Abstract
Background and Objectives:
Metabolic syndrome – a plausible precondition for type II diabetes and cardiovascular diseases is also on rise. To understand the mechanistic complexity of metabolic syndrome it is imperative to study the specific contribution of the determinants of metabolic syndrome. Such study can help to identify the most significant factor which may be of use in early detection as well as prevention efforts. Such information is scarcely available from India and especially from rural India. Hence, the present study was undertaken to explore for such factor which might be considered crucial for development of such pathogenesis particularly in rural population of Wardha.
Methods:
A cross-sectional study comprising of 300 subjects was carried out in rural area of Primary Health Center, attached to medical college with approximate 31,000 populations. The anthropometric parameters such as height, weight, waist circumference were measured. Overnight fasting samples were collected for lipid profile (total cholesterol, triglyceride, high density lipoproteins, low density lipoproteins, very low density lipoproteins) and fasting blood glucose levels. The National Cholesterol Education Programme Adult Treatment Panel, ATP-III guidelines were used to categorize the study subjects. As many of the variables are highly intercorrelated, exploratory factor analysis was carried out to reduce the data to a smaller number of independent factors that accounts for the most of the variances in the data. Principal component analysis was used as a method of extraction.
Results:
For both sexes, three factors were extracted accounting for about 71% variance in the measured variables. An adiposity factor which accounted for highest explained variance (28%), was the initial factor extracted. It was loaded positively by waist circumference, triglyceride, and very low density lipoprotein and negatively loaded by high density lipoprotein. Second factor extracted was a cholesterol factor which explained about 20% variance. It was positively loaded by total cholesterol and low density lipoprotein. Blood pressure factor was third to be extracted which again explained about 20% variance. It was positively loaded by systolic and diastolic blood pressure.
Interpretation and Conclusion:
The results clearly indicate the significance of visceral adiposity over the obesity in general or simple abdominal obesity measured anthropometrically as a pathogenic determinant of the metabolic syndrome. The most consistent factor has been found to be dyslipidemia which explained major share of the observed variance and the most significant load of this factor being rested on triglyceride and the VLDL level. Hence, we conclude measurement of triglyceride might be a rewarding screening parameter for assessment of cardio-metabolic risk in general populace and warrants a large scale study focusing into this issue.
Keywords: Metabolic syndrome, factor analysis, dyslipidemia, ATP-III, CVD, diabetes
Introduction
People with abnormal glucose metabolism, hypertension and obesity with dyslipidemia together constitute “metabolic syndrome,” posing a major public health challenge to the health systems in developed and developing countries. Metabolic syndrome may be considered as a pre-condition of two major clinical entities that are constantly on the rise: Atherosclerotic cardiovascular diseases and diabetes mellitus, which are major contributors to morbidity and mortality all over the world.(1) The National Health Examination Survey-III (NHANES) showed that people with diabetes and metabolic syndrome had the highest prevalence of coronary heart diseases.(2) The West Scotland Coronary Artery Prevention Study (WOSCOPS) found that the risk of CHD and diabetes increased with the number of metabolic syndrome risk factors.(3)
In India, diabetes and cardiovascular diseases are on the rise. Many studies have reported that there is a progressive increase in the prevalence of coronary heart disease across the country over the year.(4–6) Metabolic syndrome, a plausible precondition for type II diabetes and cardiovascular diseases, is also on the rise. Several studies reported a high prevalence of metabolic syndrome or its individual components like impaired glucose tolerance, obesity and hypertension.(7–8) However, there is considerable interplay among these components and their contribution to the pathogenesis of the diabetes and cardiovascular complications. Because of the well-known etiopathological background, altered vascular homeostasis and insulin resistance has been associated with lipid metabolic abnormality, which forms an intricate nexus of metabolic pathology.(9) To understand the mechanistic complexity of metabolic syndrome, it is imperative to study the specific contribution of the determinants of metabolic syndrome. Such study can help to identify the most significant factor that may be of use in early detection as well as prevention efforts. Such information is scarcely available from India and, especially, from rural India. Hence, the present study was undertaken to explore such factors that might be considered crucial for development of such pathogenesis, particularly in the rural population of Wardha.
Methodology
Study setting and study subjects
The present cross-sectional study was carried out in all the villages of Primary Health Center, Anji. The study site was located in rural Wardha district, about 758 km east from the state capital of Mumbai. The Primary Health Center, Anji, covers 23 villages with a population of 31,000 and is a field practice area of the Mahatma Gandhi Institute of Medical Sciences, Sewagram. All the residents of these 23 villages above the age of 18 years were included in the sampling frame. The protocol was approved by the institutional ethics committee.
Sampling method and sample size
Considering 10% prevalence of metabolic syndrome,(10) 3% absolute error, alpha = 0.1 and 10% non-response, the sample size required was 300. The study subjects were selected by simple random sampling. Sample was drawn from the enumeration list available with the Department of Community Medicine. Written informed consent was obtained from each of the participants.
Anthropometric measurements
Body weight was measured (to the nearest 0.5 kg) with the subject standing motionless on the bathroom weighing scale. The weighing scale was standardized every day with a weight of 50 kg. Height was measured (to the nearest 0.5 cm) with the subject standing in an erect position against a vertical scale of portable stadiometer and with the head positioned so that the top of the external auditory meatus was in level with the inferior margin of the bony orbit. Body mass index (BMI) was calculated as weight in kilograms divided by squared height in meter. Waist circumference (WC) was measured at the level halfway between the iliac crest and the costal margin in the mid-axillary line after exhaling with the subject in standing position. Hip circumference was measured at the level of greater trochanters with the subject in standing position and both feet together. Two consecutive recordings were made for each site to the nearest 0.5 cm using a non-stretchable fiber measuring tape on a horizontal plane without compression of the skin. The mean of the two sets of values was used.(11)
Blood pressure measurements
Blood pressure was recorded with a sphygmomanometer in the right arm, in the sitting position. Three readings were taken half an hour apart and the average was used for further analysis. Hypertension was defined as a systolic blood pressure ≥140 mmHg or a diastolic blood pressure ≥90 mmHg, or the current use of antihypertensive drug.(12)
Laboratory analysis
The previous evening, the participants were visited and written informed consent was obtained after explaining the objectives and procedures of the study. They were also counseled to be fasting overnight till the blood samples were taken the next morning by venipuncture. The samples were transported to the institutional laboratory on the same day for further analysis. The fasting blood sugar was analyzed by the glucose oxidase peroxidase (GOP-PAP) method;(13) total cholesterol was analyzed by the cholesterol oxidase (CHOD-PAP) method;(14) triglycerides by the GPO-PAP Trinder method;(15) and High Density Lipoprotein (HDL-C) by the phosphotugstic acid method.(16) Very low density lipoproteins (VLDL-C) was calculated by an indirect method as VLDL cholesterol is one-fifth of the triglyceride level.(17) LDL-C was calculated by subtracting VLDL-C and HDL-C from total cholesterol. ERBA kits supplied by Transia Biochemicals Ltd., Mumbai, India were used.
According to the National Cholesterol Education Program Adult Treatment Panel (ATP) III, the diagnosis of metabolic syndrome was made when three or more of the following risk factors were present: a WC >102 cm in men and >88 cm in women, fasting glucose ≥110 mg/ dL (6.1 mmol/L), systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg, fasting triglycerides ≥150 mg/dL (1.7 mmol/L) and HDL cholesterol <40 mg/ dL (1.0 mmol/L) in men and <50 mg/dL (1.3 mmol/L) in women.(18) ATP III definitions were based on the association of factors with subsequent coronary heart disease in Caucasian cohorts. As Indians have higher body fat content than their western counterparts for the same BMI, we used lower cut-offs of WC as suggested by Asia-Pacific guidelines. WC cut-offs were taken as >90 cm for males and >80 cm for females to define overweight.(19)
Statistical analysis
The statistical analysis was carried out using SPSS 12.0 software (SPSS, Chicago, IL, USA). The variables with skewed distribution such as triglyceride, very low-density lipoprotein, fasting blood glucose, WC, systolic blood pressure and diastolic blood pressure were log-transformed. As many of the variables are highly intercorrelated, exploratory factor analysis was carried out to reduce the data to a smaller number of independent factors that accounted for most of the variances in the data. Principal component analysis was used as a method of extraction. The number of components to be retained was based on Scree plot analysis and eigenvalue criteria of 1.0. Varimax rotation was used to obtain a set of independent interpretable factors. The resulting factor pattern was interpreted using factor loadings of more than 0.4. The procedure was carried out first for both sexes together and then for men and women individually. The result of the KMO test for sampling adequacy was 0.623, 0.536 and 0.511, respectively, for the three groups. Bartlett's test for sphericity was highly significant (P < 0.001) for all three groups, suggesting the suitability of data for factor analysis.
Results
The characteristics of anthropometric and biochemical parameters in both sexes are presented in Table 1. Data show that the mean age of men was 42.4 years and that for women was 39.3 years. The mean WC (76.82 ± 0.86 in men versus 74.90 ± 1.04 in women) was not significantly different between men and women (P > 0.05). The mean systolic and diastolic blood pressure levels did not differ significantly between the two sexes. Mean fasting blood glucose level was 89.88 mg/dL (SE = 1.47) and 90.62 mg/ dL (SE = 2.29) in men and women, respectively. Similarly, the mean triglyceride and high-density lipoprotein did not differ significantly between men and women.
Table 1.
Anthropometric and biochemical characteristics in men and women
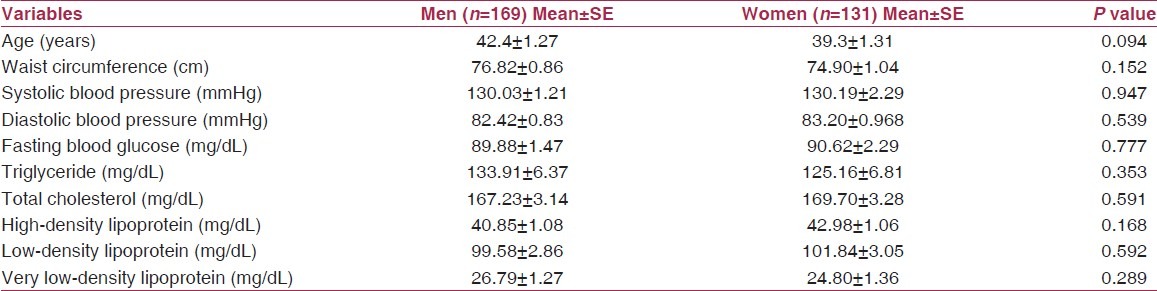
As per the ATP-III criteria, the prevalence of metabolic syndrome was 17.6% in men and 16.8% in women. Of these, 0.7% men and 7.6% women had central obesity. The prevalence of impaired fasting blood glucose level was 12% in men and 10.7% in women, whereas 31.2% of the men and 38.2% of the women had hypertension. 33.7% of the men had high triglyceride levels compared with 25.7% of women. 50.2% of the men had a low cutoff for HDL-C levels, whereas 69.7% of women had similar findings. Table 2 presents the result of factor analysis. For both sexes, three factors were extracted, accounting for about 71% variance in the measured variables. An adiposity factor that accounted for highest explained variance (28%) was the initial factor extracted. It was loaded positively by WC, triglyceride and very low-density lipoprotein and negatively loaded by high-density lipoprotein. The second factor extracted was a cholesterol factor, which explained the 20% variance. It was positively loaded by total cholesterol and low-density lipoprotein. Blood pressure factor was the third to be extracted, which again explained the 20% variance. It was positively loaded by systolic and diastolic blood pressure.
Table 2.
Factor loadings of risk variables after varimax rotation
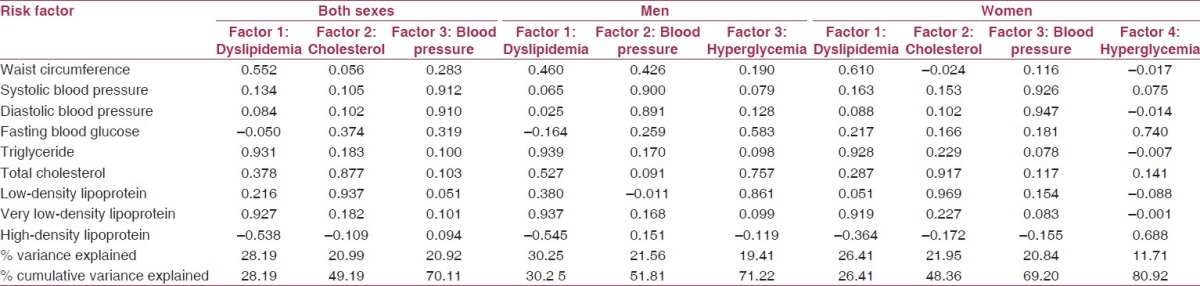
For men, again, three factors were extracted but the loadings were different. On the first factor, WC, triglycerides, total cholesterol and very low-density lipoprotein were loaded positively while high-density lipoprotein was loaded negatively. On the second factor, systolic and the diastolic blood pressure along with WC were loaded significantly. On the third factor, fasting blood glucose, low-density lipoprotein and total cholesterol were loaded significantly. Total variance explained was 71%.
For women, four factors were extracted. On first factor, the loadings were WC, triglycerides and very low-density lipoprotein. On the second factor, total cholesterol and low-density lipoprotein were loaded. The third factor was blood pressure while the fourth factor was loaded by fasting blood glucose and high-density lipoprotein. The factor explained the 81% variance.
Discussion
The present study conducted in the rural Indian populace showed a high incidence (nearly one in six persons) of metabolic syndrome as per ATPIII criteria, which is quite above the national/observed incidence in the comparable rural population.(10,18) This is particularly alarming given the fact that the majority of this study cohort belonged to the lean category, with the overall average BMI being 22.76 kg/m2.
Evidence suggests that central obesity, rather than general obesity (measured by parameters like BMI or body weight), is directly associated with this complex pathological state of metabolic syndrome.(20) Particularly in the Asian population, abdominal obesity has been implicated in the metabolic syndrome and associated insulin resistance in the form of impaired glucose tolerance.(21) Moreover, there is epidemiological evidence showing direct impact of waist girth on hypertension.(22) However, in this study, we did not record any significant variance in the WC, and the overall mean value of this parameter in the entire study population is well below the cutoff as per the ATP-III criteria.(18)
The present study result revealed that dyslipidemia (particularly high triglyceride and low-HDL cholesterol fractions) along with hypertension and impairment of glucose tolerance appeared as key players that are largely associated with such a condition, whereas the prevalence of central obesity did not figure to be a direct determinant. However, on introspection with statistical means to find out the major contributor(s) of this observed variance, the available data reflects dyslipidemia as the predominant factor that is positively contributed by WC, triglyceride and very low-density lipoprotein level. Because the overall variance of WC has already been recorded as insignificant, this parameter surfacing as a contributor in this pathology appears to be paradoxical. In this context, it is worth noting that such an anthropometric parameter for estimating abdominal subcutaneous fat is a rather crude means of assessing the true level of visceral fat content. In fact, the adipose tissue of visceral origin rather than abdominal subcutaneous fat is shown to be a major predictor of dyslipidemia associated with metabolic risk.(23) It is quite interesting that triglyceride and VLDL (which is directly calculated from triglyceride level) claimed a major share in this factor, indicating the significance of triglyceride level among the lipids. Moreover, this lipid fraction is consistently associated in both sex groups as well as in the overall study population as a major contributor. Triglyceride is the major storage form of lipid, and its level can be envisaged as a direct reflection of the visceral fat level. In support of this view, a study in the Japanese population found that insulin resistance, the major component of metabolic syndrome, is significantly associated with both visceral fat as well as serum triglyceride levels.(24) Moreover, such visceral fat was shown to be reflected by triglyceride and, consequently, VLDL level.(25)
Thus, as part of the dyslipidemia factor, the major component appeared to be triglyceride level, predictably followed by WC (representing abdominal subcutaneous fat) as the second contributor. However, as a direct marker alone, the later variable failed to mirror any significant deviation. This result strongly favors the view of visceral fat as a more reliable pathogenic correlate than simple anthropometric measure of overall adiposity (BMI) or central obesity (in terms of WC alone).
Besides cholesterol level, which may be considered as a mere component of altered lipid profile, the key factors that governed the pathogenicity of the metabolic cluster also included hypertension and hyperglycemia. In the perspective of recent evidences, these two factors should be considered in direct association with visceral adiposity, which brings in a paradigm shift in the concept of etiopathogenesis of metabolic disease cluster including insulin resistance and/or diabetes mellitus, hypertension and atherogenic cardiovascular disease.(26) There is conclusive proof that such visceral but not subcutaneous adipocytes are instrumental in the development of the pro-inflammatory environment leading to metabolic stress by elaborating adipocytokines.(27) Of late, demonstration of expression of endocanabinoid receptors in the adipose tissue along with liver and brain highlighted the coordinated impact of this system in regulation of lipid metabolism.(27–28) Pending mechanistic details, dysregulation of this system in obese individuals might be contemplated in atherogenic dyslipidemia as well as in insulin resistance. Further, an important link of adiposity with hypertension is found to be operative through the angiotensin system, with the discovery of adipose tissue as a producer of angiotensinogen, the precursor of major endogenous vasoconstrictor.(29) A complex nexus through this system reportedly lead to angiotensin-mediated generation of large dysfunctional adipocytes; those are responsible for augmented cytokine production as well as inefficient insulin sensitivity.(30–31) Yet another off-shoot of such ectopic abnormal adiposity in the form of visceral obesity also gives rise to increased leptin production, which is believed to be a direct link to sympathetic over-activity and associated hypertension.(32) This sets in a vicious cycle that paves the way for metabolic syndrome cluster. Finally, the successful prevention of atherosclerotic risk in the patients of this pathogenic cluster with fibrates and glitazones unequivocally proves the visceral obesity theory. These agents are activators of peroxisome proliferator-activated receptor (PPAR), a mediator involved in regulating glucose homeostasis and inhibiting inflammatory response, and, thus, also exerts anti-atherosclerotic properties.(33) These drugs have been shown to increase more physiologically relevant subcutaneous adipose tissue in lieu of the pathological visceral fat and, as activator of lipoprotein lipase, effectively decrease the triglyceride level, further suggesting the pivotal role of triglyceride in the entire pathology.(34–35)
Thus, the result in this study coupled with the huge body of supportive evidence, clearly indicates the significance of visceral adiposity over the obesity in general or simple abdominal obesity measured anthropometrically as a pathogenic determinant of the metabolic syndrome. Quantification of visceral adipose tissue by sophisticated tomography technique might not be a feasible option at the field-level screening condition. In this study, the most consistent factor has been found to be dyslipidemia, which explained the major share of the observed variance and the most significant load of this factor being rested on triglyceride and the VLDL level calculated thereof. Although this parameter figures in both ATPIII as well as in WHO criteria of metabolic syndrome,(20) it has not been considered significant. Of late, triglyceride assay has been given importance due to a justified metabolic rationale in predicting metabolic risk.(36) Hence, we conclude that the measurement of this analyte might be a rewarding screening parameter for assessment of cardiometabolic risk in the general populace, and warrants a large-scale study focusing this issue. However, the smaller sample size of the present study has to be kept in mind and studies with larger sample size need to be carried out to validate the present findings.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K, et al. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. 2004;164:1066–76. doi: 10.1001/archinte.164.10.1066. [DOI] [PubMed] [Google Scholar]
- 2.Alexander CM, Landsman PB, Teutsch SM, Haffner SM. Third national health and nutrition examination survey (NHANES III), national cholesterol education program (NCEP). NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–4. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 3.Sattar N, Gaw A, Scherbakova O, Ford I, O’Reilly DS, Haffner SM, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–9. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Gupta VP. Meta-analysis of coronary heart disease prevalence in India. Indian Heart J. 1996;48:241–5. [PubMed] [Google Scholar]
- 5.Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 6.Hanson RL, Imperatore G, Bennett PH, Knowler WC. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes. 2002;51:3120–7. doi: 10.2337/diabetes.51.10.3120. [DOI] [PubMed] [Google Scholar]
- 7.Mohan V, Shanthirani CS, Deepa R. Glucose intolerance (diabetes and IGT) in a selected South Indian population with special reference to family history, obesity and lifestyle factors-the Chennai Urban Population Study (CUPS 14) J Assoc Physicians India. 2003;51:771–7. [PubMed] [Google Scholar]
- 8.Pradeepa R, Mohan V. The changing scenario of the diabetes epidemic: Implications for India. Indian J Med Res. 2002;116:121–32. [PubMed] [Google Scholar]
- 9.Mathieu P, Poirier P, Pibarot P, Lemieux I, Després JP. Visceral obesity: The link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53:577–84. doi: 10.1161/HYPERTENSIONAHA.108.110320. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar S, Das M, Mukhopadhyay B, Chakrabarti CS, Majumder PP. High prevalence of metabolic syndrome and its correlates in two tribal populations of India and the impact of urbanization. Indian J Med Res. 2006;123:679–86. [PubMed] [Google Scholar]
- 11.Luepker RV, Evans A, McKeigue P, Reddy KS. Cardiovascular survey methods. 3rd ed. Geneva: World Health Organization; 2004. [Google Scholar]
- 12.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 13.Trinder P. Quantitative determination of glucose using GOP-PAP method. Clin Biochemistry. 1969;6:24–7. [Google Scholar]
- 14.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 15.Trinder P. Triglyceride estimation by GPO-PAP method. Ann Clin Biochemistry. 1969;6:24–7. [Google Scholar]
- 16.Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 1970;11:583–95. [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney, Australia: Health Communications Australia Pty Limit; 2000. WHO Western Pacific Region, IASO, IOTF. [Google Scholar]
- 20.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. American Heart Association. [DOI] [PubMed] [Google Scholar]
- 21.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world–a growing challenge. N Engl J Med. 2007;356:213–5. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 22.Poirier P, Lemieux I, Mauriège P, Dewailly E, Blanchet C, Bergeron J, et al. Impact of waist circumference on the relationship between blood pressure and insulin: the Quebec Health Survey. Hypertension. 2005;45:363–7. doi: 10.1161/01.HYP.0000155463.90018.dc. [DOI] [PubMed] [Google Scholar]
- 23.Després JP. Intra-abdominal obesity: an untreated risk factor for Type 2 diabetes and cardiovascular disease. J Endocrinol Invest. 2006;29:77–82. [PubMed] [Google Scholar]
- 24.Katsuki A, Sumida Y, Urakawa H, Gabazza EC, Murashima S, Maruyama N, et al. Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in Japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care. 2003;26:2341–4. doi: 10.2337/diacare.26.8.2341. [DOI] [PubMed] [Google Scholar]
- 25.Adiels M, Westerbacka J, Soro-Paavonen A, Häkkinen AM, Vehkavaara S, Caslake MJ, et al. Acute suppression of VLDL1 secretion rate by insulin is associated with hepatic fat content and insulin resistance. Diabetologia. 2007;50:2356–65. doi: 10.1007/s00125-007-0790-1. [DOI] [PubMed] [Google Scholar]
- 26.Mathieu P, Poirier P, Pibarot P, Lemieux I, Després JP. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53:577–84. doi: 10.1161/HYPERTENSIONAHA.108.110320. [DOI] [PubMed] [Google Scholar]
- 27.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 28.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones BH, Standridge MK, Taylor JW, Moustaïd N. Angiotensinogen gene expression in adipose tissue: analysis of obese models and hormonal and nutritional control. Am J Physiol. 1997;273:R236–42. doi: 10.1152/ajpregu.1997.273.1.R236. [DOI] [PubMed] [Google Scholar]
- 30.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo KY, Halo P, Leibel RL, Zhang Y. Effects of obesity on the relationship of leptin mRNA expression and adipocyte size in anatomically distinct fat depots in mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R112–9. doi: 10.1152/ajpregu.00028.2004. [DOI] [PubMed] [Google Scholar]
- 32.Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension. 2005;46:326–32. doi: 10.1161/01.HYP.0000175474.99326.bf. [DOI] [PubMed] [Google Scholar]
- 33.Vergès B. Clinical interest of PPARs ligands. Diabetes Metab. 2004;30:7–12. doi: 10.1016/s1262-3636(07)70083-6. [DOI] [PubMed] [Google Scholar]
- 34.Lowell BB. PPARgamma: an essential regulator of adipogenesis and modulator of fat cell function. Cell. 1999;99:239–42. doi: 10.1016/s0092-8674(00)81654-2. [DOI] [PubMed] [Google Scholar]
- 35.Auwerx J, Schoonjans K, Fruchart JC, Staels B. Regulation of triglyceride metabolism by PPARs: fibrates and thiazolidinediones have distinct effects. J Atheroscler Thromb. 1996;3:81–9. doi: 10.5551/jat1994.3.81. [DOI] [PubMed] [Google Scholar]
- 36.Brown H. Factor in triglyceride when assessing patient's visceral fat. [Accessed on February 10, 2010]. Available from: http://www.entrepreneur.com/tradejournals/article/167255989.html .


