Abstract
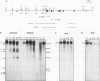
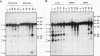
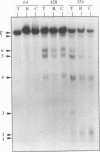
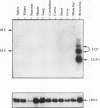
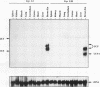
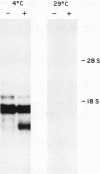
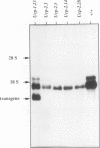
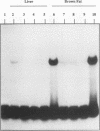
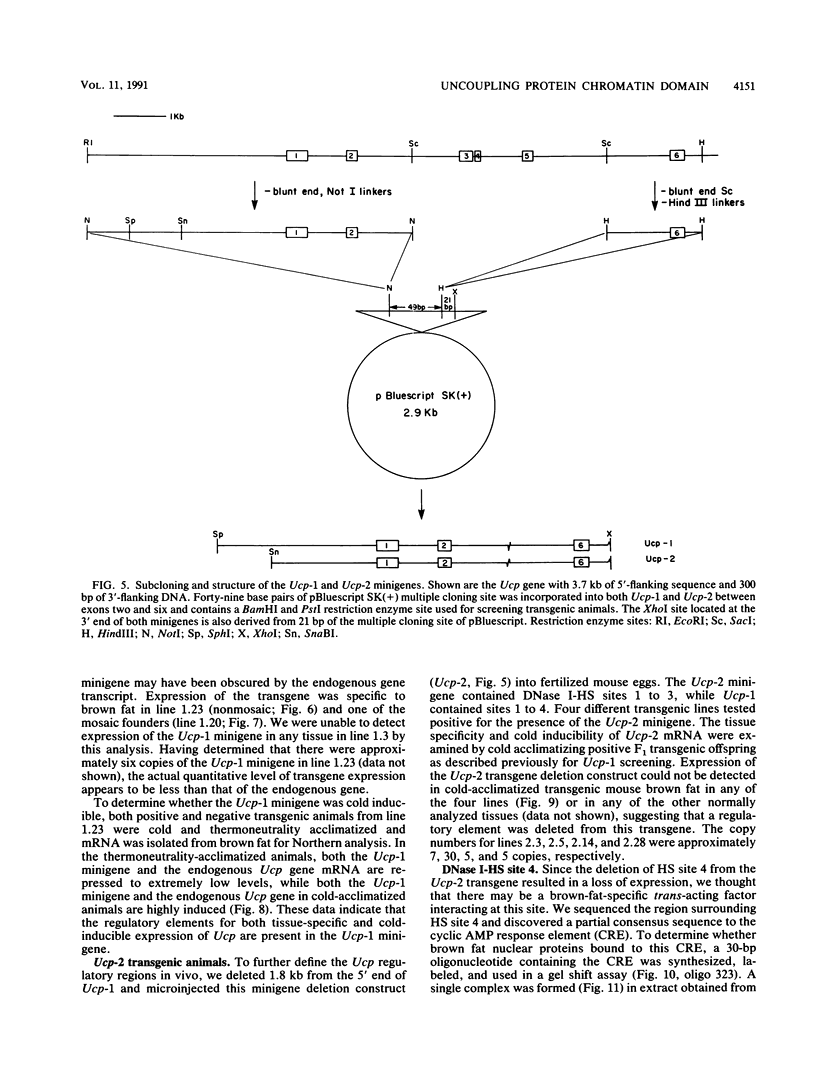
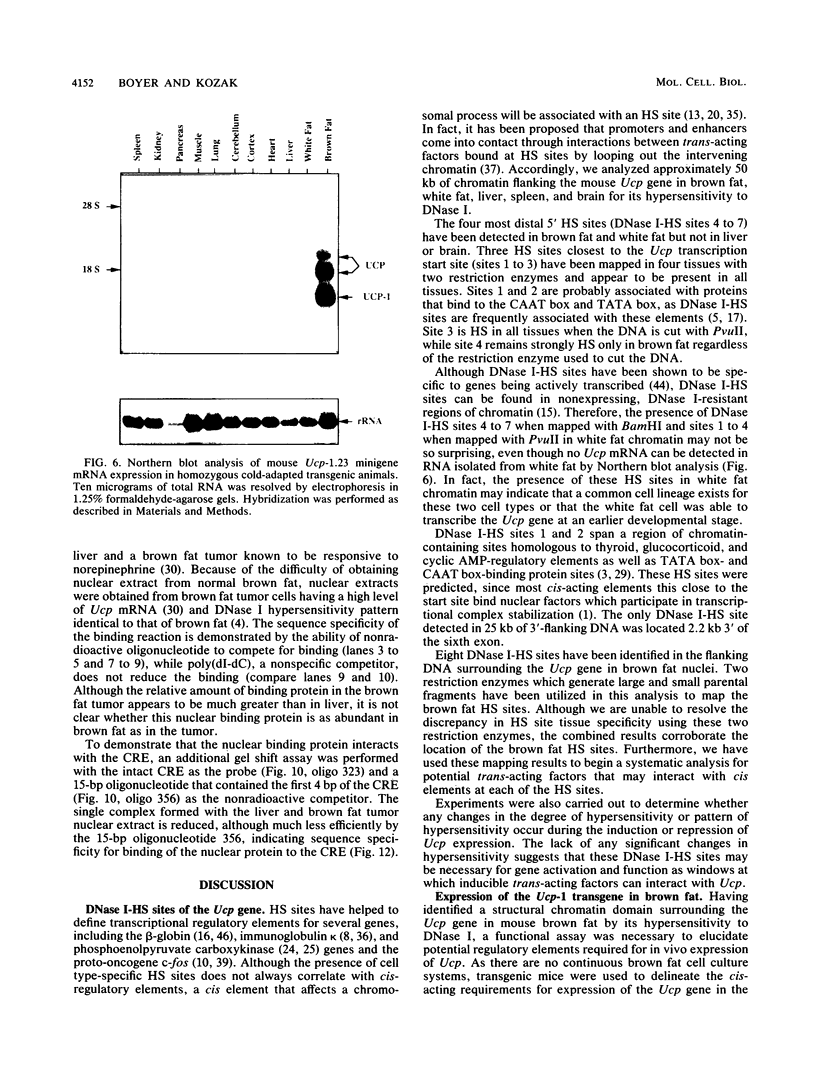
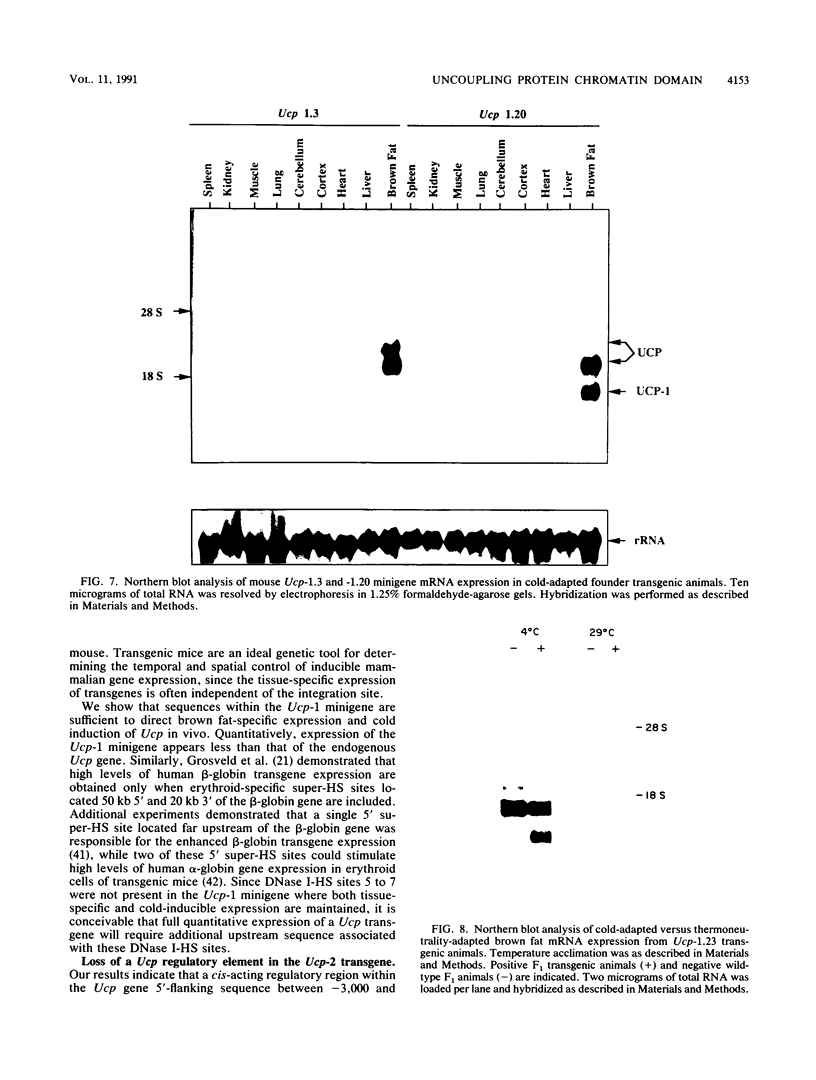
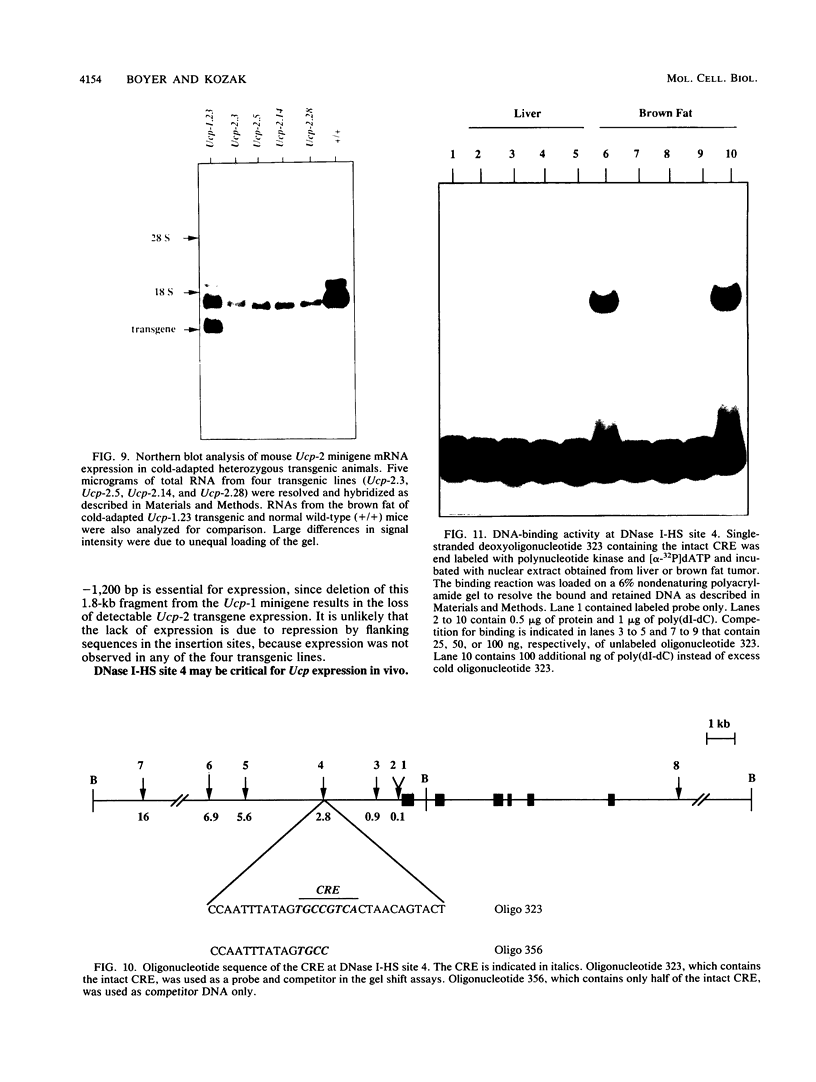
The mitochondrial uncoupling protein gene is rapidly induced in mouse brown fat following cold exposure. To identify cis-regulatory elements, approximately 50 kb of chromatin surrounding the uncoupling protein gene was examined for its hypersensitivity to DNase I. Seven DNase I-hypersensitive sites were identified in the 5'-flanking DNA, and one site was identified in the 3'-flanking DNA. Transgenic mice with an uncoupling protein minigene were generated by microinjection of fertilized eggs with a transgene containing 3 kb of 5'-flanking DNA and 0.3 kb of 3'-flanking DNA. Expression of the transgene is restricted to brown fat and is cold inducible. Four additional transgenic lines were generated with a second transgene containing a 1.8-kb deletion in the 5'-flanking DNA, and expression of this minigene is absent in all tissues analyzed. A DNase I-hypersensitive site located in the 1.8-kb deletion contains a cyclic AMP response element that binds a brown fat tumor enriched nuclear factor. On the basis of these observations, we propose that a cis-acting regulatory sequence between -3 and -1.2 kb of the 5'-flanking region, possibly at a DNase I-hypersensitive site, is required for controlling uncoupling protein expression in vivo.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham L. J., Bradshaw A. D., Shiels B. R., Northemann W., Hudson G., Fey G. H. Hepatic transcription of the acute-phase alpha 1-inhibitor III gene is controlled by a novel combination of cis-acting regulatory elements. Mol Cell Biol. 1990 Jul;10(7):3483–3491. doi: 10.1128/mcb.10.7.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard T. The ultrastructural differentiation of brown adipose tissue in the rat. J Ultrastruct Res. 1969 Nov;29(3):311–322. doi: 10.1016/s0022-5320(69)90109-9. [DOI] [PubMed] [Google Scholar]
- Bouillaud F., Ricquier D., Mory G., Thibault J. Increased level of mRNA for the uncoupling protein in brown adipose tissue of rats during thermogenesis induced by cold exposure or norepinephrine infusion. J Biol Chem. 1984 Sep 25;259(18):11583–11586. [PubMed] [Google Scholar]
- Bushel P., Rego K., Mendelsohn L., Allan M. Correlation between patterns of DNase I-hypersensitive sites and upstream promoter activity of the human epsilon-globin gene at different stages of erythroid development. Mol Cell Biol. 1990 Mar;10(3):1199–1208. doi: 10.1128/mcb.10.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chung S. Y., Folsom V., Wooley J. DNase I-hypersensitive sites in the chromatin of immunoglobulin kappa light chain genes. Proc Natl Acad Sci U S A. 1983 May;80(9):2427–2431. doi: 10.1073/pnas.80.9.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Deschamps J., Meijlink F., Verma I. M. Identification of a transcriptional enhancer element upstream from the proto-oncogene fos. Science. 1985 Dec 6;230(4730):1174–1177. doi: 10.1126/science.3865371. [DOI] [PubMed] [Google Scholar]
- Doglio A., Dani C., Grimaldi P., Ailhaud G. Growth hormone regulation of the expression of differentiation-dependent genes in preadipocyte Ob1771 cells. Biochem J. 1986 Aug 15;238(1):123–129. doi: 10.1042/bj2380123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer R. F. Morphological features of brown adipose cell maturation in vivo and in vitro. Am J Anat. 1968 Sep;123(2):255–282. doi: 10.1002/aja.1001230203. [DOI] [PubMed] [Google Scholar]
- Elgin S. C. The formation and function of DNase I hypersensitive sites in the process of gene activation. J Biol Chem. 1988 Dec 25;263(36):19259–19262. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Forrester W. C., Takegawa S., Papayannopoulou T., Stamatoyannopoulos G., Groudine M. Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin-expressing hybrids. Nucleic Acids Res. 1987 Dec 23;15(24):10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritton H. P., Sippel A. E., Igo-Kemenes T. Nuclease-hypersensitive sites in the chromatin domain of the chicken lysozyme gene. Nucleic Acids Res. 1983 Jun 11;11(11):3467–3485. doi: 10.1093/nar/11.11.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
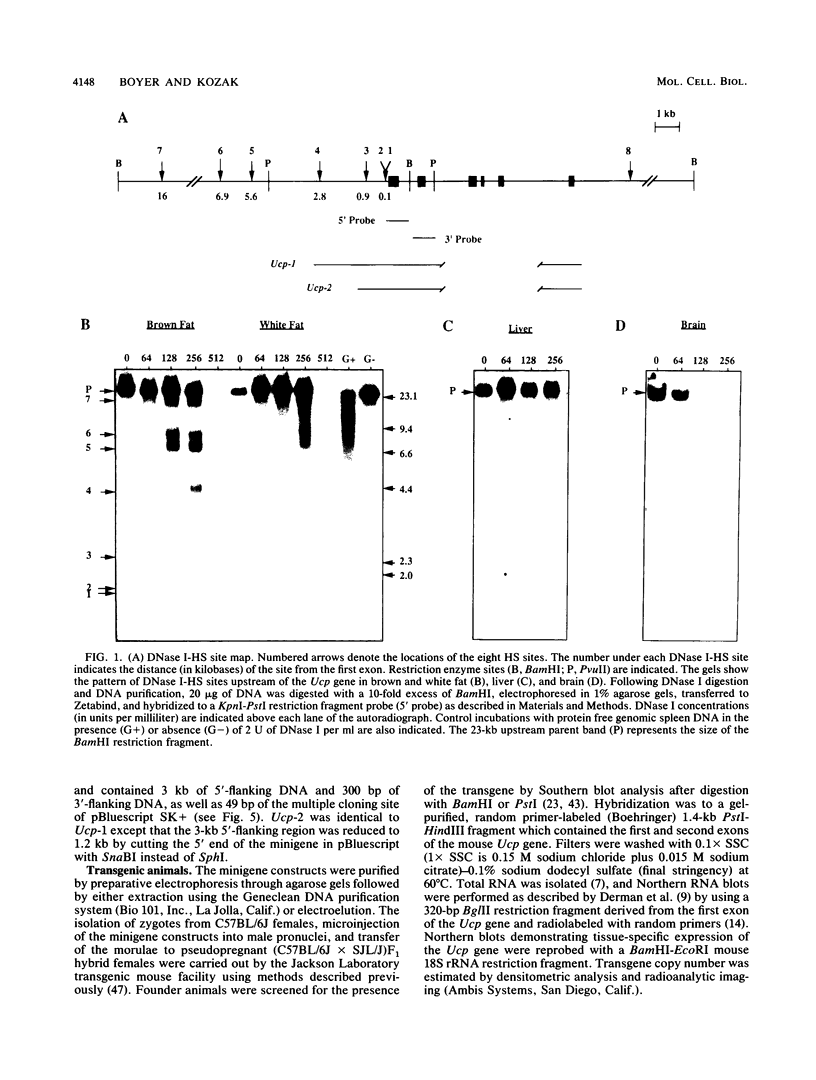
- Gross D. S., Garrard W. T. Nuclease hypersensitive sites in chromatin. Annu Rev Biochem. 1988;57:159–197. doi: 10.1146/annurev.bi.57.070188.001111. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Guthrie P. D., Freeman M. R., Liao S. T., Chung L. W. Regulation of gene expression in rat prostate by androgen and beta-adrenergic receptor pathways. Mol Endocrinol. 1990 Sep;4(9):1343–1353. doi: 10.1210/mend-4-9-1343. [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Fournier R. E., Chalkley R. Extinction of phosphoenolpyruvate carboxykinase gene expression is associated with loss of a specific chromatin-binding protein from a far upstream domain. Mol Cell Biol. 1990 Jul;10(7):3782–3787. doi: 10.1128/mcb.10.7.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip Y. T., Poon D., Stone D., Granner D. K., Chalkley R. Interaction of a liver-specific factor with an enhancer 4.8 kilobases upstream of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1990 Jul;10(7):3770–3781. doi: 10.1128/mcb.10.7.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki K., Sukhatme V. P., Shubeita H. E., Chien K. R. Alpha- and beta-adrenergic stimulation induces distinct patterns of immediate early gene expression in neonatal rat myocardial cells. fos/jun expression is associated with sarcomere assembly; Egr-1 induction is primarily an alpha 1-mediated response. J Biol Chem. 1990 Aug 15;265(23):13809–13817. [PubMed] [Google Scholar]
- Jacobsson A., Nedergaard J., Cannon B. alpha- and beta-adrenergic control of thermogenin mRNA expression in brown adipose tissue. Biosci Rep. 1986 Jul;6(7):621–631. doi: 10.1007/BF01114756. [DOI] [PubMed] [Google Scholar]
- Jacobsson A., Stadler U., Glotzer M. A., Kozak L. P. Mitochondrial uncoupling protein from mouse brown fat. Molecular cloning, genetic mapping, and mRNA expression. J Biol Chem. 1985 Dec 25;260(30):16250–16254. [PubMed] [Google Scholar]
- Kozak L. P., Britton J. H., Kozak U. C., Wells J. M. The mitochondrial uncoupling protein gene. Correlation of exon structure to transmembrane domains. J Biol Chem. 1988 Sep 5;263(25):12274–12277. [PubMed] [Google Scholar]
- Lefkowitz R. J., Caron M. G. Adrenergic receptors. Models for the study of receptors coupled to guanine nucleotide regulatory proteins. J Biol Chem. 1988 Apr 15;263(11):4993–4996. [PubMed] [Google Scholar]
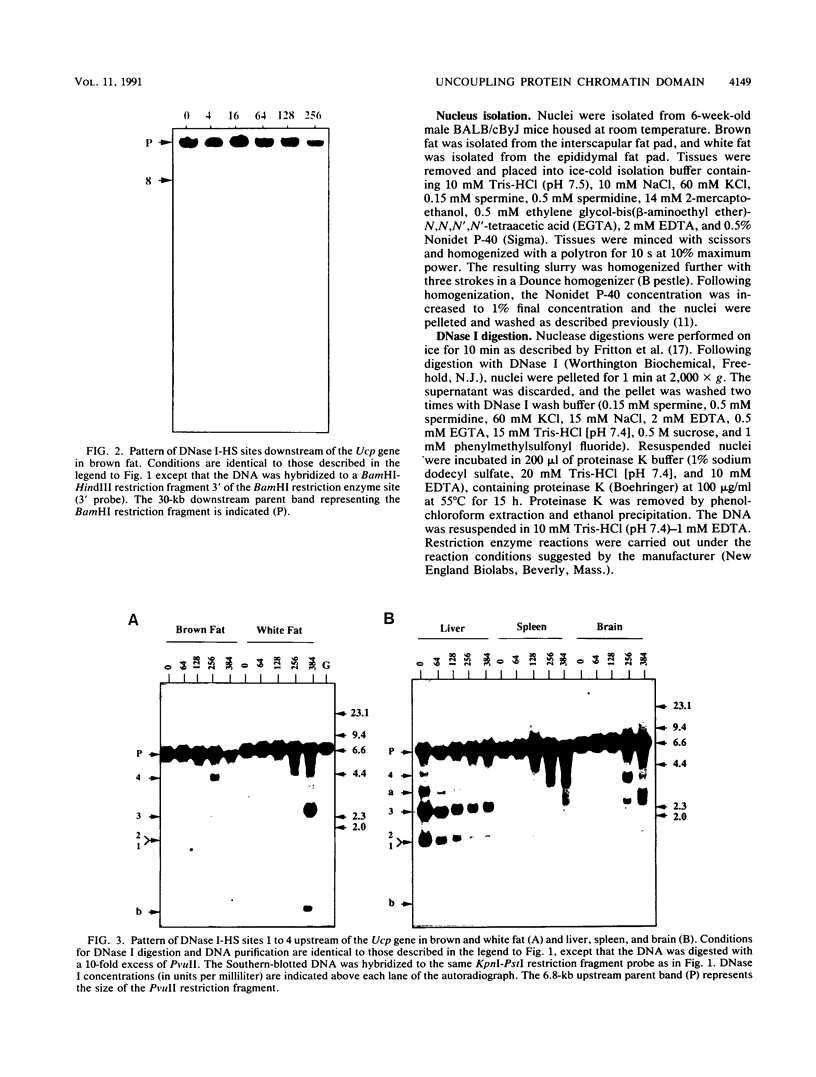
- MacDonald R. J., Hammer R. E., Swift G. H., Ornitz D. M., Davis B. P., Palmiter R. D., Brinster R. L. Tissue-specific expression of pancreatic genes in transgenic mice. Ann N Y Acad Sci. 1986;478:131–146. doi: 10.1111/j.1749-6632.1986.tb15527.x. [DOI] [PubMed] [Google Scholar]
- Maire P., Wuarin J., Schibler U. The role of cis-acting promoter elements in tissue-specific albumin gene expression. Science. 1989 Apr 21;244(4902):343–346. doi: 10.1126/science.2711183. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Locke R. M. Thermogenic mechanisms in brown fat. Physiol Rev. 1984 Jan;64(1):1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Nitsch D., Stewart A. F., Boshart M., Mestril R., Weih F., Schütz G. Chromatin structures of the rat tyrosine aminotransferase gene relate to the function of its cis-acting elements. Mol Cell Biol. 1990 Jul;10(7):3334–3342. doi: 10.1128/mcb.10.7.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parslow T. G., Granner D. K. Chromatin changes accompany immunoglobulin kappa gene activation: a potential control region within the gene. Nature. 1982 Sep 30;299(5882):449–451. doi: 10.1038/299449a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986 Aug 21;322(6081):697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Reitman M., Felsenfeld G. Developmental regulation of topoisomerase II sites and DNase I-hypersensitive sites in the chicken beta-globin locus. Mol Cell Biol. 1990 Jun;10(6):2774–2786. doi: 10.1128/mcb.10.6.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz M., Neuberg M., Kurz C., Bravo R., Müller R. Regulation of c-fos transcription in mouse fibroblasts: identification of DNase I-hypersensitive sites and regulatory upstream sequences. EMBO J. 1985 Dec 30;4(13B):3711–3716. doi: 10.1002/j.1460-2075.1985.tb04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricquier D., Bouillaud F., Toumelin P., Mory G., Bazin R., Arch J., Pénicaud L. Expression of uncoupling protein mRNA in thermogenic or weakly thermogenic brown adipose tissue. Evidence for a rapid beta-adrenoreceptor-mediated and transcriptionally regulated step during activation of thermogenesis. J Biol Chem. 1986 Oct 25;261(30):13905–13910. [PubMed] [Google Scholar]
- Ryan T. M., Behringer R. R., Martin N. C., Townes T. M., Palmiter R. D., Brinster R. L. A single erythroid-specific DNase I super-hypersensitive site activates high levels of human beta-globin gene expression in transgenic mice. Genes Dev. 1989 Mar;3(3):314–323. doi: 10.1101/gad.3.3.314. [DOI] [PubMed] [Google Scholar]
- Ryan T. M., Behringer R. R., Townes T. M., Palmiter R. D., Brinster R. L. High-level erythroid expression of human alpha-globin genes in transgenic mice. Proc Natl Acad Sci U S A. 1989 Jan;86(1):37–41. doi: 10.1073/pnas.86.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
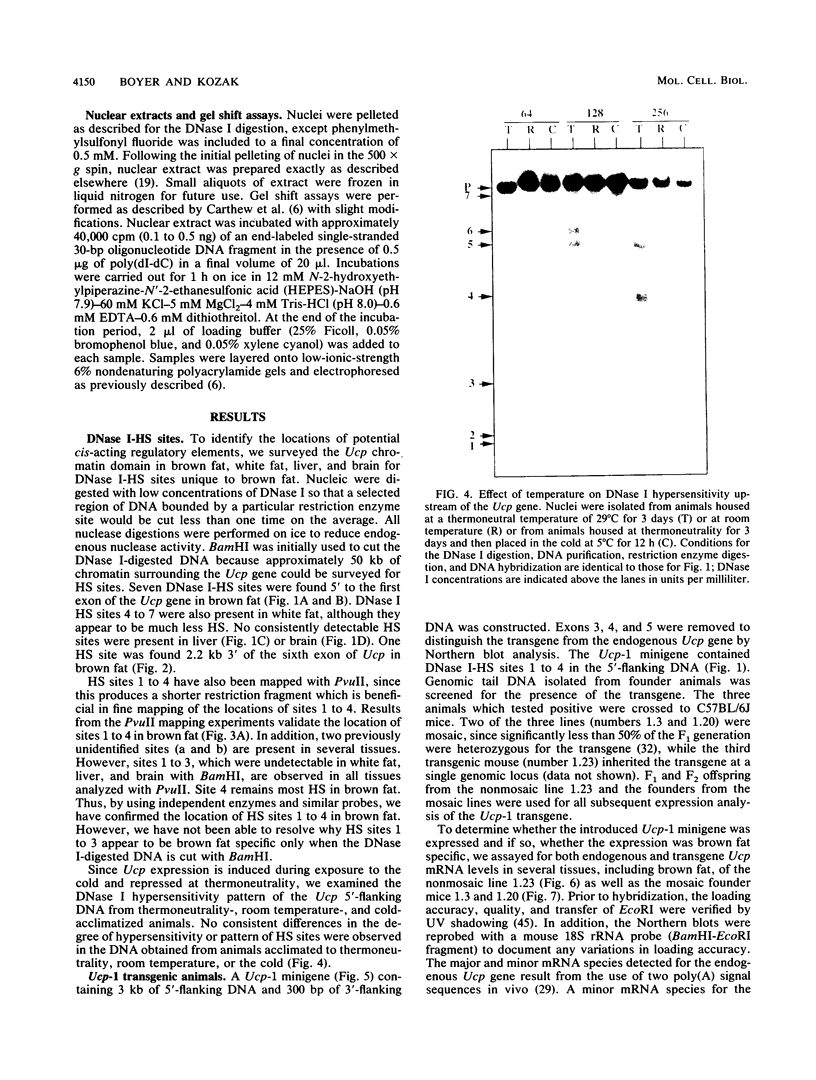
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Thurston S. J., Saffer J. D. Ultraviolet shadowing nucleic acids on nylon membranes. Anal Biochem. 1989 Apr;178(1):41–42. doi: 10.1016/0003-2697(89)90353-9. [DOI] [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T. E., Hoppe P. C., Jollick J. D., Scholl D. R., Hodinka R. L., Gault J. B. Microinjection of a rabbit beta-globin gene into zygotes and its subsequent expression in adult mice and their offspring. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6376–6380. doi: 10.1073/pnas.78.10.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]