Sir,
Adverse drug reactions (ADRs) are one of the leading causes of death among hospitalized patients and occur in 0.3 to 7 per cent of all hospital admissions1. These may vary from mild rashes to severe reactions such as Stevens-Johnson syndrome (SJS). Five to eight per cent of hospitalized patient develop serious adverse drug reaction1. However, these may often go undetected and unreported. SJS is a severe life threatening mucocutaneous syndrome caused by drugs like antimicrobials, antiepileptics, and analgesics2. We present here findings of a retrospective analysis of the relationship between drug induced SJS, causative agents and the patients’ characteristics done in a tertiary care hospital in Ahmedabad, Gujarat, India.
This retrospective study comprised 59 cases of SJS reported over a period of four and a half years (from July 2005 to December 2009) at B.J. Medical College, Ahmedabad. The condition was diagnosed by a dermatologists using Bastuji Garin classification3. Relevant details were recorded including the clinical presentation, date of starting and stopping of event, relevant laboratory investigations, other relevant history including pre-existing diseases, suspected medication (including dose, frequency, route of administration, dates and duration of administration and indications for use), dechallenge and rechallenge data (if available) and concomitant medicines (including self medication and herbal remedies). Causality assessment was carried out using WHO- UMC criteria and Naranjo's algorithm4,5. ALDENS algorithm was also used as this method is specific for SJS6. Preventibility score was determined using Schumocks and Thorontons criteria7.
A total of 1769 ADRs were reported during the study period, of which 59 were drug induced SJS. The occurrence was 3.33 per cent (95% confidence interval, 2.91 to 4.17), which was less as compared to another report from India (13.9%)8.
Age of the patients suffering from SJS ranged from 2 months to 85 years (median age 35 yr). Of the 59 patients, 15 (25%) were children or young adults (age <20 yr) and nine (15%) were more than 50 yr old. SJS is reported to be common at extremes of ages9. The male to female ratio was 2.10. The onset of SJS was seen within 1 to 39 days of starting drug therapy with a median time being 5 days. The interquartile range was 14 to 2. Thirty six patients developed SJS in the first week of therapy. The average onset of SJS in our study was 10.5 days (8.4 days for phenytoin and 7.5 days for carbamazepine). Others have reported the mean reaction time of six days10. For the aromatic anticonvulsants, the risk is shown to be highest during the first two months of treatment11. A knowledge of this possibility should help prescriber remain vigilant during this period. The patients receiving treatment for epilepsy (25), HIV (14) and treatment of upper respiratory tract infection (6) were common victims. Nearly half of the children in our study who developed SJS had upper respiratory or non specific viral infection. Others have reported that 56 per cent of SJS patients had an antecedent upper respiratory tract infection or non specific viral infection12,13. The mechanism of development of SJS in patients suffering from viral infections is probably due to a decrease in immunity12. Of the 25 patients on antiepileptic treatment, 16 were actually being treated for epilepsy while remaining nine received these drugs prophylactically. Mortality was observed more commonly in elderly patients in our study. It is possible that the severity of SJS is greater at the extremes of ages perhaps due to a poor immune response as compared to adults10.
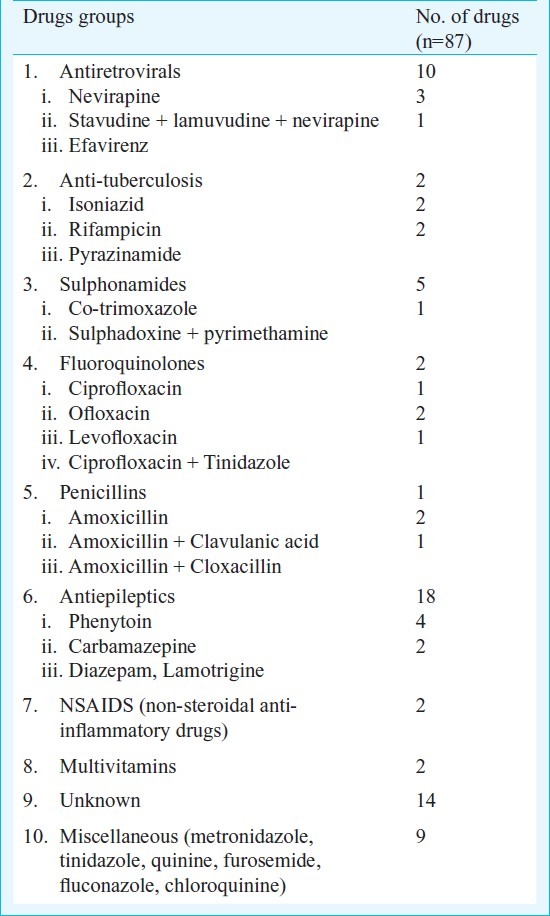
Eighty seven drugs were prescribed to 59 patients suffering from SJS (Table). These drugs included antimicrobials (40) antiepileptics (25), analgesics (6) and multivitamins (2). Interestingly, 14 drugs were of unknown nature. Of these unidentified drugs, 11 were being used for fever, two as analgesics and one for renal failure. Amongst antimicrobials, antiretrovirals (14) were the most common group, followed by antituberculosis drugs (6), sulphonamides, flouroquinolones (6) and penicillins (4). Antimicrobials were the most commonly suspected drugs (45%) as has also been reported in Australia10. Amongst antimicrobials, antiretrovirals were the most common group. Nearly all cases receiving antiretrovirals were exposed to nevirapine which is similar to observations made before15,16. Phenytoin (18) was the most commonly implicated antiepileptic drug followed by carbamazepine (4), gabapentin and diazepam (1 each). Phenytoin as a common cause of SJS has also been observed earlier17. Ibuprofen and paracetamol were prescribed in two patients. Amongst the unknown agents, most of the drugs (11) were used for the treatment of fever, and were available as over the counter drugs. Majority (56) of the suspected drugs were stopped after reaction. The symptoms resolved in 42 cases, while in the remaining 14 patients the reaction continued unabated. The serious nature of ADR was evident from the fact that of the 59 patients, four had a fatal outcome, while the condition was life threatening in 18 patients. Of the four deaths reported, three were elderly (>55 yr old). The suspected drug prescribed were phenytoin, roxithromycin, amoxicillin, cloxacillin, cefotaxime and nevirapine.
Table.
List of suspected drugs to cause the Stevens-Johnson syndrome
Risk of developing SJS remains unidentifiable in most of the cases18. Drugs with long half-lives are more likely to cause such fatal reactions than those with short half-lives20. Sulphonamides and antiepileptic drugs are metabolized to toxic substances that are subsequently detoxified in most individuals21. It is postulated that in some individuals, due to a genetic defect, the metabolites may bind to the proteins and trigger an immune response that leads to the cutaneous reactions of SJS22. A recent study in Chinese population has shown that HLA-B*1502 may act as a genetic marker for carbamazepine induced SJS19.
Causality assessment using the WHO UMC criteria showed that it was possible for 45 drugs, and probable for 28 drugs. The causality was unassesible in 14 cases as the nature of the drug was not known. Causality assessment by Naranjo's algorithm showed possible causality for 53 drugs and probable for 34 drugs. The causality was not ‘certain’ in any case as there was no rechallange and the drug levels were also unknown as required for Naranjio's algorithm. With ALDENS algorithm the causality assessment was ‘very probable’ for 25 drugs. These patients were prescribed either phenytoin, nevirapine or lamotrigine. The causality was ‘possible’ for 19 drugs, ‘unlikely’ in three and ‘very unlikely’ for one. Approximately 50 per cent of the patients in our study were prescribed more than one drug. In such cases, determination of the causative agents becomes difficult.
ADRs were preventable in five cases. These included fixed drug combinations of ciprofloxacin with tinidazole and metronidazole, fluconazole with amoxicillin and concomitant use of cefixime with quinine for fever. The drugs were irrationally prescribed in four cases. These included, irrational fixed dose combination (e.g. ciprofloxacin with tinidazole and metronidazole). It was also observed that drugs were prescribed in absence of proper indications like use of both cefixime and quinine for fever. Polypharmacy was also evident in a few cases. It is important to obtain a history of allergy also as it was missed in one patient before prescribing the causal drug.
SJS is a life threatening adverse drug reaction. In conclusion, our study showed that SJS was more commonly seen in children who were susceptible to viral infections, mortality was higher in the elderly. This serious ADR when caused by unknown drugs or those used as self medication may cause difficulties in diagnosis and management. A robust ADR monitoring system with a feedback to and the education of the prescribers can help prevent, identify and manage this life threatening condition much more effectively.
References
- 1.Doshi MS, Patel PP, Shah SP, Dikshit RK. Intensive monitoring of adverse drug reactions in hospitalized patients of two medical units at a tertiary care teaching hospital. J Pharmacol Pharmacother. 2012;3:308–13. doi: 10.4103/0976-500X.103687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. Medication Use and the Risk of Stevens-Johnson Syndrome or Toxic Epidermal Necrolysis. N Engl J Med. 1995;333:1600–8. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- 3.Bastuji Garin S, Rzany B, Stern R S. A clinical classification of cases of toxic epidermal necrolysis, Stevens Johnson Syndrome and erythema multiforme. Arch Dermatol. 1993;129:92–6. [PubMed] [Google Scholar]
- 4.World Health Organization. Sweden. [accessed on November 12, 2010]. Available from: http://www.who-umc.org .
- 5.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 6.Hartwig S, Seigel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–32. [PubMed] [Google Scholar]
- 7.Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, et al. ALDEN, algorithm for assessment of drug causality in Stevens Johnsons syndrome and toxic epidermal necrolysis: Comparision with case control analysis. Nature. 2010;88:60–7. doi: 10.1038/clpt.2009.252. [DOI] [PubMed] [Google Scholar]
- 8.Jhaj R, Uppal R, Malhotra S, Bhargava VK. Cutaneous adverse drug reactions in in-patients in a tertiary care hospital. Indian J Dermatol Venereol Leprol. 1999;65:14–7. [PubMed] [Google Scholar]
- 9.Chan HL, Stern RS, Arndt KA, Langlois J, Jick SS, Jick H, et al. The incidence of erythema multiforme, Stevens - Johnson Syndrome and Toxic Epidermal Necrolysis. Arch Dermatol. 1990;126:43–7. [PubMed] [Google Scholar]
- 10.Sanmarkan AD, Sori T, Thappa DM, Jaisankar TJ. Retrospective analysis of Stevens-Johnsons syndrome and toxic epidermal necrolysis over a period of 10 years. Indian J Dermatol. 2011;56:25–9. doi: 10.4103/0019-5154.77546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens Johnsons Syndrome and Toxic Epedermal Necrolysis: Assessment of medication risks with emphasis on recently marked drugs. The Euro SCAR- study. J Invest Dermatol. 2008;128:35–44. doi: 10.1038/sj.jid.5701033. [DOI] [PubMed] [Google Scholar]
- 12.Ginsburg CM. Stevens-Johnson syndrome in Children. Pediatr Infect Dis. 1982;1:155–8. doi: 10.1097/00006454-198205000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Choy AC, Yarnold PR, Brown JE, Kayaloglou GT, Greenberger PA, Patterson R. Virus induced erythema multiforme and Stevens-Johnson syndrome. Allergy Asthma Proc. 1995;16:157–61. doi: 10.2500/108854195778666847. [DOI] [PubMed] [Google Scholar]
- 14.Wong KC, Kennedy PJ, Lee S. Clinical manifestations and outcomes in 17 cases of Stevens-Johnson syndrome and toxic epidermal necrolysis. Australas J Dermatol. 1999;40:131–4. doi: 10.1046/j.1440-0960.1999.00342.x. [DOI] [PubMed] [Google Scholar]
- 15.Fagot, Jean-Paul, Mockenhaupt, Maja A, Bouwes-Bavinck, Jan-Nico B, et al. for the EuroSCAR study group. Nevirapine and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. AIDS. 2001;15:1843–8. doi: 10.1097/00002030-200109280-00014. [DOI] [PubMed] [Google Scholar]
- 16.Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H, Tripathi C. Drug-induced Stevens-Johnsons syndrome (SJS), Toxic epidermal necrolysis (TEN)< and SJS_ TEN overlap: A multicentric retrospective study. J Postgrad Med. 2011;57:115–9. doi: 10.4103/0022-3859.81865. [DOI] [PubMed] [Google Scholar]
- 17.Rzany B, Correia O, Kelly J, Naldi L, Auquier A, Stern R, et al. Risk of Stevens – Johnson syndrome and Toxic Epidermal Necrolysis during the first weeks of antiepileptic therapy: a case control study. Lancet. 1999;353:2190–4. doi: 10.1016/s0140-6736(98)05418-x. [DOI] [PubMed] [Google Scholar]
- 18.Pushker N, Tandon R, Vajpayee RB. Stevens-Johnsons syndrome in India- risk factors, ocular manifestations and management. Ophthalmologica. 2000;214:285–8. doi: 10.1159/000027505. [DOI] [PubMed] [Google Scholar]
- 19.Chang CC, Too CL, Murad S, Hussein SH. Association of HLA-B*1502 allele with carbamazepine-induced toxic epidermal necrolysis and Stevens-Johnson syndrome in the multi-ethnic Malaysian population. Int J Dermatol. 2011;50:221–4. doi: 10.1111/j.1365-4632.2010.04745.x. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson Syndrome: Does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136:323–7. doi: 10.1001/archderm.136.3.323. [DOI] [PubMed] [Google Scholar]
- 21.Carroll OM, Bryan PA, Robinson RJ. Stevens Johnsons syndrome associated with long acting sulfonamides. JAMA. 1966;195:691–3. [PubMed] [Google Scholar]
- 22.Ting HC, Adam BA. Stevens-Johnson syndrome. A review of 34 cases. Int J Dermatol. 1985;24:587–91. doi: 10.1111/j.1365-4362.1985.tb05857.x. [DOI] [PubMed] [Google Scholar]


