use of statins in the secondary prevention group based on the potential for statins to mitigate adverse cardiovascular events in this subpopulation. A slightly higher proportion of survivors in the secondary prevention cohort continued refills over time. However, the proportion of postdiagnosis refills, days between diagnosis and last refill, and days from last refill to death were comparable between groups. Thus, it is unclear whether persistent refills are related to indications for statin use. The low number of cardiovascular causes of death in both treatment and prevention groups did not inform any conclusions about the cardiovascular effects of continued statin use in either group.
If clinical inertia reflects failure to intensify treatment in the face of appropriate clinical indications, “clinical momentum” may reflect failure to de-intensify treatment in the face of a changing clinical context.13,14 Such clinical momentum may be useful as part of a long-term care plan, but may have unintended consequences at the end of life. This is especially true in settings in which patients have high adherence to chronic medications.
Several criteria have been proposed to guide medication management at the end of life. These include an understanding of drug metabolism and of patients' prognoses, accurate estimates of benefits and harms of medications, clear treatment targets, adequate time to anticipated benefit, and consistency with overall goals of care.1,15,16 Even if medication discontinuation is appropriate, clinicians may shy away from such discussions. However, it is possible to use patient-centered approaches to decrease medication burden while at the same time reassuring patients of continued care and attention.15
Our study has several limitations. As mentioned above, medication refills are only a rough proxy for medication prescribing and use. It is possible that end-of-life statin use could differ in other care settings. We did not have information on the prior duration of statin treatment for individuals with cardiovascular diagnoses; or the statistical power to use number of refills after diagnosis as an outcome. In any setting, cause of death data are at best a rough proxy for more detailed clinical information. It will be important to study other end-of-life populations (including those with terminal cardiovascular diagnoses) to further inform management of cardiovascular risk at the end of life. A randomized controlled trial is underway to evaluate the outcomes of statin discontinuation during hospice care.12 This trial concentrates on persons taking statins for primary prevention, and will inform use of statins in that subpopulation.
Conclusions
Our longitudinal evaluation of a large, retrospective cohort of persons with incident poor-prognosis cancer describes diminished but persistent statin refills after diagnosis. There may be an opportunity to reevaluate medication burden in persons taking statins for primary prevention, and it is unclear whether continuing statins prescribed for secondary prevention affects cardiovascular outcomes.
Acknowledgments
This project was funded through the Agency for Healthcare Research and Quality MCC Research Network grant number R21HS019520. The AHRQ Multiple Chronic Conditions Research Network, made up of AHRQ funded researchers and a Technical Assistance Center, has been brought together to generate new knowledge about patients with multiple chronic conditions (MCC).The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Holmes HM. Hayley DC. Alexander GC. Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166:605–609. doi: 10.1001/archinte.166.6.605. [DOI] [PubMed] [Google Scholar]
- 2.Currow DC. Stevenson JP. Abernethy AP. Plummer J. Shelby-James TM. Prescribing in palliative care as death approaches. J Am Geriatr Soc. 2007;55:590–595. doi: 10.1111/j.1532-5415.2007.01124.x. [DOI] [PubMed] [Google Scholar]
- 3.LaRosa JC. He J. Vupputuri S. Effect of statins on risk of coronary disease: A meta-analysis of randomized controlled trials. JAMA. 1999;282:2340–2346. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GG. Olsson AG. Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: The MIRACL study: A randomized controlled trial. JAMA. 2001;285:1711–1718. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 5.Spencer FA. Allegrone J. Goldberg RJ, et al. Association of statin therapy with outcomes of acute coronary syndromes: The GRACE study. Ann Intern Med. 2004;140:857–866. doi: 10.7326/0003-4819-140-11-200406010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Spencer FA. Fonarow GC. Frederick PD, et al. Early withdrawal of statin therapy in patients with non-ST-segment elevation myocardial infarction: National registry of myocardial infarction. Arch Intern Med. 2004;164:2162–2168. doi: 10.1001/archinte.164.19.2162. [DOI] [PubMed] [Google Scholar]
- 7.Vollrath AM. Sinclair C. Hallenbeck J. Discontinuing cardiovascular medications at the end of life: Lipid-lowering agents. J Palliat Med. 2005;8:876–881. doi: 10.1089/jpm.2005.8.876. [DOI] [PubMed] [Google Scholar]
- 8.Maddison AR. Fisher J. Johnston G. Preventive medication use among persons with limited life expectancy. Prog Palliat Care. 2011;19:15–21. doi: 10.1179/174329111X576698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silveira MJ. Kazanis AS. Shevrin MP. Statins in the last six months of life: A recognizable, life-limiting condition does not decrease their use. J Palliat Med. 2008;11:685–693. doi: 10.1089/jpm.2007.0215. [DOI] [PubMed] [Google Scholar]
- 10.Riechelmann RP. Krzyzanowska MK. Zimmermann C. Futile medication use in terminally ill cancer patients. Support Care Cancer. 2009;17:745–748. doi: 10.1007/s00520-008-0541-y. [DOI] [PubMed] [Google Scholar]
- 11.Tanvetyanon T. Choudhury AM. Physician practice in the discontinuation of statins among patients with advanced lung cancer. J Palliat Care. 2006;22:281–285. [PubMed] [Google Scholar]
- 12.Abernethy AP. Aziz NM. Basch E, et al. A strategy to advance the evidence base in palliative medicine: Formation of a palliative care research cooperative group. J Palliat Med. 2010;13:1407–1413. doi: 10.1089/jpm.2010.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmittdiel JA. Uratsu CS. Karter AJ, et al. Why don't diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med. 2008;23:588–594. doi: 10.1007/s11606-008-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips LS. Branch WT. Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825–834. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson J. Abernethy AP. Miller C. Currow DC. Managing comorbidities in patients at the end of life. BMJ. 2004;329:909–912. doi: 10.1136/bmj.329.7471.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Currow DC. Abernethy AP. Frameworks for approaching prescribing at the end of life. Arch Intern Med. 2006;166:2404. doi: 10.1001/archinte.166.21.2404-a. [DOI] [PubMed] [Google Scholar]