Abstract
Malaria control in India has occupied high priority in health sector consuming major resources of the Central and State governments. Several new initiatives were launched from time to time supported by foreign aids but malaria situation has remained static and worsened in years of good rainfall. At times malaria relented temporarily but returned with vengeance at the local, regional and national level, becoming more resilient by acquiring resistance in the vectors and the parasites. National developments to improve the economy, without health impact assessment, have had adverse consequences by providing enormous breeding grounds for the vectors that have become refractory to interventions. As a result, malaria prospers and its control is in dilemma, as finding additional resources is becoming difficult with the ongoing financial crisis. Endemic countries must contribute to make up the needed resources, if malaria is to be contained. Malaria control requires long term planning, one that will reduce receptivity and vulnerability, and uninterrupted financial support for sustained interventions. While this seems to be a far cry, the environment is becoming more receptive for vectors, and epidemics visit the country diverting major resources in their containment, e.g. malaria, dengue and dengue haemorrhagic fevers, and Chikungunya virus infection. In the last six decades malaria has taken deep roots and diversified into various ecotypes, the control of these ecotypes requires local knowledge about the vectors and the parasites. In this review we outline the historical account of malaria and methods of control that have lifted the national economy in many countries. While battles against malaria should continue at the local level, there is a need for large scale environmental improvement. Global Fund for AIDS, Tuberculosis and Malaria has provided huge funds for malaria control worldwide touching US$ 2 billion in 2011. Unfortunately it is likely to decline to US$ 1.5 billion in the coming years against the annual requirement of US$ 5 billion. While appreciating the foreign assistance, we wish to highlight the fact that unless we have internal strength of resources and manpower, sustained battles against malaria may face serious problems in achieving the final goal of malaria elimination.
Keywords: Drug resistance, malaria elimination, malaria profile, malaria vectors, MDG, MPO, urban malaria scheme
Introduction
Malaria in India is an ancient disease, killing 1 million and 100 million people suffered from bouts of malaria annually in the British India. Malaria situation remained hopeless due to its ravages and at the time of independence in 1947 about 75 million episodes and 0.8 million deaths were estimated to have occurred in normal years1. There are no estimates of deaths due to malaria superimposed by other infections, and periodical epidemics during favourable climatic conditions. For the first time in India in 1847 Surgeon General T.E. Dempster used spleen to measure malaria in the community2. The role of Anopheles mosquitoes in malaria transmission remained elusive until Ronald Ross while serving in the army succeeded in demonstrating malaria parasite in the mid gut wall of the Anopheles mosquito on 20th August 18973. The following year Ross demonstrated in Calcutta (now Kolkata) the malaria parasite cycle in the birds. For his work on malaria, Ronald Ross was awarded the Nobel Prize in Medicine or Physiology in 1902. The first field experiment on malaria control was undertaken at the military cantonment at Mian Mir (near Lahore, now in Pakistan) from 1902 to 1909; although the field experiment did not succeed in demonstrating malaria control, but the principle of malaria control remained valid and required further work4. Later the techniques of larval control and space spraying were applied in cantonment areas and sites of economic importance, although these made no difference in the overall picture of malaria in India. At the time of independence in 1947, malaria remained a major concern as it was the single biggest misfortune with which Indians had to live5. Malaria led to complete collapse of all economic development activities in agriculture, industry, education, commerce etc6. Indian agriculture was worse hit, and unable to support the growing population with imminent disasters of famine. Paul H. Muller discovered dichlorodiphenyltrichloroethane (DDT) residual action in killing vectors of diseases. For this discovery Paul Muller was awarded Nobel Prize in Medicine or Physiology in 19487. This discovery of residual action of DDT led to scientific basis of malaria control. Soon after World War II, DDT was released for use in public health, and DDT has been in use since 1946. Initial trials in southern Indian States were highly successful, and wherever DDT was sprayed malaria was wiped out. This unprecedented success led to euphoria among all ranks of malaria workers. Following the initial trials, the National Malaria Control Programme (NMCP) was launched in 1953. While malaria control was progressing satisfactorily, NMCP was re-designated as the National Malaria Eradication Programme (NMEP) to eradicate malaria before the onset of insecticide resistance in malaria vectors. The success in malaria eradication was so spectacular that India was heading towards malaria eradication. At that time in 1963, there were an estimated 0.1 million cases and deaths due to malaria were completely eliminated. Since malaria was disappearing, the Government of India de-emphasized all work on malaria including research on malaria. At that time, wiping out the residual malaria foci required intensified campaign to detect and eliminate each case of malaria; instead NMEP moved into the complacency8. In absence of any control measures, malaria started to raise its ugly head all over the country, and resurgence started building up in hard core areas and towns, resulting in widespread malaria resurgence1,9. Eventually malaria occupied new niches and intensified establishing new ecotypes viz., rural malaria, urban and peri-urban malaria, industrial malaria, irrigation malaria, forest malaria, migration malaria, border malaria10. Each ecotype comprised vast terrain with distinct malaria ecology. At one time umbrella approach to malaria control (i.e. spray DDT regardless ecotype) was no longer successful in decimating transmission. Instead malaria control now required in-depth knowledge of malaria epidemiology at the local level for a focused attack on malaria.
Urban malaria scheme (UMS): At the time of launching of the NMCP in 1953 and NMEP in 1958, urban malaria was considered a trivial problem. Malaria control was, therefore, assigned to the local governments, municipalities and corporations. Covell11 had already developed strategies for malaria control in the towns. Urban malaria control required to implement the recommendations contained in “Malaria Control in Bombay”. During the decades that followed towns witnessed exponential growth, haphazard development, settlements in low lying areas, mushrooming of hutments, tropical aggregation of labour, erratic water supply and poor drainage, and water logging, etc12. Malaria cases were seen multiplying with majority of cases treated by the private sector. Malaria was diffusing to rural areas that were freed from the disease13. To contain the malaria outbreaks in urban areas, Urban Malaria Scheme (UMS) was launched in 1970-1971 in 131 towns with >40,000 populations (later increased to >50,000 population) and two or more annual parasite incidence (API)14. These towns were identified on the basis of 10 per cent annual blood examination rate (ABER) and 2API. Implementation of UMS was very slow. For example, 23 towns were brought under UMS in 1971-1972 and another five towns in 1972-1973; another 28 in 1976-1977; 38 in 1977-1978; 37 in 1978-1979; 12 in 1979-1980 and 17 in 1980-1981 making it a total of 131 towns (total population 115.1 million in 19 States and union territories), and since then no new town has been added under the urban malaria scheme15. There are general guidelines by the National Vector Borne Disease Control Programme (NVBDCP) for adoption of municipal bylaws and building bylaws but their implementation is wanting in almost all towns. Malaria control guidelines provide the control of vectors by source reduction, filling and leveling, channelizing, de-silting, de-weeding, periodical cleaning of drains, solid waste disposal, sanitation, empty water container once in a week, recurrent anti-larval measures like Temephos and Fenthion; biological control i.e. application of Guppy and Gambusia fishes and bio-larvicides (Bacillus thuringiensis & Bacillus sphaericus); minor engineering works; malathion thermal fogging, indoor residual spraying in peri-urban settlements; repellents-DEET/Neem oil, coils, mats, vaporizers; anti-parasitic measures, malaria clinics for diagnosis and early treatment; information, malaria month, education and communication (IEC) campaigns, etc. The technology of malaria control in towns was not fully developed, particularly in detecting An. stephensi breeding due to lack of trained and experienced staff and inadequacy of funds14–16. UMS was not reviewed and strengthened in the background of expansion of towns and unprecedented growth of population throughout the country. Existing towns were growing horizontally in an unplanned manner often in low lying lands without the adequate provision of drainage, water supply, sanitation, solid waste disposal as required in municipal and adjacent areas12.
Inadequacy in urban planning and interventions led to epidemics of communicable diseases e.g. diarrhoeal diseases, malaria, dengue, chikungunya, jaundice etc17. There was no active surveillance in urban areas and private sector flourished and masked the outbreaks. Data of the towns under the UMS indicated that malaria situation was fast deteriorating and required immediate remedial measures. The existing tools and strategy were failing in the containment of malaria. NVBDCP has further reduced the towns from 131 to 28 as the most malarious towns (Fig. 1), and also Programme's thrust in control of urban malaria. These towns have migratory labour population and fall in the belt of industrial development (personal communication from R.S. Sharma, NVBDCP, presentation in Jabalpur 2011)16. Important disease vectors in these towns are: An. stephensi and An. culicifacies (malaria), Aedes aegypti [dengue & dengue haemorrhagic fever (DHF); and Chikungunya]; and Culex quinquefasciatus (lymphatic filariasis), and mosquito nuisance is very high and unbearable in most situations.
Fig. 1.
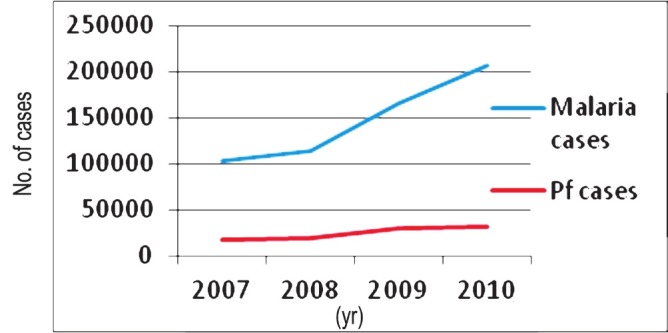
Rising trend of malaria cases [total and Pf (Plasmodium falciparum)] cases in towns under the Urban Malaria Scheme (Source: NVBDCP, India16.
Modified Plan of Operation (MOP): The worsening malaria situation forced the government to abandon eradication and revert to malaria control. Thus in 1977 the Modified Plan of Operation (MPO) was launched with the main objective to (i) prevent malaria mortality and morbidity, (ii) protect the green revolution, and (iii) retain the achievements gained from malaria eradication18. Under the MPO, a focused attack on malaria was made country-wide and NMEP was strengthened through research and development. At that time an epidemiological study revealed that bulk of P. falciparum cases was confined to northeastern States. Besides chloroquine resistance first detected from Karbi Anglong district in Assam19, these parasites continued to multiply and spread to occupy larger geographical territories. At that time P. falciparum was largely confined to some districts in the north eastern region. Therefore, it was still possible to contain and liquidate P. falciparum from the northeastern States, and thus save the mainland from P. falciparum. Swedish International Development Agency (SIDA) financed the Plasmodium falciparum Containment Progrmme (PfCP) starting in 197720. The focus of PfCP was on 28 million populations in the northeast, but over the years P. falciparum and chloroquine resistant mutant strains steadily spread to the mainland and gradually covered >100 million populations21. PfCP failed in the containment of P. falciparum and drug resistant malaria, and was terminated in 1988 after 11 years of field operations (Fig. 2). In general, malaria situation had deteriorated all over the country, malaria outbreaks were reported from many regions and malaria deaths started visiting the country as a regular feature of the NVBDCP1,9,22–25. At that time malaria control tools had blunted and become unreliable coupled with the chronic problems of shortages in supplies and lack of experienced staff26,27. During the MPO malaria outbreaks had become commonplace, vector and drug resistance continued to intensify, and malaria occupied the centre stage among the prevalent communicable diseases28,29. At the turn of the present century, new technologies raised hopes for malaria control30. Additional funds were made available through the Global Fund for AIDS, Tuberculosis and Malaria (GFATM). This review article brings out missed opportunities by the NMEP (now named National Vector Borne Disease Control Programme, NVBDCP), and road map for the future malaria elimination programme.
Fig. 2.
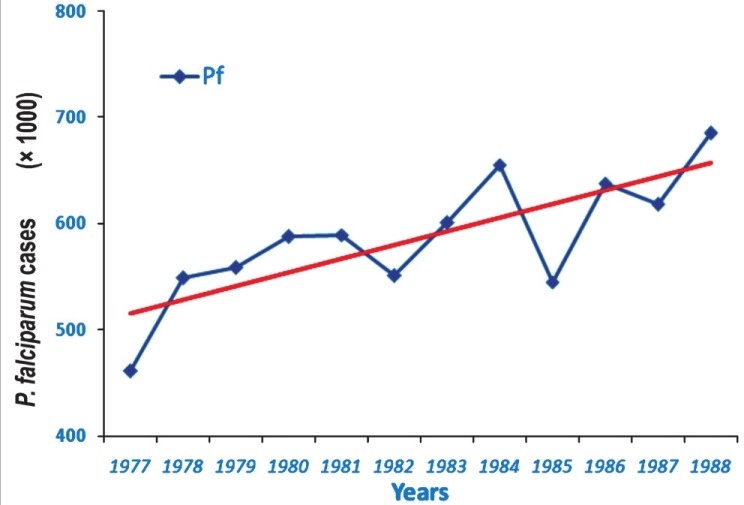
Showing yearly P. falciparum cases for the duration of P. falciparum Containment Programme (1977 to 1988). There was a rising trend of Pf cases (see the trend line) and the spread of chloroquine resistance in P. falciparum(Source: NVBDCP, India)16.
Malaria profile
In the world 99 countries have ongoing transmission, 43 countries recorded decreases of more than 50 per cent between 2000 and 2010, and another eight countries recorded decrease of more than 25 per cent cases. There were 216 million cases of malaria worldwide in 2010, 81 per cent of these in the WHO Africa Region. An estimated 3.3 billion people were at risk of malaria in 201031. An estimated 6,55,000 persons died of malaria in 2010, 86 per cent of these were children under 5 yr, and 91 per cent of malaria deaths occurred in the WHO African Region. A routine activity of the NVBDCP is to organize active and passive surveillance, collate these data from all the States and use this information in the planning and implementation of the malaria control countrywide. Fig. 3 gives the malaria incidence in India since 1970, long enough time to reveal malaria trends. Malaria situation continues to be bad enough raising health crisis as it not only causes high morbidity and mortality but also retards economic development in the country29. In the background of prevailing malaria situations priority interventions with modern technologies are required32. The NVBDCP's epidemiological data for 2010 from the predominantly tribal States with persistent malaria transmission show high API, slide positivity rate (SPR) and very high Pf%. viz., Odisha (population 42 million), Chhattisgarh (population 25.5 million), Jharkhand (population 33 million) and Madhya Pradesh (population 72.6 million)16. These States with a total population of 173.1 million (out of a total of the country's 1.21 billion population) represent 14.3 per cent population. Northeastern States of India always had high P. falciparum including drug resistance, and nearly 4 per cent of country's population in northeastern States contributes more than 10 per cent reported malaria cases. It may be noted that Pf is a killer parasite, and there is a growing resistance in Pf to antimalarials drugs33. Fig. 4 shows district-wise malaria indices of Odisha State in 2010. Mining is an important commercial activity in Odisha that attracts labour from all over the country, thus helping the parasites to disseminate. As per the NVBDCP (Fig. 3), malaria is showing a declining trend since the implementation of the MPO in 1977, the percentage of P. falciparum is rising steadily from about 10-15 per cent and gradually risen to 50 per cent. Two factors have combined to produce this picture viz., sensitivity of P. vivax and resistance of P. falciparum to chloroquine. NVBDCP is collecting 80 to 110 million blood smears annually. Based on the blood smear examination, P. vivax and P. falciparum cases are reported each year. For example, in 2010, 108,679,429 blood smears were examined and 1,599,986 were found positive, of which 834,364 were P. falciparum, and 1,018 malaria deaths (malaria death requires demonstration of malaria parasite in the peripheral blood)16. There are several interesting features of this reporting i.e. (i) malaria situation is almost stable with minor downward disease trend, (ii) there is no information on the sex and age of the patients, (iii) mixed infections are not reported, the percentage varies from 30-40 and not <2 as had been reported through routine surveillance data34–36, (iv) fate of >100 million fever cases negative for malaria is not known, and (v) deaths due to malaria need a more realistic case definition. Malaria incidence figures have been contested by research institutions, but the system has continued to follow the old and unreliable surveillance system and reporting. There are more recent studies reporting malaria morbidity and mortality figures in India. The difference in the reported and estimated cases is large enough to question the very system of surveillance and reporting. Epidemiological indices of malaria in Madhya Pradesh revealed a very dismal picture of malaria (Fig. 5). An international team of experts reported a very high incidence of malaria in pregnancy (MiP)37. For example in Madhya Pradesh (rural) 183,000-1.5 million per year contract malaria in pregnancy, and result in 73,000-629,000 lost foetuses and 1,500 to 12,600 maternal deaths. Authors state “Plausible estimate of 220,000 MiP cases per year (136,000-305,000), 95,800 lost foetuses (56,800-147,600) and 1,000 maternal deaths (650-1,600)”37.
Fig. 3.
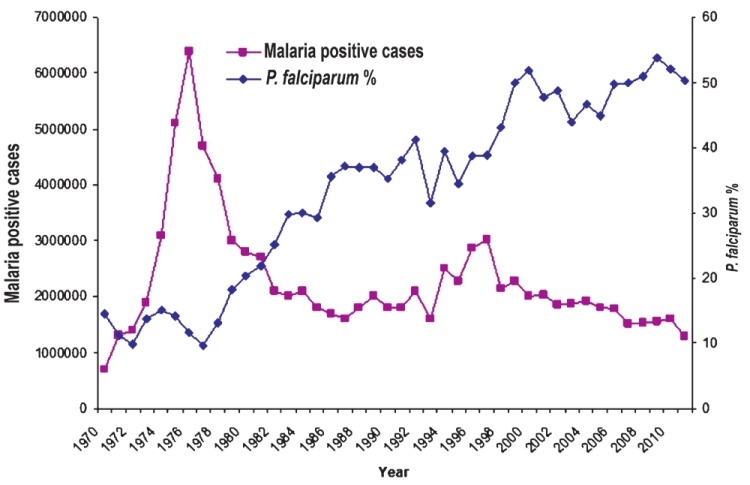
Malaria cases in India (1970-2011) as recorded by the NVBDCP. Cases started rising in 1970, peaking in 1976 to 6.45 million cases and thereafter following the implementation of the Modified Plan of Operation in 1977, malaria cases declined but mainly P. vivax malaria due to its sensitivity to chloroquine. P. falciparum % was about 10 in 1977 but due to fall in vivax malaria, this % has risen to about 50 and the parasite has become mono- to multi-drug resistant (Source: Ref. 28 reproduced with permission).
Fig. 4.
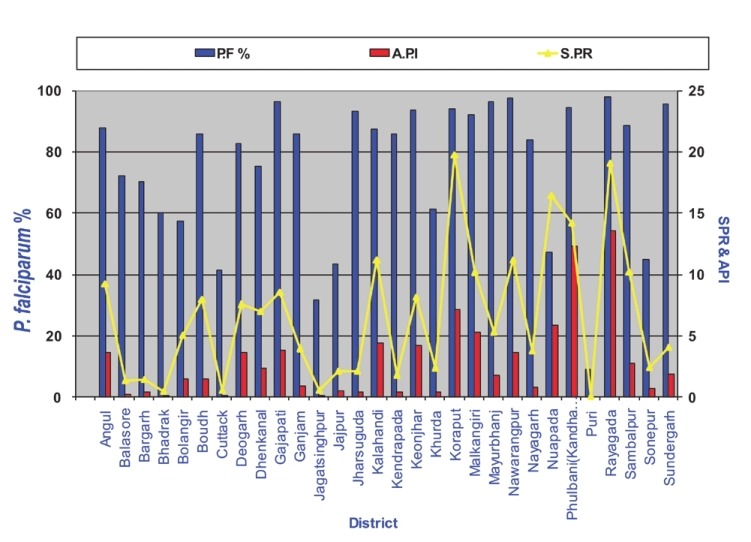
District-wise annual parasite incidence (API), slide positivity rate (SPR) and Pf% in Odisha State in 2010 (Source: NVBDCP, India)16.
Fig. 5.
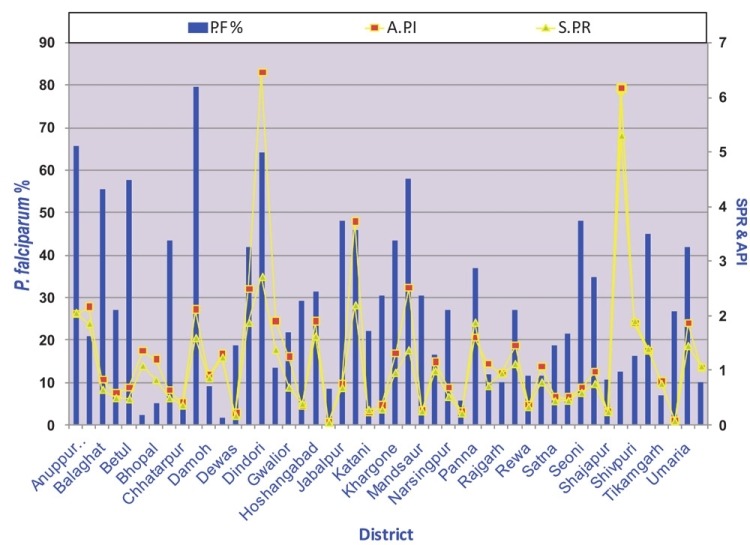
Showing epidemiological data (API.SPR and Pf%) of Madhya Pradesh in 2010. (Source: NVBDCP, India)16.
Estimates of malaria mortality38 show “205,000 malaria deaths per year in India before age 70 yr (55 000 in early childhood, 30 000 at ages 5-14 yr, 120 000 at ages 15-69 yr); 1·8 per cent cumulative probability of death from malaria before age 70 yr”. Sarakar39 estimated approximately 180 million malaria cases, half of these P. falciaprum. WHO reports 28 million cases and 38,000 deaths in SEA (South East Asia) Region40, 70 per cent of these occur in India. P. vivax was the dominant infection in India but in the last 2-3 decades P. falciparum has been rising in all areas of the country. The proportion of P. falciparum varies seasonally and from place to place. P. vivax that used to be 80 per cent or more has come down to 50 per cent. Vivax malaria was also considered to be innocuous infection and all deaths due to malaria were due to P. falciparum. P. vivax (Pv) produces relapses and some people have to suffer several rounds of relapses unless the parasite is removed from the body by giving primaquine. But primaquine is a toxic drug and should not be given to G6PD deficient cases, pregnant women and children under 5 yr16. In the last decade, Pv is also showing resistance to chloroquine and short, medium and long term relapses, and cases of severe malaria in Pv infections41. There are several reports of severe malaria caused by P. vivax including deaths42–49. Therefore, malaria treatment should not be delayed and appropriate treatment given to prevent patient becoming severe before developing life threatening illness.
Drug resistant malaria
Chloroquine resistance in P. falciparum was first detected from Karbi Anglong district in Assam19. The problem of drug resistance is one of the most serious obstacles in malaria control33. In India, chloroquine resistance in P. falciparum is widespread and to overcome this problem, drug policy was changed to sulphadoxine-pyrimethamine (SP) combination. But eventually resistance developed to SP drugs as well16. WHO recommended the use of Artemisinin+Lumefantrine combination therapy (ACT). The experience from malaria endemic countries in the control of malaria has been very encouraging. In India, ACT is a combination of artemisinin and sulphadoxine-pyrimethamine. The combination has a risk of failure in areas with SP resistance. Reduced sensitivity to this combination has already surfaced along Indo-Bangladesh border in northeast India40. It would be advisable to use ACT with other combination drugs recommended by the WHO. Fig. 6 shows that the problem of drug resistance started from Thai-Cambodia border in 1961, moved to Bangladesh, northeastern States, Odisha, Madhya Pradesh and worldwide chloroquine resistance originated from at least six centers in the world50. During this period malaria resurgence facilitated the multiplication of drug resistance foci that spread to the entire country. Chloroquine and SP resistance was detected in all countries of the South East Asia Region (SEAR). This Region was, therefore, recognized as the epicenter of drug resistant malaria. The drug policy to combat drug resistant malaria in India now recommends Artemisinin combination therapy (ACT) (AS+SP) combination in 117 districts that includes 50 high endemic districts of Andhra Pradesh, Chhattisgarh, Jharkhand, Madhya Pradesh and Odisha plus 67 in north eastern States. Additionally, 253 primary health centres (PHCs) of 46 districts were included on the basis of chloroquine resistance and the surrounding clusters of blocks16. In Sonapur, Assam (India), independent monitoring revealed poor response to all the antimalarials including the AS+SP combination16. Artemisinin combination therapy (ACT) is the most promising therapy for the treatment of malaria particularly drug resistant malaria. Successful treatment leads to reduction in malaria transmission as artemisinin has gametocytocidal action. As happened with chloroquine, artemisinin resistance in P. falciparum also originated from the Thai-Cambodia borders and has started spreading. WHO launched artemsinin containment programme but some resistant parasites escaped and started to multiply, and as a consequence resistant specimens have been found in Myanmar31 and Vietnam51. Artemisinin resistant parasites may follow the same route as the chloroquine and SP resistance. It is absolutely essential to prevent drug resistance against artemisinin, as this is the best drug in our armamentarium used in all malaria endemic countries of the world. Monitoring and containment of artemisinin resistance should be our foremost priority. WHO recommends that the use of artemisinin monotherapy must be stopped to prevent the build-up of resistance. Unfortunately, despite of WHO's recommendation 25 countries still allow the marketing of artemisinin motherapies, and 28 pharmaceutical companies continue to market these products31. The marketing of sub-standard and fake drugs estimated to be about 38 per cent in SEA Region continues to be a serious threat in preventing drug resistance and treatment failures52. P. vivax has remained susceptible to chloroquine but in the last two decades there are increasing reports of chloroquine resistance in P. vivax53. In the past, cases of severe malaria caused by P. vivax were rarely reported, but severe malaria cases are being encountered with increasing frequency in most countries with vivax malaria54,55. In India, resistance to chloroquine in P. vivax has been reported from several States, and cases of severe malaria have been reported with increasing frequency56–59. Vivax malaria is the major infection in India and its prevalence, biology, response to drugs, pattern of resistance and severity including deaths need to be investigated to develop an in-depth for a rational strategy in the management of vivax malaria.
Fig. 6.
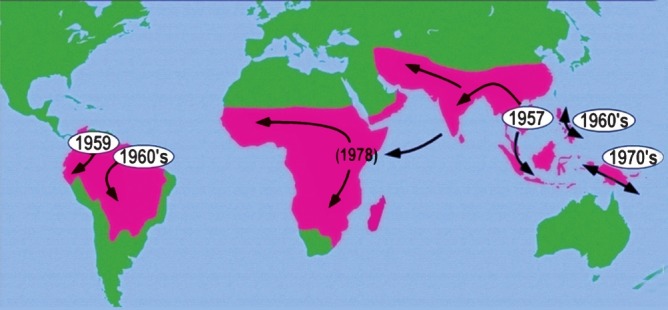
Chloroquine resistant P. falciparum originated from Thai Cambodia border in South East Asia region and spread worldwide from at least six known origins50.
Malaria vectors
Mosquito abundance depends on the availability of water bodies for breeding and also the ambient temperatures for development and adult survival. As a general rule, minimum temperature required for mosquito development of immature is between 8-10°C whereas, optimum temperature varies between 25-27°C. Minimum temperature for parasite development is between 14-19°C. P. vivax survives better at lower temperatures than P. falciparum. Temperatures exceeding 30°C progressively retard and at 40°C become lethal to both the vectors and parasite. Ambient temperatures are, therefore, critical in the transmission of malaria. At temperatures below 20°C extrinsic cycle of P. falciparum is arrested in the Anopheles mosquitoes; but P. vivax may be prevalent because of its tolerance of lower temperatures. Duration of extrinsic cycle decreases as temperatures increase from 21 to 27°C. Development of aquatic stages of the mosquito also depends on temperature. A higher temperature increases the number of blood meals taken by the mosquito, and also increases the egg laying frequency of the gravid female mosquitoes60,61. There are 58 Anopheline species, of which 6 are major and 2 secondary malaria vectors. Vector distribution map is given in Fig. 7. Of these vectors, three major malaria vectors (An. culicifacies, An. stephens, An. baimaii) are a formidable challenges in the control of malaria in India. Descriptions of these vectors with the challenges they offer are briefly described below.
Fig. 7.
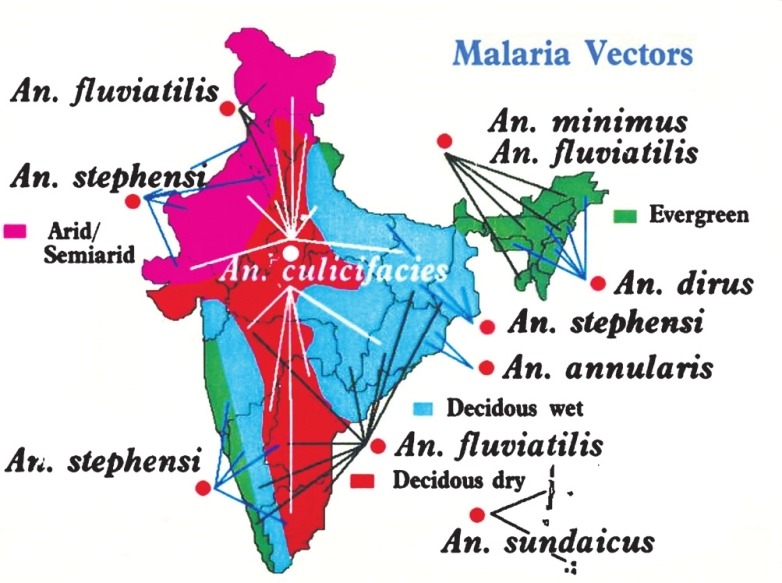
Distribution of major malaria vectors of India (Courtesy: NIMR, New Delhi) (Available from: http://www.mrcindia.org/contact.htm, assessed on May 13, 2012).
Anopheles culicifacies: Major rural malaria vector
An. culicifacies is the vector of rural malaria throughout the plains of India except urban areas. Epidemiological analysis of malaria revealed that An. culicifacies alone transmits 65 per cent malaria and 55 per cent of all P. falciparum annually16. Bulk of the malaria outbreaks/epidemics in India are caused by An. culicifacies. Control of An. culicifacies consumes about 80 per cent budget of the malaria control25,27. Since last six decades spraying to control An. culicifacies transmitted malaria has become an annual feature, and malaria returns year after year in more aggressive form62. Although DDT indoor residual spraying (IRS) was highly successful in malaria control but eventually the impact was transient inter alia due to insecticide resistance. Resistance to insecticides is not uniform but varies depending on the proportion and nature of resistant genes. Monitoring of resistance is incomplete but the available data revealed that An. culcifacies has become resistant to DDT in 141 districts, malathion in 71 districts and to pyrethroids in 21 districts26. Fighting rural malaria has led us to believe that control of An. culicifacies has remained a formidable challenge63. Furthermore, spraying is an important source of environmental contamination with associated hazards to men, animals and wildlife. Indoor residual spraying must be undertaken annually to keep the vectors under check, and eventually IRS produces diminishing returns, and set back in malaria control64.
An. culicifacies breeds in a variety of fresh dig pits, wells, coconut and casuarina plantation pits and in rock pools, fresh water accumulations in the fields, concrete reservoirs, tanks, rainwater pools, ponds, and grassy margins in sunlit field channels devoid of predators. It rapidly colonizes agricultural lands under development, road construction areas, new housing sites, industrial belts, etc. An. culicifacies has invaded new territories and participated in malaria transmission from areas where it was never reported e.g. Odisha, Assam. An. culicifacies is a complex of 5 sibling species designated as A, B, C, D and E65. Sibling species B is a non-vector, but all other sibling species have varying transmission potential. Species E is the most efficient vector of malaria, and also breeds in brackish water in Sri Lanka66,67. Larval biology of different sibling species differs depending on the species complex, and so also the adult biology and behaviour. It transmits P. falciparum and P. vivax, both susceptible and resistant strains to drugs. Sustained control of An. culicifacies below critical level of transmission is one of the most difficult tasks in malaria control. Unfortunately, current tools provide partial control, and that leaves behind hidden parasite reservoir in the community27,63,68. Information on the current status of vectors and their biology and ecology is outdated and has lost relevance.
Anopheles stephensi: Urban malaria vector
An. stephensi Liston is an important urban malaria vector in India. In early 1950s, An. stephensi distribution was limited to port cities. An. stephensi is an invasive species and has been expanding its territories. It has been incriminated in the transmission of malaria, often leading to epidemics, while variety mysorensis is rarely involved in malaria transmission and breeds in ponds in forested areas, pits, river margins and river-bed pools, margins of streams, and rain water collections. Mysorensis is a zoophilic species with poor parity rates. An. stephensi Type form breeding has been observed in stored water in homes, cement tanks, tankas, curing tanks, disused wells, jharias, earthen pots, room coolers, cisterns, roof gutters and stagnant rain water collections, etc. Type form and Mysorensis feed indoor and outdoor, on animals and human beings. Adults rest in wells, barrels, walls of tankas and hanging objects indoors. Finding adult resting specimen is difficult68.
Before the launching of the NMCP malaria was a problem in Bombay (now Mumbai) and a few more towns but its control was managed largely by source reduction and biological control11. This is an invasive species and has colonized a large number of urban areas. So far urban malaria control has received low priority and its control is organized by the local governments with token financial support from the NVBDCP. Urban malaria control requires human resource development, sound planning and strengthening of interventions, particularly taking note of the growing urbanization of rural India, industrialization and expansion of cities and peri-urban areas. Covell11 developed urban malaria control strategy and maintained efficient control of malaria in Mumbai. During the eradication phase when success in malaria control was at its peak, epidemiological investigations revealed that while rural malaria was sharply declining, it was raising its ugly head in towns. To contain this rising trend NMEP launched Urban Malaria Scheme (UMS) in 1971-1972 in 131 towns (100 million population) with >40,000 population showing two or more API, contributing 12.3 per cent cases in the country in 2005. Census 201112 reports 7,935 towns in India with >377 million population (31.16%). Class I urban agglomerations/towns with a population of at least 100,000 are 468. Urban decadal population growth was 31.2 per cent with 25.7 per cent urban population poor, mostly living in slums. Rapid and haphazard urbanization leading to degraded environment lacking adequate infrastructure is at the root of vector proliferation. Vector problems largely arise and multiply in degraded environment. Multiplicity of agencies further complicates the situation. Malaria is increasing in urban areas, e.g., in 2007, 102,829 cases; in 2008, 113,810 cases; in 2009, 166,065 cases and in 2010, 207,094 cases16.
Blood meal analysis of An. stephensi collected from the urban areas shows tendency for human blood meal60,64–69. Temperature is an important factor in extrinsic cycle of the parasite. Studies in urban areas have revealed high variability in temperatures from one area to another, and generally high human density has higher temperature. Mallick and Rahman70 reported a strong positive correlation between night surface temperature and human population density. Results of temperature records in Delhi revealed 23.90°C to 40.01°C in urban Delhi, Delhi suburbs (30°C to 34°C) which have less build up area, and industrial areas have temperature 30°C to 40°C. It may be noted that temperature has profound effect on the basic reproduction rate of malaria. Since 1970, An. stephensi has entered a large number of urban areas and tremendous opportunities have been provided for its breeding, but the UMS has stuck to 131 towns, and field operations have been reduced to 28 towns69. In almost all towns one can find innumerable breeding grounds that may support vector breeding. Many breeding habitats of An. stephensi and Aedes aegypti overlap and both vectors are present in abundance in urban environment. The situation is further complicated by the transmission of dengue and chikungunya virus in the last two decade or so71. The present situation of vector borne diseases is most notable and worrisome. Culex quinquefasciatus, vector of lymphatic filariasis is ubiquitous mosquito and the cause of intense nuisance in urban and rural areas. Urban malaria control relies on source reduction at weekly intervals, chemical larvicidng with Baytex, Fenthion and biological larvicides viz., B. thuringiensis and B. sphaericus, release of larvivorous fishes e.g. Guppy and Gambusia, de-weeding, de-silting, minor engineering interventions, IRS in slums, IEC and legislative measures.
Anopheles baimaii: Vector of forest malaria
An. baimaii is found in the northeastern States in jungles, and is the most efficient malaria vector comparable to An. gambiae of Africa. It colonizes deep jungles and retracts to interior portion of the forests after forest clearance. Entomological surveys revealed that An. baimaii (known earlier as An. dirus species D in the northeastern region of India) populations occur in abundance in monsoon season compared to pre-monsoon and post-monsoon seasons72. Man biting rate is high during the post-monsoon period, and transmission occurs both indoor and outdoors. Prakash et al73 reported 36.1 mean biting rate/person/night, sporozooite 1.9 per cent and parous rate 58.7 per cent in forest fringe villages of Assam; and 21 per cent mean inoculation rate took place before 2100 h pointing out the need of additional protective measures along with long-lasting insecticide nets (LLINs) during the pre-bed time in An. baimaii areas in the northeast India. An. baimaii is a hygrophilic and exophilic species and its natural populations occur in clusters. It rests on tree trunks/creepers in forests, and has high breeding potential in jungle pools in clear and turbid water, elephant foot prints, cattle foot prints, disused wells and other stagnant water, drains covered with foliage73,74. Most forests are closed forest and rich in wild life threatening the life of the workers. An. baimaii requires high humidity and spreads to cover larger areas during the rainy season but retracts to mother foci during dry season. In SEA Region it is widely spread in jungles and a major malaria vector maintaining stable malaria. There is almost negligible surveillance and scanty primary health care system in the deep jungles. For these reasons the real malaria situation remains hidden, and it would be reasonable to speculate that almost all tribal populations settled in forests under the influence of An. baimaii contract malaria and survivors have high immunity. In SEA Region forest area under closed forest is 951,356 km sq. or 79.9 per cent at the risk of malaria. Geographically, this is the lowest area in the world but has highest persons at risk of malaria (PARM) i.e. 70,879,923, and this is due to high human density per sq km. An. baimaii is the only malaria vector in closed forests, whereas in the outer periphery three other major vectors operate viz., An. culicifacies, An. fluviatilis and An. minimus75. An. baimaii populations expand during the rainy season while mother foci is alive in the deep forets. The populations in mother foci are long lived with high parity rates and harbour sporozoites. The species demonstrate horizontal and vertical pulsation influenced by the micro- and macro-environment76–78. Malaria control is, therefore, complex and problematic particularly when preservation of the forests is considered absolutely essential. As of now there are no appropriate technologies applicable to control An. baimaii transmitted malaria. This is an area of priority research when considering malaria elimination79.
Other important vectors (An. fluviatilis, An. minimus, An. sundaicus, An. annularis)
An. fluviatilis, an efficient vector of malaria that causes 15 per cent malaria cases, and 30 per cent P. falciparum cases annually, is widely distributed in the foothills and plains and breeds in slow flowing streams. An. fluviatilis control is a problem because of its natural occurrence in hilly degraded and thin forest with streams, as for example, in Odisha. Of the four sibling species identified in An. fluviatilis complex, species S is highly anthropophagic and an efficient vector, species T highly zoophagic with a few sporozoite positives in some areas; species U is highly zoophagic and not found sporozoite positives so far, and species V is yet to be studied.
An. minimus breeds in streams in the northeastern region of the country. It has retracted from UP Terai and many other endemic areas such as Nepal. It is highly susceptible to DDT and other insecticides. It had invaded northeastern States but has retracted under the pressure of insecticide treated bed nets in Assam80. Control of An. minimus is feasible and has been demonstrated in the country81,82.
An. sundaicus has retracted from the main land and confined to the Andman and Nicobar islands. This vector is susceptible to DDT. An. sundaicus breeds in brackish water mainly in the creeks in the island. A variant sweet water species also occurs in the same region. Vector control of An. sundaicus has been demonstrated by installing one way sluice gates to prevent the mixing of sea water with rain water and the use of Guppy and Gambusia fish in the control of vectors in wells. The existing technologies of vector control are good enough for sustainable malaria control in An. sundaicus areas. An. annularis is a vector of secondary importance and integrated vector management methods can control vector populations to below critical levels of disease transmission.
Vector control
Vector control is one of the most important activities of the NVBDCP. WHO recommends integrated vector management, and towards this objective evidence based data have been collected and disseminated for member countries to take advantage of this global and regional experience83. Every year before the onset of malaria, season dwellings at risk of malaria are sprayed with the residual insecticide by the NVBDCP. Insecticides in use are DDT, malathion and synthetic pyrethroids. Initially dieldrin was used but because of the toxicity it was discontinued and replaced with hexacyclohexane (HCH). Later HCH was also found toxic due to the presence of other isomers which had no role in vector control; only the gamma isomer which has the insecticidal properties (6.5% gamma isomer in 50% HCH) is insecticidal, and therefore, HCH was also discontinued. The current policy for IRS is to spray the insecticides in vector populations that are endophilic and susceptible to the insecticide in use. As per the norms, all structures should be sprayed with the desired dosage, and two or sometimes three rounds are sprayed. The coverage should be maximum possible, but all precautions should be taken to avoid food contamination in the households, and spray man should be trained and given protective clothing.
In 2010 preventive spraying data showed that 2023 mt 50 per cent DDT (50% WP) was sprayed (@150 mt/m population) in 52.89 million population with 72.63% coverage; 123 mt malathion (25% WP) was also sprayed (@900 mt/m population) to protect 0.7 million population with 86.92 per cent coverage, and 197 mt synthetic pyrethroids was sprayed to protect 15.65 million population with 92.36 per cent coverage. A total of 69.25 million population was covered with IRS an overall coverage of 77.16 per cent population (Dr R.C. Dhiman, personal communication). The estimated population of India in 201031 was 1,224 million. Of this population, 220 million (18%) is free from malaria risk, while 218 million (26%) population is at high to medium malaria risk and 686 million (56%) to low malaria risk31. Even if we take high to medium risk population requiring IRS, spray coverage comes to 31.7 per cent and that also based on 77 per cent coverage of the sprayed population. Besides these calculations, IRS is a demanding field operation and its performance depends on the quality of spray on the walls. This is often far below the desired standards. It is, therefore, obvious that field operations for rural malaria control are grossly inadequate and unlikely to provide any reasonable protection from malaria transmission. The impact of poor IRS is often reported by the media when epidemics strike year after year, otherwise the real malaria situation remains hidden under the cover of poor surveillance23,84–86. This is reflected by the NVBDCP data also, for example, district-wise data of Jharkhand and Chhattisgarh for 2010 show high SPR and Pf% invariably exceeding 70 to 80 (Figs 8 and 9). A similar picture is seen in other predominantly tribal areas of the country. For example, in the northeastern States Pf% is very high, average Pf% of all the 7 States is 75.78 per cent (Table). In some States surveillance is very poor, e.g. Assam, Manipur, Nagaland and Mizoram.
Fig. 8.
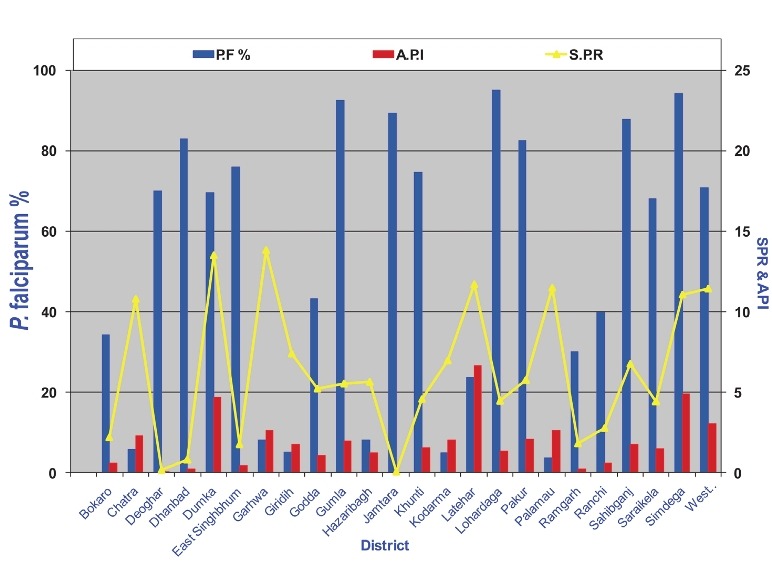
District-wise data of malaria API, SPR and Pf% of Jharkhand for 2010 (Source: NVBDCP, India)16.
Fig. 9.
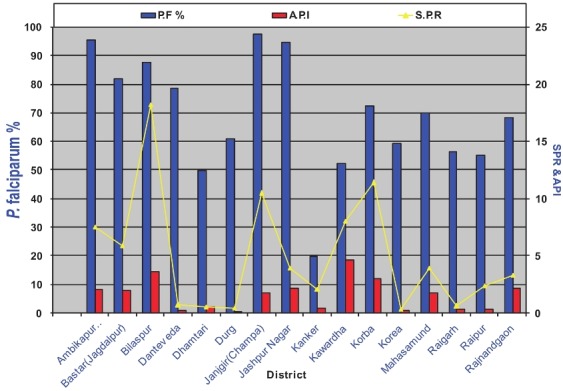
District-wise data of malaria API, SPR and Pf% of Chhattisgarh for 2010 (Source: NVBDCP, India)16.
Table.
Malaria situation in northeastern States in 2010
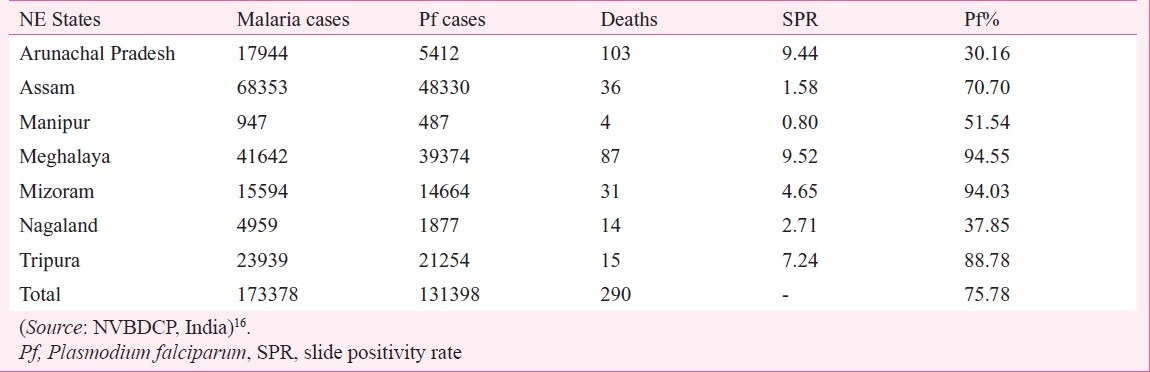
As described earlier, chloroquine resistance steadily travelled from northeastern States to Odisha and then to Madhya Pradesh and Chhattisgarh. With so high transmission in the tribal belt from northeastern States across central Indian States, it would be very difficult to contain the spread of artemisinin resistance as and when it arises. This high transmission belt should receive priority in the containment of P. falciparum.
ROAD MAP FOR MALARIA ELIMINATION
Millennium Development Goals (MDGs)
Malaria control objectives of the NVBDCP should coincide with the Millennium Development Goals (MDGs)87. Here we shall confine presentation on malaria situation in India and likely future scenario in the next plan period and beyond. MDG Goal 6: Combat HIV/AIDS, malaria, and other diseases. Target 6C: Have halted by 2015 and begun to reverse the incidence of malaria and other major diseases. While in some isolated local areas it is possible to meet the MDG but for the country as a whole we will definitely miss the deadline. This is true for the rural and urban malaria, and other ecotypes as well. Sustainable malaria control requires scientific approach based on evidence, cost-effective and sustainable interventions, and concurrent monitoring and evaluation. Organization of such an approach would require partnership with national and international agencies, NGOs, matching resources, trained and experienced manpower, community participation, inter-sectoral coordination, and appropriate technologies supported by research and development. Some possible approaches to improve environment and control malaria and other vector borne diseases are discussed here.
Drainage
Even before Sir Ronald Ross demonstrated the role of mosquitoes in malaria transmission, people settled in endemic areas knew how to protect themselves from fevers. In Europe they protected themselves from malaria by avoiding to settle near the marshy areas; and wherever possible lived up the hills to avoid mosquito bites, and mosquito nets were in use. Drainage was an important means to control mosquito production. In India irrigation projects lack proper drainage and water is allowed to stagnate. Floods are an annual feature causing large scale devastation. Permanent solution to flood control needs planning at the national level and mobilization of national resources to organize drainage and reclaim the wasteland and related resources. Health impact assessment (HIA) must become mandatory for all development projects so that the environment remains healthy and do not produce mosquito borne diseases. Project labour must be provided safe housing with drainage and adequate water supply. Paul F. Russel88 commented in his paper that malaria persisted due to defective and untidy irrigation lacking drainage. Although Sir Ronald Ross established the role of mosquitoes in malaria transmission in India, yet British engineers did not create drainage channels in the flooded fields. ‘Today [in 1938] the situation as regards irrigation and malaria is probably worse than ever before . . . For natural laws still obtain’: Each malarial area had its own special problems: there are more than a dozen anopheles types capable of bearing a lethal Plasmodium. Some breed and bite after the flooding of a region through irrigation; others bite after rainfall and natural flooding; others bite during dry seasons89. The situation has not changed in 2012, rather it has aggravated due to abject poverty which may be at the root of perennial malaria90. The strong bond between poverty and malaria must be broken, and it is possible by scientific planning of flood control to reclaim the waste land and put it to productive use, as has happened in Europe and America. Some case studies are briefly described below:
Pontine marshes: Historically drainage has played a major role in malaria control that eventually led to the expansion of agriculture, industries and sustenance of human and animal life. For example, malaria in Pontine marshes near Rome was the major cause of the vicious cycle of disease and poverty. In 1932 Benito Mussolini realized the importance of drainage in converting the malaria waste lands to healthy and productive lands. He deployed troops to drain Pontine marshes and successfully controlled An. labranchiae, the malaria vector. The entire marsh lands became productive and disease free. The new towns of Latina (1932) Sabaudia (1934), Pontinia (1935), Aprilia (1937), and Pomezia (1939) were founded. These towns with their architecture and town planning even today stand out as marvels. Agricultural economy received a boost and Mussolini became an unchallenged popular national leader. In 1943, Adolf Hitler had fallen with Mussolini and used malaria as biological war weapon. His army destroyed pumps and filled the Pontine marshes with water. Malaria returned with vengeance. Long afterwards in 1962 Pontine marshes were again drained that finally eliminated malaria from Italy91,93.
Tennessee Valley Authority (TVA): In USA Tennessee river's potential for irrigation, hydroelectric power generation and waterways was going waste while communities suffered from food shortages, poverty and unemployment. Malaria was responsible for about 30 per cent people's illnesses, and valley was almost de-populated due to malaria and abject poverty93. Mosquito breeding was eliminated by regulating water levels, drainage, and insecticide application94. President Franklin D. Roosevelt signed the Tenneessee Valley Authority (TVA) Act in 1933. In 10 year period, the entire area was reclaimed. Malaria was controlled for good and the region became healthy. TVA provided boost to the national economy. TVA engineers were in demand all over the world, and TVA had become an important source of national wealth. Malaria control in Tennessee valley transformed the American economy95.
Panama canal construction: Panama canal construction could not be undertaken due to high death rates from malaria and yellow fever. For example, in 1906 21,000 of the 26,000 work force were hospitalized due to malaria and yellow fever. Integrated methods of malaria and yellow fever control led to the construction of Panama Canal (1905-1910). By 1912 of the 50,000 workers, only 5,600 were hospitalized and both diseases eventually collapsed. Drainage of marshes remained the principal method of mosquito control. The areas remained healthy and had become an important source of commerce97.
Eradication of An. gambiae: An. gambiae, one of the most notorious mosquito responsible for the highest number of malaria cases in the world invaded Brazil. Rockfeller foundation eliminated An. gambiae from north east coast of Brazil in 1939-194098. Later An. gambiae from Nile Valley of Egypt was eradicated (1942-1945)99. This is a classical example of species sanitation. In India seepage control and drainage in Banbasa headworks (Uttrakhand State) was a successful malaria control activity that helped in colonization of Terai that led to green revolution99. Boelee et al100 provided several examples of drainage in malaria control from India, Sri Lanka101, Malaysia102, Indonesia and African countries. Siphons have been used to flush the mosquito breeding in endemic countries103–105.
Irrigation: At the time of independence irrigated area under agriculture was 28.9 million hectares (m ha) and remarkable developments in irrigation have increased the irrigated area to >90 m ha. Irrigation is an important source of mosquito production leading to malaria transmission106–109. In India lack of drainage in agricultural fields and on farm development of irrigation has become an important source of mosquito production. Keiser et al110 reported that about 40,000 large and 800,000 small dams have been built in over 50 year period world wide. This has brought 272 m ha million hectares of land under irrigation. These irrigated tracts have become receptive to several vector borne diseases and often encounter epidemics111. Construction of dams and irrigation systems ought to have included health impact assessment, and remedial measures put in place concurrently along with the development in irrigation. Unfortunately as we see it today, bulk of malaria is localized in the irrigation tracts. Crops requiring irrigation are given flood irrigation and water along the flooded fields stagnate to produce harmful insects, some of these are the vectors of human diseases. The more insecticide we use to control mosquitoes, the problem of resistance and environmental pollution magnifies to dangerous levels. Malaria control now requires sound water management practices and integrated malaria control measures to mitigate malaria burden in locations near the dam sites and in proximity with irrigation tracts112–114. Total floods affected land in the country is 31.94 m ha. Investments to reclaim this land and convert it for productive use would overhaul the national economy and provide healthy environment for the marginalized and neglected population113.
Health impact assessment (HIA): Health impact assessment in all development projects must become an important legal requirement before undertaking any construction or dealing with water115. Remedial measures must be incorporated at the design stage and applied along with the construction to ensure that there would be no adverse impact of the project.
Land use pattern: In the background of major ecological changes related to agricultural practices, it is essential that agriculture sector is closely associated in the planning of malaria control. Land use pattern can mitigate many situations that otherwise would be receptive and vulnerable to malaria. This is an area of great opportunity and should not be missed. For example, Malnad region in southern India (Karnataka-Kerala) measures about 50,000 sq km. was hot bed of prior to 1950s. An. fluviatilis was the only malaria vector in Malnad. Aquatic stages of An. fluviatilis used to breed profusely in slow running perennial streams in the forests. The source of perennial streams was rainwater absorbed by the thick vegetation cover on the ground. Arrival of DDT paved the way for colonization of Malnad and converted the malaria waste land to coffee plantations. Jungle clearance removed thick forest. As a result, litter on the ground gradually disappeared and so also the seepage. This natural change in the ecology of the region removed the breeding habitats of An. fluviatilis, the only vector of malaria prevalent in that area. Since then the region has become healthy, and there are no cases of indigenous malaria transmission in Malnad (N.L. Kalra, personal communication).
Bioenvironmental malaria control: Malaria was rampant in the rural areas in Karnataka. Streams with big boulders form a natural ecology of the area. Natural populations of An. culicifacies sibling species A and B are encountered in Karnataka. Control of breeding in streams was very difficult. Studies revealed that sibling species B breeds in streams exclusively, and in the wells and other stagnant water sibling species A was present. Based on this information mosquito control in streams was not attempted as sibling species B is a non-vector. Introduction of fish to control mosquito in wells led to successful malaria control at negligible cost116. Inspired by the success story in Karnataka as role model, other malaria endemic States are contemplating use of larvivorous fish in vector control117. Studies on bioenvironmental malaria control successfully eliminated mosquito nuisance and led to malaria control in many ecological settings in India118–122. Studies on bioenvironmental malaria control have revealed that convergence of various development agencies, for example, agriculture, irrigation and drainage, construction agencies, and public health can yield heavy dividends in malaria control, and the process can be accelerated and made sustainable with community participation.
Malaria elimination: During 1960s malaria defeated the mankind, and the available technologies could not meet the emerging challenges in malaria control. The turn of the century has brought new hope by re-defining old tools and developing new tools. The strategy has been tested in many endemic countries and found to be yielding good results. In the South East Asia Region malaria is at the verge of elimination in Sri Lanka, Bhutan, Nepal, and sharply declined in DPR Korea and East Timor31. Steady progress is being made in other countries of the Southeast Asia. But the real battle lies ahead in wiping out the remaining malaria from India, Bangladesh, Myanmar, and Indonesia. The environmental management methods (modification, manipulation and habitat management) received a set back with the use of indoor residual insecticides e.g. spraying of DDT and other residual insecticides to control malaria. The multifaceted problems in the use of insecticides in malaria control led to a realization that instead of depending on the chemicals alone, integrated vector management (IVM) would be a more scientific and sustainable strategy for malaria control123.
World Malaria Report 201131 provides goals, targets, policies and strategies for malaria control and elimination. Malaria targets have been revised while maintaining the overall vision of “malaria-free world”. These targets are: (i) reduce malaria deaths to near zero by the end of 2015, (ii) reduce malaria cases by 75 per cent from 2000 base line by the end of 2015, and (iii) eliminate malaria in 10 new countries by the end of 2015. While pursuing these targets, special emphasis should be given to combat resistance against the artemisinin-based medicines and insecticides. The strategy envisages malaria prevention through malaria vector control with two-fold goals viz., to protect individuals from contracting malaria, and reduce the intensity of local transmission by reducing longevity, vector density and human vector contact. These goals should be achieved by treated bed nets and/or long lasting insecticide nets (LLINs)124–127, indoor residual spraying128–130 and control of mosquito breeding131. IRS should be undertaken based on the biology of the vector species under attack. If spraying is not possible treated lining may be provided132,133. The insecticide selected should be effective in killing vectors i.e. resistance should not interfere in control, the vectors should be indoor resting, and the same insecticide should not be used on the LLINs and for the IRS. These interventions can be integrated with larval control to reduce vector populations.
Malaria diagnosis and treatment involve early confirmation of malaria parasite, preferably within 24 h either by (i) microscopic examination of blood smear, or (ii) use of bivalent RDTs134,135. For the first time in India rapid diagnostic tests “dipsticks” were tested in Mandla district villages used by the daily wage workers in Madhya Pradesh, and results were compared with the microscopy136. Rapid diagnostic tests were equally good compared to microscopic examination and sensitivity of the test depended on the parasite density. Later several RDTs were developed which are fast, reliable and simple in use and can detect P. falciparum and non-falciparum infections or both. FIND Foundation137 provides results of evaluation of all the RDTs available for malaria testing. A study138 was conducted in tribal areas of central India to measure the overall performance of several RDTs for diagnosis of P. falciparum and non-falciparum infections in comparison with traditional and molecular techniques. Results unequivocally supported the performance of first response (bivalent) RDTs. First response RDTs require 20 min compared to 30 min by other RDTs and require two drops of blood instead of four by other RDTs. Thus the “First response RDT” clearly has an advantage over other RDTs and high value in surveillance and in the treatment of patients where laboratory services are not readily available138.
Artemisinin is the drug of choice for all P. falciparum uncomplicated malaria, but should not be given as monotherapy. P. vivax should be treated with chloroquine, and P. vivax resistant cases with ACT. All fever cases should be diagnosed within 24 h or as early as possible. Malaria cases should be identified for parasite species or the mixed infection134,139,140. Soon after the diagnosis, uncomplicated P. falciaprum cases should be treated with ACT (as per national drug policy) to be given with a single dose of primaquine (not to be given to children under 5, pregnant women and G6PD deficient cases). In P. vivax cases chloroquine or ACT (not the SP combination) should be given along with 14 day primaquine treatment. Five ACTs currently recommended by WHO are: artemether + lumafantrine; artesunate + amodiaquine; artesunate + mefloquine; artesunate + sulphadoxine pyremethamine; and dihydroartemisinin + piperaquine. In India, the ACT selected by the NVBDCP is artesunate + sulphadoxine pyremethamine. The drug policy should follow the Global Plan for Artemisinin Resistance Containment (GPARC) as outlined in the WHO document on the subject134,141.
Global Fund for AIDS, Tuberculosis and Malaria (GFATM) in the last decade has pooled huge funds and distributed as per the needs of the endemic countries for the control of AIDS, Tuberculosis and Malaria. In 2011 malaria control world-wide has received US$ 2 billion from global fund. Unfortunately, it is likely to decline to US$ 1.5 billion, although projected annual requirement is US$ 5 billion. GFATM funding has already improved the malaria situation throughout the world. Malaria mortality rates have fallen by more than 25 per cent since 2000, with largest percentage reductions seen in the European (99%), American (55%) and Western Pacific (42%) and African Regions (33%). Out of 99 countries with ongoing malaria transmission, 43 recorded decreases of more than 50 per cent in the number of malaria cases between 2000 and 2010. Another 8 countries recorded decreases of more than 25 per cent31.
Counting malaria out142 would require universal coverage of all interventions143. Malaria control field work must be closely monitored and mid-course corrections incorporated. New and innovative well tested technologies should be continuously introduced into the programme, particularly for many situations and vectors that are difficult to control, with the ultimate aim of attaining the “freedom from malaria”.
Acknowledgment
Author thanks his former colleagues Drs Neeru Singh, R.C. Dhiman and Vas Dev for the review of the manuscript and for constructive suggestions.
References
- 1.Sharma VP. Re-emergence of malaria in India. Indian J Med Res. 1996;103:26–45. [PubMed] [Google Scholar]
- 2.Graham JD. Collected Memoranda on the Subject of Malaria. Reprinted from Government of India Reports (1847-1924) Rec Malar Survey India. 1930;1:vi+1–203. [Google Scholar]
- 3.Sir Ronald Ross (1857-1932) [accessed on 14 January, 2013]. Available from: http://www.malariasite.com/malaria/ross.htm .
- 4.Bynum WF. An experiment that failed: malaria control at Mian Mir. Parassitologia. 1994;36:107–20. [PubMed] [Google Scholar]
- 5.Madhok R. Report of the special committee to review the working of the National Malaria Eradication Programme and to recommend measures for improvement. New Delhi: Ministry of Health, Family Planning & Urban Development; 1970. [Google Scholar]
- 6.Sinton JA. What malaria costs India, nationally, socially and economically. Rec Malar Survey India. 1935;5:223–64. 413-89. [Google Scholar]
- 7.Müller Paul. “trihcorethane insecticidal composition and 7. methods”, assigned to JR Geigy AG. US patent 2329074. 1943 Sep 3; [Google Scholar]
- 8.Rao TR. The Anophelines of India. Delhi: Malaria Research Centre, Indian Council of Medical Research; 1984. [Google Scholar]
- 9.Sharma VP, Mehrotra KN. Malaria resurgence in India: a critical study. Soc Sci Med. 1986;22:835–45. doi: 10.1016/0277-9536(86)90238-8. [DOI] [PubMed] [Google Scholar]
- 10.Pattanayak S, Sharma VP, Kalra NL, Orlov VS, Sharma RS. Malaria paradigms in India and control strategies. Indian J Malariol. 1994;31:141–99. [PubMed] [Google Scholar]
- 11.Covell G. Malaria in Bombay. Bombay: Central Government Press; 1928. [Google Scholar]
- 12.New Delhi: Ministry of Home Affairs, Government of India; Census of India 2011. Available from: http:#www.imainmor.com/census-of-India-2011.html . [Google Scholar]
- 13.Roy RG, Panchapakesan A, Sitaraman NL, Ganesan AV, Ghosh RB. The urban malaria problem in Tamilnadu state. Indian J Med Sci. 1976;30:313–6. [PubMed] [Google Scholar]
- 14.Pattanayak S, Roy RG, Samnotra KG, Bendley MS. Urban malaria scheme of the National Malaria Eradication Programme of India. Indian J Malariol. 1981;18:21–7. [Google Scholar]
- 15.Sharma RS. Conference held at Regional Medical Research Centre for Tribals, Jabalpur, M.P. Delhi: NVBDCP; 2011. Urban malaria and its control. [Google Scholar]
- 16.National Vector Borne Disease Control Programme, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India, Delhi. [accessed on January 14, 2012]. Available from: http://nvbdcp.gov.in/
- 17.New Delhi: Communicable Diseases, Planning Commission, Government of India; 2011. Report of the working group on disease burden for the 12th Five Year Plan. [Google Scholar]
- 18.Pattanayak S, Roy RG. Malaria in India and the modified plan of operations for its control. J Commun Dis. 1980;12:1–13. [PubMed] [Google Scholar]
- 19.Sehgal PN, Sharma MI, Gogai S. Resistance to chloroquine in falciparum malaria in Assam state. India. J Commun Dis. 1973;5:175–80. [Google Scholar]
- 20.Ray AP, Narasimham MVVL, Kondrashin AV, Anna-Kari Bill. P. falciparum containment programme. Ten years of operation in India (1978-1988) Delhi: PfCP/Directorate of NMEP/WHO/SIDA; 1998. [Google Scholar]
- 21.Ray AP. Some aspects of P. falciparum containment programme. Indian J Med Res. 1979;70(Suppl):1–13. [PubMed] [Google Scholar]
- 22.Sharma VP. Hidden burden of malaria in Indian women. Malar J. 2009;8:281. doi: 10.1186/1475-2875-8-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma VP. Battling the malaria iceberg with chloroquine in India. Malaria J. 2007;6:105. doi: 10.1186/1475-2875-6-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma VP, Mehrotra KN. Return of malaria. Nature. 1982;298:210. doi: 10.1038/298210a0. [DOI] [PubMed] [Google Scholar]
- 25.Sharma VP, Mehrotra KN. Malaria resurgence. Nature. 1982;300:212. doi: 10.1038/300212d0. [DOI] [PubMed] [Google Scholar]
- 26.Sharma GK. Review of malaria and its control in India. In: Sharma VP, editor. Proceedings of the Indo-UK workshop on malaria in November 14-19, 1983. Delhi: Malaria Research Centre (ICMR); 1984. pp. 13–40. [Google Scholar]
- 27.Sharma GK. A critical review of the impact of insecticidal spray under NMEP on malaria situation in India. J Commun Dis. 1987;19:187–290. [PubMed] [Google Scholar]
- 28.John TJ, Dandona L, Sharma VP, Kakkar M. Continuing challenge of infectious diseases in India. Lancet. 2011;377:252–69. doi: 10.1016/S0140-6736(10)61265-2. [DOI] [PubMed] [Google Scholar]
- 29.Sharma D. Thar desert: sitting on the tip of a malarial iceberg. Lancet Infect Dis. 2004;4:322. doi: 10.1016/s1473-3099(04)01037-0. [DOI] [PubMed] [Google Scholar]
- 30.World Malaria Report 2011. Geneva: World Health Organization; 2011. WHO. [Google Scholar]
- 31.Sharma VP. Accelerating the pace of roll back malaria in India. Proc Natl Acad Sci India. 2009;79:13–21. [Google Scholar]
- 32.Yadav RS, Bhatt RM, Kohli VK, Sharma VP. The burden of malaria in Ahmedabad city, India - a retrospective analysis of reported cases and deaths. Ann Trop Med Parasitol. 2003;97:793–802. doi: 10.1179/000349803225002642. [DOI] [PubMed] [Google Scholar]
- 33.Shah NK, Dhillon GP, Dash AP, Arora U, Meshnick SR, Valecha N. Antimalarial drug resistance of Plasmodium falciparum in India: changes over time and space. Lancet Infect Dis. 2011;11:57–64. doi: 10.1016/S1473-3099(10)70214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta B, Gupta P, Sharma A, Singh V, Dash AP, Das A. High proportion of mixed-species Plasmodium infections in India revealed by PCR diagnostic assay. Trop Med Intl Health. 2010;15:819–24. doi: 10.1111/j.1365-3156.2010.02549.x. [DOI] [PubMed] [Google Scholar]
- 35.Mayxay M, Pukrittayakamee S, Chotivanich K, Imwong M, Looareesuwan S, White NJ. Identification of cryptic coinfection with Plasmodium falciparum in patients presenting with vivax malaria. Am J Trop Med Hyg. 2001;65:588–92. doi: 10.4269/ajtmh.2001.65.588. [DOI] [PubMed] [Google Scholar]
- 36.Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed-species malaria infections in humans. Trends Parasitol. 2004;20:233–40. doi: 10.1016/j.pt.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Diamond-Smith N, Singh N, DasGupta PK, Dash A, Thimasarn K, Campbell OM, et al. Estimating the burden of malaria in pregnancy: a case study from rural Madhya Pradesh, India. Malar J. 2009;8:24. doi: 10.1186/1475-2875-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhingra N, Jha P, Sharma VP, Cohen AA, Jotkar RM, Rodriguez PS, et al. Adult and child malaria mortality in India: a nationally representative mortality survey. Lancet. 2010;376:1768–74. doi: 10.1016/S0140-6736(10)60831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarkar J. Malaria in pregnancy. Curr Sci. 2012;103:15. [Google Scholar]
- 40.Ortega LI. Malaria situation in South East Asia Region, SEARO, WHO. [accessed on December 17, 2012]. Available from: http://www.mmv.org/sites/default/files/uploads/docs/events/2012/Stakeholder_meeting_presentations/Ortega_Southeast_Asia.pdf .
- 41.Adak T, Sharma VP, Orlov VS. Studies on the Plasmodium vivax relapse pattern in Delhi, India. Am J Trop Med Hyg. 1998;59:175–9. doi: 10.4269/ajtmh.1998.59.175. [DOI] [PubMed] [Google Scholar]
- 42.Anstey NM, Russell B, Yeo TW, Price RN. The pathophysiology of vivax malaria. Trends Parasitol. 2009;25:220–7. doi: 10.1016/j.pt.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Kochar DK, Saxena V, Singh N, Kochar SK, Kumar SV, Das A. Plasmodium vivax malaria. Emerg Infect Dis. 2005;11:132–4. doi: 10.3201/eid1101.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, et al. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg. 2009;80:194–8. [PubMed] [Google Scholar]
- 45.Kochar DK, Singh P, Agarwal P, Kochar SK, Pokharna R, Sareen PK. Malarial hepatitis. J Assoc Physicians India. 2003;51:1069–72. [PubMed] [Google Scholar]
- 46.Choi HJ, Lee SY, Yang H, Bang JK. Retinal haemorrhage in vivax malaria. Trans R Soc Trop Med Hyg. 2004;98:387–9. doi: 10.1016/j.trstmh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Prakash J, Singh AK, Kumar NS, Saxena RK. Acute renal failure in Plasmodium vivax malaria. J Assoc Physicians India. 2003;51:265–7. [PubMed] [Google Scholar]
- 48.Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg. 1997;91:256–62. doi: 10.1016/s0035-9203(97)90066-3. [DOI] [PubMed] [Google Scholar]
- 49.Chugh KS, Jha V, Sakhuja V, Joshi K. Acute renal cortical necrosis - a study of 113 patients. Ren Fail. 1994;16:37–47. doi: 10.3109/08860229409044846. [DOI] [PubMed] [Google Scholar]
- 50.Wellems TE, Hayton K, Fairhurst RM. The impact of malaria parasitism: from corpuscles to communities. J Clin Invest. 2009;119:2496–505. doi: 10.1172/JCI38307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thanh NV, Toan TQ, Cowman AF, Casey GJ, Phuc BQ, Tien NT, et al. Monitoring for Plasmodium falciparum drug resistance to artemisinin and artesunate in Binh Phuoc Province, Vietnam 1998-2009. Malar J. 2010;9:181. doi: 10.1186/1475-2875-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newton P, Proux S, Green M, Smithuis F, Rozendaal J, Prakongpan S, et al. Fake artesunate in south east Asia. Lancet. 2001;357:1948–50. doi: 10.1016/S0140-6736(00)05085-6. [DOI] [PubMed] [Google Scholar]
- 53.Rieckmann KH, Davis DR, Hutton DC. Plasmodium vivax resistance to chloroquine? Lancet. 1989;2:1183–4. doi: 10.1016/s0140-6736(89)91792-3. [DOI] [PubMed] [Google Scholar]
- 54.Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, Karyana M, et al. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 2008;5:e128. doi: 10.1371/journal.pmed.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Price RN, Douglas NM, Anstey NM. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis. 2009;22:430–5. doi: 10.1097/QCO.0b013e32832f14c1. [DOI] [PubMed] [Google Scholar]
- 56.Dua VK, Sharma VP. Plasmodium vivax relapses after 5 days of primaquine treatment, in some industrial complexes of India. Ann Trop Med Parasitol. 2001;95:655–9. doi: 10.1080/00034980120103225. [DOI] [PubMed] [Google Scholar]
- 57.Dua VK, Kar PK, Sharma VP. Chloroquine resistant Plasmodium vivax malaria in India. Trop Med Int Health. 1996;1:816–9. doi: 10.1111/j.1365-3156.1996.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 58.Shah I. Chloroquine resistant vivax malaria in an infant: a report from India. J Vector Borne Dis. 2008;45:176–7. [PubMed] [Google Scholar]
- 59.Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, et al. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg. 2009;80:194–8. [PubMed] [Google Scholar]
- 60.Githeko AK. Malaria and climate change. Special Feature. Climate Human Health Research Unit, Centre for Global Health Research, Kenya, Medical Research Institute. Commonwealth Health Ministers update. 2009/2010. [accessed on December 7, 2012]. pp. 40–3. Available from: http://www.thecommonwealth.org/files/190385/FileName/Githeko_2009.pdf .
- 61.Alemu A, Abebe G, Tsegaye W, Golassa L. Climatic variables and malaria transmission dynamics in Jimma town, South West Ethiopia. Parasite Vectors. 2011;4:30. doi: 10.1186/1756-3305-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mathur KK, Harpalani G, Kalra NL, Murthy GG, Narasimham MV. Epidemic of malaria in Barmer District (Thar desert) of Rajasthan during 1990. Indian J Malariol. 1992;29:1–10. [PubMed] [Google Scholar]
- 63.Sharma VP. DDT: the fallen angel. Curr Sci. 2003;85:1532–7. [Google Scholar]
- 64.Barik TK, Sahu B, Swain V. A review on Anopheles culicifacices: from bionomics to control with special reference to Indian subcontinent. Acta Trop. 2009;109:87–97. doi: 10.1016/j.actatropica.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 65.Anopheline species complexes in South and South-East Asia. New Delhi: WHO-SEARO Technical publication no. 57. SEARO publication; 2007. WHO-SEARO. [Google Scholar]
- 66.Kar I, Subbarao SK, Eapen A, Ravindran J, Satyanarayana TS, Raghavendra K, et al. Evidence for a new malaria vector species, species E, within the Anopheles culicifacies complex (Diptera: Culicidae) J Med Entomol. 1999;36:595–600. doi: 10.1093/jmedent/36.5.595. [DOI] [PubMed] [Google Scholar]
- 67.Jude PJ, Dharshini S, Vinobaba M, Surendran SN, Ramasamy R. Anopheles culicifacies breeding in brackish waters in Sri Lanka and implications for malaria control. Malar J. 2010;9:106. doi: 10.1186/1475-2875-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kar I, Eapen A, Ravindran KJ. Domestic breeding sources and their contribution in Anopheles stephensi breeding in Dindigul, Tamil Nadu. Indian J Malariol. 1996;33:191–9. [PubMed] [Google Scholar]
- 69.National Vector Borne Disease Control Programme. New Delhi: Ministry of Health and Family Welfare, Government of India; [accessed on September 18, 2011]. NVBDCP. Available from: http://www. nvbdcp.gov.in/ [Google Scholar]
- 70.Mallick J, Rahman A. Impact of population density on the surface temperature and micro-climate of Delhi. Curr Sci. 2012;102:1708–13. [Google Scholar]
- 71.Sharma VP. Dengue haemorrhagic fever epidemic in Delhi. Curr Sci. 1996;72:10. [Google Scholar]
- 72.Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasite Vectors. 2011;4:89. doi: 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prakash A, Bhattacharyya DR, Mohapatra PK, Mahanta J. Malaria transmission risk by the mosquito Anopheles baimaii (formerly known as An. dirus species D) at different hours of the night in North-east India. Med Vet Entomol. 2005;19:423–7. doi: 10.1111/j.1365-2915.2005.00592.x. [DOI] [PubMed] [Google Scholar]
- 74.Sharma VP. Forest-related malaria, malaria among tribal communities and malaria in development projects in South East. SEARO Working paper presented during the Regional Consultation on Malaria Control and Malaria Elimination in Bhubaneswar, Orissa, India October 11-14, 2011 [Google Scholar]
- 75.Guerra CA, Snow RW, Hay SI. A global assessment of closed forests, deforestation and malaria risk. Ann Trop Med Parasitol. 2006;100:189–204. doi: 10.1179/136485906X91512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kondrashin AV, Kalra NL. Malaria as anthropo-ecosystem. Part I: General concept. J Commun Dis. 1988;20:79–88. [PubMed] [Google Scholar]
- 77.Kondrashin AV, Kalra NL. Malaria as anthropo-ecosystem. Part III: Diversity of MAES. J Commun Dis. 1989;21:62–70. [PubMed] [Google Scholar]
- 78.Kondrashin AV, Kalra NL. Malaria as anthropo-ecosystem (MAES): Part V. Self-regulation and stability of MAES. J Commun Dis. 1990;22:12–22. [PubMed] [Google Scholar]
- 79.Sarma DK. Molecular population genetics and physiographic studies on Anopheles dirus (Diptera: Culicidae) complex malaria vector mosquito in north east India. Ph. D. thesis. Guwahati: Gauhati University; 2012. [Google Scholar]
- 80.Dev V, Bhattacharyya PC, Talukdar R. Transmission of malaria and its control in the northeastern region of India. J Assoc Physicians India. 2003;51:1073–6. [PubMed] [Google Scholar]
- 81.Dev V, Phookan S, Sharma VP, Anand SP. Physiographic and entomologic risk factors of malaria in Assam, India. Am J Trop Med Hyg. 2004;71:451–6. [PubMed] [Google Scholar]
- 82.Jana-Kara BR, Jihullah WA, Shahi B, Dev V, Curtis CF, Sharma VP. Deltamethrin impregnated bednets against Anopheles minimus transmitted malaria in Assam, India. J Trop Med Hyg. 1995;98:73–83. [PubMed] [Google Scholar]
- 83.Sharma VP. Sixty Years of WHO commemorative volume. New Delhi: South East Asia Regional Office World Health Organization; 2008. Work of WHO: Malaria. [Google Scholar]
- 84.Bouma MJ, van der Kaay HJ. Epidemic malaria in India and the El Niño southern oscillation. Lancet. 1994;344:1638–9. doi: 10.1016/s0140-6736(94)90432-4. [DOI] [PubMed] [Google Scholar]
- 85.Bouma MJ, Sondorp HE, van der Kaay HJ. Climate change and periodic epidemic malaria. Lancet. 1994;343:1440. doi: 10.1016/s0140-6736(94)92569-0. [DOI] [PubMed] [Google Scholar]
- 86.Kalra NL. Status report on malaria and other health related aspects of the SSP projects and recommendations regarding short-term and long-term remedial measures. New Delhi: World Bank; 1992. [Google Scholar]
- 87.Millennium Development Goals (MDGs) [accessed on May 15, 2012]. Available from: http://en.wikipedia.org/wiki/Millennium_Development_Goals .
- 88.Russell PF. Malaria due to defective and untidy irrigation. A preliminary discussion. J Malar Inst India. 1938;1:339–49. [Google Scholar]
- 89.Watts S. British development policies and malaria in India: 1897-c.1929. Past present. 1999;165:141–81. doi: 10.1093/past/165.1.141. [DOI] [PubMed] [Google Scholar]
- 90.Sharma VP. Malaria and poverty in India. Curr Sci. 2003;84:513–5. [Google Scholar]
- 91.Caprotti F. Malaria and technological networks: medical geography in the Pontine marshes, Italy, in the 1930s. Geogr J. 2006;172:145–55. [Google Scholar]
- 92.Frost RS. The reclamation of Pontine marshes. Geogr Rev. 1934;24:584–95. [Google Scholar]
- 93.Vector Control Program Environmental Statement. Technical Report. Knoxville, Tennessee: TVA; 1974. Tennessee Valley Authority (TVA) [Google Scholar]
- 94.Derryberry OM, Gartrell FE. Trends in malaria control program of the Tennessee Valley Authority. Am J Trop Med Hyg. 1952;1:500–7. doi: 10.4269/ajtmh.1952.1.500. [DOI] [PubMed] [Google Scholar]
- 95.Mills DB. TVA Handbook (Revised) Tennessee Valley, Knoxville: Tennessee, Authority; 1984. [Google Scholar]
- 96.La Prince JAA, Orenstein AJ. Mosquito control in Panama: the eradication of malaria and yellow fever in Cuba and Panama. New York: Putnam; 1916. [Google Scholar]
- 97.Soper FL, Wilson B, Lima S, Antunes WS. The organization of permanent nation-wide anti-Aedes aegypti measures in Brazil. New York: Rockefeller Foundation; 1943. [Google Scholar]
- 98.Shousha AT. Species-eradication. The eradication of Anopheles gambioe from Upper Egypt 1942-1945. Bull World Health Organ. 1948;1:309–52. [PMC free article] [PubMed] [Google Scholar]
- 99.Barrett J. Panama Canal: what it is, what it means. Washington: Pan American Union; 1913. [Google Scholar]
- 100.Boelee E, Konradsen F, van der Hoek W, editors. Malaria in irrigated agriculture. Working Paper 47. International Water Management Institute SIMA Document 2. Papers and Abstracts for the SIMA Special Seminar at the ICID 18th International Congress on Irrigation and Drainage, Montreal, July 23, 2002 [Google Scholar]
- 101.Worth HN, Subrahmanyam K. Anti-larval flushing of rivers and streams in Ceylon. J Malar Inst India. 1940;3:81–92. [Google Scholar]
- 102.Fourth report of the WHO Expert Committee on Vector Biology and Control Tech Rep Ser No. 649. Geneva: World Health Organization; 1980. WHO Expert Committee on Vector Biology and Control: Environment management for vector Control; pp. 1–75. [PubMed] [Google Scholar]
- 103.Ramsay GC, Anderson IR. An investigation on the use of automatic siphon sluices on a group of tea estates in northern Bengal. J Malar Inst India. 1940;3:93–7. [Google Scholar]
- 104.Manual on larval control operations in malaria programmes. Geneva: WHO; 1973. WHO; p. 1975. [Google Scholar]
- 105.Ejercito A. “Pampana Siphon” for automatic flushing of a stream as a naturalistic method of malaria control. (Design I) Riv Malariol. 1940;19:345–69. [Google Scholar]
- 106.Sharma VP, Uprety HC. Preliminary studies on irrigation malaria. Indian J Malariol. 1982;19:139–42. [Google Scholar]
- 107.Tyagi BK. A review of the emergence of Plasmodium falciparum-dominated malaria in irrigated areas of the Thar Desert, India. Acta Trop. 2004;89:227–39. doi: 10.1016/j.actatropica.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 108.Amerasinghe FP. Irrigation and mosquito-borne diseases. J Parasitol. 2003;89(Suppl):s14–22. [Google Scholar]
- 109.Singh N, Mehra RK, Sharma VP. Malaria and the Narmada- river development in India: a case study of the Bargi dam. Ann Trop Med Parasitol. 1999;93:477–88. doi: 10.1080/00034989958212. [DOI] [PubMed] [Google Scholar]
- 110.Keiser J, De Castro ML, Maltese MF, Bos R, Tanner M, Singer BH, et al. Effect of irrigation and large dams on the burden of malaria on a global and regional scale. Am J Trop Med Hyg. 2005;72:392–406. [PubMed] [Google Scholar]
- 111.Tyagi BK, Yadav SP. Correlation of irrigation and flood water management with malaria in the Thar desert. Reg Health Forum. 1997;2:5–11. [Google Scholar]
- 112.Singh N, Mehra RK, Sharma VP. Malaria and the Narmada-river development in India: a case study of the Bargi dam. Ann Trop Med Parasitol. 1999;93:477–88. doi: 10.1080/00034989958212. [DOI] [PubMed] [Google Scholar]
- 113.Oomen JMV, deWolf Jobin WR. Health and Irrigation Incorporation of disease-control measures in irrigation, a multi-faceted task in design construction, operation. Publication 45. International Institute for Land Reclamation and Improvement/ILRI.P.O. Box 45,6700 AA Wageningen, The Netherlands. [accessed on September 1, 2012]. Available from: http://www.mapsofindia.com/top-ten/geography/india-flood.html .
- 114.Sharma VP. Malaria and other vector borne diseases in South Asia. In: Menon MGK, Sharma VP, editors. Sustainable management of water resources: emerging science and technology issues in South Asia General Assembly of SCOPE (Scientific Committee on Problems of the Environment), New Delhi, India. February 7-11, 2005. New Delhi: Indian National Science Academy; 2009. pp. 337–58. [Google Scholar]
- 115.Mindell J, Biddulph J, Taylor L, Lock K, Boaz A, Joffe M, et al. Improving the use of evidence in health impact assessment. Bull World Health Organ. 2010;88:543–50. doi: 10.2471/BLT.09.068510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ghosh SK, Tiwari SN, Sathyanarayan TS, Sampath TR, Sharma VP, Nanda N, et al. Larvivorous fish in wells target the malaria vector sibling species of the Anopheles culicifacies complex in villages in Karnataka, India. Trans R Soc Trop Med Hyg. 2005;99:101–5. doi: 10.1016/j.trstmh.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 117.Dev V, Dash AP, Hojai D. Fishing out malaria in Assam, northeastern India: hope or hype? Trans R Soc Trop Med Hyg. 2008;102:839–40. doi: 10.1016/j.trstmh.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 118.Sharma VP. Community-based malaria control in India. Parasitol Today. 1987;3:222–6. doi: 10.1016/0169-4758(87)90066-4. [DOI] [PubMed] [Google Scholar]
- 119.Sharma VP, Dua VK, Sharma SK. Bioenvironmental control of industrial malaria. ICMR Bull. 1987;17:59–62. [Google Scholar]
- 120.Sharma VP. Community based bioenvironmental control of malaria in India. Ann Natl Acad Med Sci (India) 1988;24:157–69. [Google Scholar]
- 121.Sharma VP, Sharma RC. Community based bioenvironmental control of malaria in Kheda district, Gujarat, India. J Am Mosq Control Assoc. 1989;5:514–21. [PubMed] [Google Scholar]
- 122.Singh N, Sharma VP, Mishra AK, Singh OP. Bioenvironmental control of malaria in a tribal area of Mandla district, Madhya Pradesh, India. Indian J Malariol. 1989;26:103–2o. [PubMed] [Google Scholar]
- 123.Core structure for training curricula on the integrated vector management. Geneva: WHO; 2012. World Health Organization (WHO) [Google Scholar]
- 124.Yadav RS, Sampath TR, Sharma VP, Adak T, Ghosh SK. Evaluation of lambdacyhalothrin-impregnated bednets in a malaria endemic area of India. Part 3. Effects on malaria incidence and clinical measures. J Am Mosq Control Assoc. 1998;14:444–50. [PubMed] [Google Scholar]
- 125.Insecticide-treated mosquito nets: a WHO position statement. Geneva, World Health Organization, Global Malaria Programme. 2007. [accessed on February 2, 2012]. Available from: http://www.who.int/malaria/publications/atoz/itnspospaperfinal.pdf .
- 126.WHO Pesticide Evaluation Scheme (WHOPES) Geneva: World Health Organization; 2011. [accessed on February 2, 2012]. WHO recommended long-lasting insecticidal mosquito nets. Available from: http://www.who.int/whopes/Long_lasting_insecticidal_nets_Jul_2011.pdf . [Google Scholar]
- 127.Geneva: World Health Organization; 2011. [accessed on February 2, 2012]. Guidelines for monitoring the durability of long-lasting insecticidal mosquito nets under operational conditions. Available from: http://whqlibdoc.who.int/publications/2011/9789241501705_eng.pdf . [Google Scholar]
- 128.Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010;(4):CD006657. doi: 10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.WHO recommended insecticides for indoor residual spraying against malaria vectors. [accessed on October 1, 2011]. Available from: http://www.who.int/whopes/Insecticides_IRS_Malaria_09.pdf .
- 130.Geneva: World Health Organization; 2011. [accessed on April 17, 2012]. The use of DDT in malaria vector control: WHO position statement Global Malaria Programme. Available from: http:..whqlibdoc.who.int/hq/2011/WHO_HTM_GMP_2011_eng.pdf . [Google Scholar]
- 131.Geneva: World Health Organization; 2011. The role of larval source management for malaria control, with particular reference to Africa. [Google Scholar]
- 132.Messenger LA, Miller NP, Adeogun AO, Awolola TS, Rowland M. The development of insecticide-treated durable wall lining for malaria control: insights from rural and urban populations in Angola and Nigeria. Malar J. 2012;11:332. doi: 10.1186/1475-2875-11-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Diabate A, Chandre F, Rowland M, N’guessan R, Duchon S, Dabire KR, et al. The indoor use of plastic sheeting pre-impregnated with insecticide for control of malaria vectors. Trop Med Int Health. 2006;11:597–603. doi: 10.1111/j.1365-3156.2006.01605.x. [DOI] [PubMed] [Google Scholar]
- 134.Geneva: World Health Organization; 2011. [accessed on August 15, 2012]. Universal access to malaria diagnostic testing: an operational manual. Available from: http://whqlibdoc.who.int/publications/2011/9789241502092_eng.pdf . [Google Scholar]
- 135.Geneva: World Health Organization; 2011. [accessed on October 10, 2012]. Good practices for selecting and procuring rapid diagnostic tests for malaria. Available from: http://whqlibdoc.who.int/publications/2011/9789241501125_eng.pdf . [Google Scholar]
- 136.Singh N, Valecha N, Sharma VP. Malaria diagnosis by field workers using on immunochromatographic test. Trans R Soc Trop Med Hyg. 1997;91:396–7. doi: 10.1016/s0035-9203(97)90254-6. [DOI] [PubMed] [Google Scholar]
- 137.WHO. The Foundation for Innovative New Diagnostics-FIND, Geneva. [accessed on November 22, 2012]. Available from: http://www.who.int/trypanosomiasis_african/partners/find/en/index.html .
- 138.Singh N, Shukla MM, Shukla MK, Mehra RK, Sharma S, Bharti PK, et al. Field and laboratory comparative evaluation of rapid malaria diagnostic tests versus traditional and molecular techniques in India. Malaria J. 2010;9:191. doi: 10.1186/1475-2875-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sharma VP. Artemisinin drugs in the treatment of Plasmodium falciparum malaria in India. Curr Sci. 2006;90:1323–4. [Google Scholar]
- 140.Whitty CJ, Chandler C, Ansah E, Leslie T, Staedke SG. Deployment of ACT antimalarials for treatment of malaria: challenges and opportunities. Malar J. 2008;7(Suppl 1):S7. doi: 10.1186/1475-2875-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Global plan for artemisinin resistance containment (GPARC) World Health Organization. 2011. [accessed on September 2, 2012]. Available from: http://www.who.int/malaria/publications/atoz/artemisinin_resistance_containment_2011.pdf .
- 142.de Silva N, Wickremasinghe R. Counting malaria out – what progress? Indian J Med Res. 2010;131:475–7. [PubMed] [Google Scholar]
- 143.Kant R. Global malaria burden and achieving universal coverage of interventions: a glimpse on progress and impact. Curr Sci. 2011;101:286–92. [Google Scholar]


