Abstract
Background & objectives:
Optic neuritis (ON) is characterized by sudden and rapid impairment of vision. Bartonella henselae is a known aetiological agent of cat scratch disease (CSD), which is a common cause of neuroretinitis, the least common type of optic neuritis. The present study was carried out to determine the microbiological aetiology of optic neuritis in patients attending a tertiary care eye hospital in north India, which was later confirmed with molecular characterization.
Methods:
Of the 50 patients suffering from optic neuritis reported to the Ophthalmology OPD of a tertiary care eye hospital in New Delhi, India, 29 were included in the study. Blood culture from these patients were processed for aerobic and anerobic cultures to rule out infective aetiology. Subsequently, PCR was done on archive, glycerol-stocked cultures.
Results:
Gram-negative pleomorphic coccobacilli grew in four of 29 patients tested. Characterization of these revealed Bartonella like organism as tested by the API 20E, API Staph, API Strept and RapID ANA systems. Electron microscopy revealed presence of polar flagella and bleb like projection all over the bacterial surface. PCR performed on preserved culture confirmed these as Bartonella sp.
Interpretation & conclusions:
Infections with Bartonella like organisms have not been demonstrated from India in cases of optic neuritis or in any of the other clinical syndromes in the past. The present study shows the isolation and characterization of Bartonella like organisms from optic neuritis patients. From clinical point of view it will be important to look for these organisms as aetiological agents in ON cases in order to treat with appropriate antibiotics.
Keywords: Bartonella, demyelinating disease, optic neuritis, vision impairment
Optic neuritis (ON) is an inflammatory, infective or demyelinating process affecting the optic nerve and is characterized by sudden and rapid impairment of vision in one or both eyes. While demyelination is the most important cause, it may follow viral infection of childhood such as mumps, measles and chicken pox (herpes zoster), viral encephalitis and multiple sclerosis1. Neuroretinitis is the least common type of optic neuritis and is most frequently associated with viral infections and cat scratch fever. Other infectious causes include HIV infections2 and less commonly, Lyme disease, Toxoplasma gondii3 and Treponema pallidum4. Bartonella henselae is known to be the aetiological agent of cat scratch disease (CSD)4. In the present study, the microbiological aetiology of optic neuritis was presumptively determined in patients attending a tertiary care eye hospital in New Delhi. The initial finding was confirmed later on by rapid automated systems and molecular techniques which were not available at the time of initiation of the study.
Material & Methods
A total of 50 consecutive patients of optic neuritis who attended the Ophthalmology OPD at Dr R.P. Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, during the study period (1994-1995), were included in the study. Blood samples could be obtained from 29 of these 50 patients and hence could be included in the study. About 5 ml blood was collected from all patients Brain Heart Infusion (BHI) broth for culture of both aerobic and anaerobic bacteria. Attempts were made to look for Bartonella sp. by subculturing BHI broth by plating onto BHI agar with 5 per cent sheep blood and Columbia blood agar. The plates were incubated with 10 per cent CO2 for a prolonged period of 14 days. Suitable control (A standard strain of Bartonella henselae, kindly provided by Diane Hensel, Clinical Microbiology Laboratory, University Hospitals, Oklahoma City, USA, which was later inducted into the ATCC panel as standard strain of B.henselae and was given the number 49882) was used. In addition, 2-3 ml blood was also collected in plain vial for serology. Serum samples were subjected to Toxoplasma indirect haemagglutination assay (IHA) and Venereal Disease Research Laboratory (VDRL) test for syphilis (antigen obtained from the Institute of Serologists, Kolkata). Representative serum samples (n=6) from these patients were randomly chosen and sent to the Centers for Disease Control (CDC), Atlanta, for serology of B. henselae.
All isolated organisms were identified by standard methods described previously5. Bartonella sp. were identified based on morphology, motility, biochemical tests using automated rapid identification systems which utilise pre-formed enzymes like RapID ANA system (Innovative Diagnostic Systems, Norgross, GA, USA) and others like API 20E (Bio-Merieux, Vitek Inc., USA), API Staph/Strept systems (Bio-Merieux, Vitek Inc., USA) as well as electron microscopy. Antimicrobial susceptibility testing was done by performing disc diffusion test on Brain Heart Infusion (BHI) blood agar in line with other Gram negative anerobic organism. Subsequently, PCR was done on archived, glycerol-stocked cultures for confirmation of the aetiology.
The organisms were isolated in 1994-1995, but the molecular confirmation was done later. Blood samples from patients were collected for routine investigations with informed consent. However, the study protocol was later approved by the Institute's Ethics Committee.
Results
A total of 29 patients with provisional diagnosis of optic neuritis were included in the study. Twenty of these patients (68.97%) were males and nine (31.03%) were females. The age of the patients ranged from 5-66 yr with the mean age being 27.93 yr. Atypical presentation like symptoms of headache, fever and pain around eyeball and conjunctivitis were associated with the chief complaints of vision loss. History of fever preceding the eye symptoms were given by nine patients (31.03%) and conjunctivitis was present in 10 patients (34.48%).
Of these 29 cases, 18 (62.07%) presented with unilateral eye involvement while bilateral eye involvement was seen in the remaining 11 (37.93%). The initial visual acuity was perception of light (PL) negative (8/29 cases, 27.59%), <6/60 (15/29 cases, 51.72%), 6/36-6/28 (3/29 cases, 10.34%) and 6/12-6/9 (3/29 cases, 10.34%). A clinical diagnosis of papillitis was made in 16 patients (55.17%), 11 were diagnosed as retrobulbar neuritis (37.93%) while the remaining two (6.9%) were diagnosed as neuroretinitis. It could be possible that the patients diagnosed as papillitis presented at an early stage of disease, when the macular star was not developed. The patients diagnosed as papillitis were treated with oral ciprofloxacin and intravenous (iv) pulse steroids and eight of 16 patients reported improvement in vision after one month of treatment.
Culture: Of the 29 blood samples tested, Gram-negative coccobacilli were grown in four samples (13.79%) after 7-10 days of incubation in 10 per cent CO2. The colonies were either minute or rough, irregular dry type in primary culture. The isolates were all motile, non-fermenting, indole and nitrate negative and could not be identified by conventional biochemical tests. The clinical features and other relevant data on the four patients who were blood culture positive are given in the Table.
Table.
Clinical presentations and relevant clinical and laboratory findings in the four culture positive patients
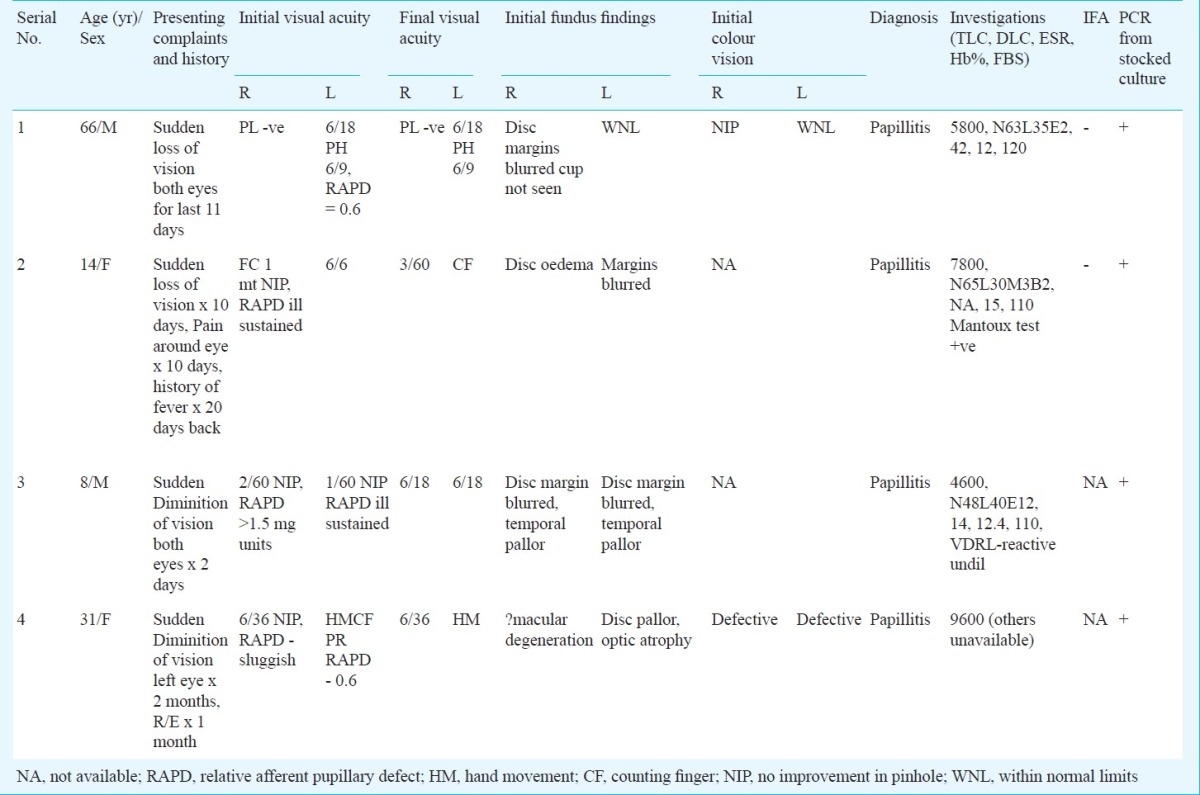
There was no growth in the blood agar and MacConkey agar plates from the samples of these patients thereby ruling out other non-fermenters like Pseudomonas or Acinetobacter.
Antimicrobial susceptibility: Although no standard methods for doing antibiotic susceptibility exist for Bartonella, disc diffusion test was performed on BHI blood agar in lines with other Gram-negative anerobic organisms5. It was found that the organism was sensitive to ciprofloxacin (5 μg), tetracycline (30 μg), piperacillin (100 μg) and amikacin (30 μgm) but was resistant to penicillin (10 IU), vancomycin (30 μg) and chloramphenicol (30 μgm).
Electron microscopy: Transmission electron microscopy following negative staining with 2 per cent phosphotungstic acid (PTA) revealed that the organism has a single polar flagella and bleb like projections all over the bacterial surface (Fig.).
Fig.
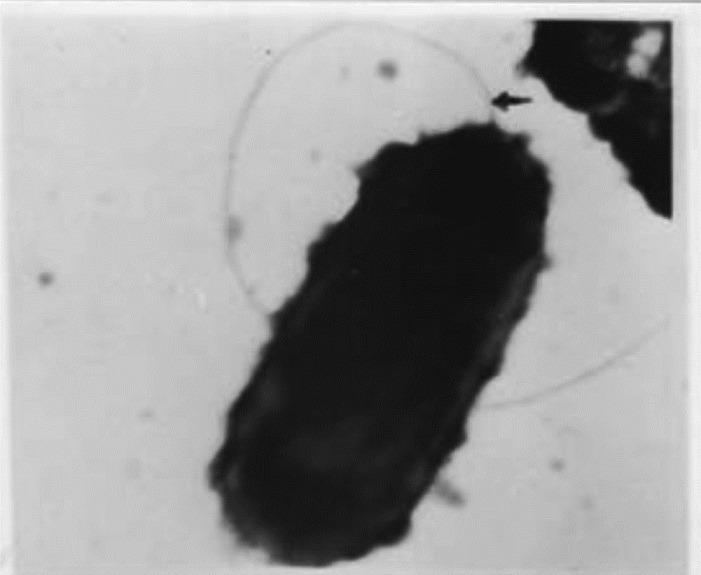
Electron micrograph of Bartonella showing single polar flagella and bleb like projections all over the bacterial surface. 40,000x.
Serology: Antibody to Toxoplasma gondii by IHA was positive in one patient and VDRL test was reactive in two patients (titre 16 and 32 dil). However, the IFA test for antibody to B. henselae was reported to be negative by CDC, Atlanta in the six referred serum samples.
Rapid automated identification systems: The isolates were tested by various rapid identification systems like RapID ANA, API 20E, API Staph and API Strept. The codes obtained were 000671 (RapID ANA), 0000004 (API 20E), 0004000 (API Staph) and 006000 (API Strepto). By these identification codes, the organism resembled B. henselae. B. henselae ATCC 49882 was used as the control strain.
Molecular techniques: In order to confirm the aetiology, PCR was performed on the archived, glycerol-stocked cultures. Previously published primers by Matar et al6 were used. Also, previously standardized PCR conditions were chosen for the study. The primers used for the PCR amplification were primer RPC5 (5’-AAG TCG TAA CAA GGT A-3’) and primer R23S2693 (5’-TAC TGG TTC ACT ATC GGT CA-3’). PCR amplification of the spacer + 23S sequence generated an approximately 1,600 bp fragment from all Bartonella species except B. bacilliformis which was approximately 1,000 bp. In the present study, a band size of approximately 1.1 kbps was obtained in all the four isolates. The amplification confirmed the isolates as belonging to Bartonella sp.
Discussion
Bartonella sp. are fastidious, slow growing, aerobic, short, pleomorphic, Gram-negative coccobacillary or bacillary rods. It is biochemically inert and hence cannot be identified by conventional biochemical tests. It is difficult to diagnose clinically because the ophthalmologic findings are often indistinguishable from those of other infections and results of antibody test can be non-diagnostic7. Bartonella is a member of α-2 subgroup of class proteobacterium8,9. The genus combines all the species of the three genera Bartonella, Rochalimaea and Grahamella and is the only genus in the family Bartonellaceae, which has been removed from the order Rickettsiales10. The various species in the genus are associated with a variety of clinical conditions such as bacillary angiomatosis, parenchymal bacillary peliosis and prolonged fever associated with persistent bacteremia11–13.
B.henselae has been implicated in the pathogenesis of cat scratch disease14,15 and optic neuritis. In the present study, serology in six serum samples for B. henselae by IFA test was reported to be negative. Similar observation of seronegative B. henselae has been reported by various workers7,16. Drancourt et al16 attributed seronegative results to antigenic variability.
The organisms recovered in four cases in the present study although consistent with B.henselae biochemically, were oxidase positive. This isolates seem to resemble a new α-2 proteobacterium as reported earlier from a patient of sepsis17, which was also oxidase positive and showed single polar flagella. Neuritis in B. henselae infections has been reported earlier7,9,18. Association of B. henselae with neuritis in previous studies was based on serology, direct demonstration, silver staining and PCR. Isolation of the organism from blood confirms the diagnosis as also shown by Wong et al18 in three patients of optic neuritis where bacteremia with B.henselae was reported. They also reported history of preceding fever in one of his patients of neuroretinitis, a finding observed in nine patients in our study. The isolates obtained in our study showed morphological and biochemical similarity with B. henselae, a fact also confirmed by rapid automated systems. Daly et al19 suggested that organisms that are difficult to identify and appear to be Rochalimaea (Bartonella)-like species should be tested for pre-formed enzymes using RapID ANA system. Their isolate showed profile number 000671 on RapID ANA. Although, the system may be useful for identifications to the genus level, it does not differentiate between the species of Rochalimaea (Bartonella)20.
In India, reports of Bartonella infections are rare.In one study the diagnosis was confirmed by IF and Western blot21, others involved only histopathology suggestive of B. bacilliformis22 or mere hypothesis23.
The organisms isolated in pure culture were stocked in glycerol and later used for confirmation by PCR. All the four isolates were confirmed as belonging to Bartonella sp. by PCR. Further, these were suggestive of B. henselae by their biochemical characteristics in rapid automated systems, but did not morphologically resemble with it as EM picture clearly showed polar flagella in this organism which is missing in B. henselae but is found in other Bartonella species like B. bacilliformis, B. clarridgeiae, B. capreoli and B. schoenbuchensis which are motile by unipolar flagella24. Moreover, the band size obtained in the four isolates was 1.1 kbps, which is intermediate between B. bacilliformis (1 kbps) and other Bartonella like B. vinsonui and B. henselae. Thus, the putative isolation of a new, as yet uncharacterized Bartonella sp. as an aetiological agent of optic neuritis cannot be ruled out. The clinical presentation was not typical neuroretinitis, but presented as papillitis. According to Golnick et al9, lack of previous cat scratch or lymphadenopathy does not preclude the presence of Bartonella infections.
In conclusion, this study underlines the importance of careful search for aetiological agents in patients with intraocular inflammation manifesting as vitritis, retinitis, neuroretinitis and optic neuritis of unknown origin. It is also worthwhile to search for these organisms from clinical point of view as B. henselae and/or Bartonella-like organisms can be treated with appropriate antibiotic therapy more easily as compared to other untreatable causes of optic neuritis9,25.
Acknowledgment
Authors acknowledge the help from Dr Ramesh Kumar, former Head of Department of Microbiology, AIIMS, Dr Diane Hensel, Clinical Microbiology Laboratory, Oklahoma and Dr Russel Regnery, CDC, Altanta.
References
- 1.Kanski JJ. Clinical ophthalmology- A systemic approach. 5th ed. Oxford: Butterworth Heinemann; 2006. [Google Scholar]
- 2.Bloom JN, Palestine AG. The diagnosis of cytomegalovirus retinitis. Ann Intern Med. 1988;109:963–9. doi: 10.7326/0003-4819-109-12-963. [DOI] [PubMed] [Google Scholar]
- 3.Holland GN, Engstrom RE, Glasgow BJ. Ocular toxoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1988;106:653–67. doi: 10.1016/0002-9394(88)90697-6. [DOI] [PubMed] [Google Scholar]
- 4.Passo MS, Rosenbaum JT. Ocular syphilis in patients with human immunodeficiency virus. Am J Ophthalmol. 1988;106:1–6. doi: 10.1016/s0002-9394(14)76378-0. [DOI] [PubMed] [Google Scholar]
- 5.Sutter VL, Citron DM, Finegold SM. Wadsworth anerobic bacteriology manual. St. Louis: C.V. Mosby; 1980. [Google Scholar]
- 6.Matar GM, Swaminathan B, Hunter SB, Slater LN, Welch DF. Polymerase chain reaction-based restriction fragment length polymorphism analysis of a fragment of the ribosomal operon from Rochalimaea species for subtyping. J Clin Microbiol. 1993;31:1730–4. doi: 10.1128/jcm.31.7.1730-1734.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keith W, Goldstein E, Hung VS, Kochler JE, Richardson W. Use of retinal biopsy to diagnose Bartonella henselae (formerly Rochalimeae) retinitis in an HIV infection patient. Arch Ophthalmol. 1998;116:937–40. doi: 10.1001/archopht.116.7.937. [DOI] [PubMed] [Google Scholar]
- 8.Hensel DM. The genus Bartonella. Clin Microbiol Newsletter. 1995;17:9–14. [Google Scholar]
- 9.Golnick KC, Marotto ME, Fanous MM, Hetter D, King LP, Halpern JI, et al. Ophthalmic manifestations of Rochalimeae sp. Am J Ophthalmol. 1994;118:145–51. doi: 10.1016/s0002-9394(14)72893-4. [DOI] [PubMed] [Google Scholar]
- 10.Brenner DJ, O’Connor SP, Winkler HH, Steigerwalt AG. Proposals to unify the Genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov, and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int J Syst Bacteriol. 1993;13:777–86. doi: 10.1099/00207713-43-4-777. [DOI] [PubMed] [Google Scholar]
- 11.Slater LN, Welch DF, Hensel D, Coody DW. A newly recognised fastidious Gram negative pathogen as cause of fever and bacteremia. N Engl J Med. 1990;232:1587. doi: 10.1056/NEJM199012063232303. [DOI] [PubMed] [Google Scholar]
- 12.Relman DA, Loutit JS, Schmidt TM, Falkow S, Thompkins LS. The agent of bacillary angiomatosis: An approach to the identification on uncultured pathogens. N Engl J Med. 1990;323:1573. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- 13.Welch DF, Pickett DA, Slater LN, Steigerwalt AG, Brenner GJ. Rochalimeae henselae a cause of septicemia, bacillary angiomatosis and parenchymal bacillary peliosis. J Clin Microbiol. 1992;30:275. doi: 10.1128/jcm.30.2.275-280.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regnery RL, Olsen JG, Perkins BA, Bibb W. Serological response to Rochalimeae henselae antigen in suspected cat-scratch disease. Lancet. 1992;339:1443. doi: 10.1016/0140-6736(92)92032-b. [DOI] [PubMed] [Google Scholar]
- 15.Perkins BA, Swaminathan B, Jackson LA, Brenner DJ, Wenger JD, Regnery RL, et al. Pathogenesis of cat scratch disease. N Eng J Med. 1992;327:1599. doi: 10.1056/NEJM199211263272215. [DOI] [PubMed] [Google Scholar]
- 16.Drancourt M, Birtles R, Chaumentin G, Vandenesch F, Etienne J, Raoult D. New serotype of Bartonella henselae in endocarditis and cat scratch disease. Lancet. 1996;347:441–3. doi: 10.1016/s0140-6736(96)90012-4. [DOI] [PubMed] [Google Scholar]
- 17.Blomovist G, Wesslen L, Pahlson E, Hjelm E, Petterson B, Nikkila T, et al. Phylogenetic placement and characterisation of a new alpha-2 proteobacterium isolated from a patient with sepsis. J Clin Microbiol. 1997;35:1988–95. doi: 10.1128/jcm.35.8.1988-1995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong MT, Dolan MJ, Lttuada CP, Regnery RL, Garcia ML, Mokulis EC. Neuroretinitis, aseptic meningitis and lymphadenitis associated with Bartonella (Rochalimeae) henselae infections in immunocompetent patients and patients infected with human immunodeficiency virus type 1. Clin Infect Dis. 1995;21:352–60. doi: 10.1093/clinids/21.2.352. [DOI] [PubMed] [Google Scholar]
- 19.Daly JS, Worthington MG, Brenner DJ, Moss CW, Hollis DG, Weyant RS. Rochalimaea elizabethae sp. nov., isolated from a patient with endocarditis. J Clin Microbiol. 1993;31:872–81. doi: 10.1128/jcm.31.4.872-881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regnery RL, Anderson BE, Clarridge JE, Rodriquez-Barradas MC, Jones DC. Characterisation of a novel Rochalimaea sp. R.henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus positive patient. J Clin Microbiol. 1991;30:265–74. doi: 10.1128/jcm.30.2.265-274.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balakrishnan N, Menon T, Fournier PE, Raoult D. Bartonella quintana and Coxiella burnetii as causes of endocarditis, India. Emerg Infect Dis. 2008;14:1168–9. doi: 10.3201/eid1407.071374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asharaf M, Letha S. Cutaneous bacillary angiomatosis. Indian J Pediatr. 2002;69:1003–5. doi: 10.1007/BF02726028. [DOI] [PubMed] [Google Scholar]
- 23.Sood FH, Chaudhari MS. Bartonella bacilliformis or a similar organism and cardiovascular disease. Med Hypotheses. 1994;43:135–7. doi: 10.1016/0306-9877(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 24.Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA. Manual of clinical microbiology. 9th ed. Washington: ASM Press; 2007. [Google Scholar]
- 25.Weiss AH, Beck RW. Neuroretinitis in children. J Pediatr Ophthalmol Strabismus. 1989;26:198. doi: 10.3928/0191-3913-19890701-10. [DOI] [PubMed] [Google Scholar]


