Abstract
Introduction
Atherosclerosis is the leading cause of death in the Western world. Despite huge advances in understanding its pathophysiological mechanisms, current treatment is mostly based on ‘traditional’ risk factors. The introduction of statins more than 20 years ago reduced morbidity and mortality of atherosclerosis by 30%, leaving a residual cardiovascular risk. Therefore, efforts continue toward the development of novel therapies that can be added to established treatments. Besides targeting dyslipidemia, recent focus has been put on preventing or resolving inflammatory processes involved in atherosclerosis.
Areas covered
The article discusses therapeutic and diagnostic targets in atherosclerosis and how they can be discovered and studied in preclinical animal models. The roles of immune cells, specifically macrophages and monocytes, in plaque inflammation are discussed. The article also describes current preclinical models of atherosclerosis, specifically the mouse, study designs (for progression and regression studies), basic and advanced methods of analysis of atherosclerotic lesions, and discusses the challenges of translating the findings to humans.
Expert opinion
Advances in genomics, proteomics, lipidomics and the development of high-throughput screening techniques help to improve our understanding of atherosclerosis disease mechanisms immensely and facilitate the discovery of new diagnostic and therapeutic targets. Preclinical studies in animals are still indispensable to uncover pathways involved in atherosclerotic disease and to evaluate novel drug targets. The translation of these targets, however, from animal studies to humans remains challenging. There is a strong need for novel biomarkers that can be used to prove the concept of a new target in humans.
Keywords: atherosclerosis, drug discovery, inflammation, laser capture microdissection, progression of atherosclerosis, regression of atherosclerosis
1. Introduction
Coronary artery disease (CAD) is the most common cause of death in the US and other Western countries and is a direct result of atherosclerosis. Hypercholesterolemia, especially the increase in plasma low-density lipoprotein (LDL), is a major risk factor for atherosclerosis and the associated complications. Cholesterol-lowering statins have long been the hallmark treatments for LDL hypercholesterolemia, but in clinical trials typically reduce morbidity and mortality of atherosclerosis by only 30%. This residual risk is attributed to other known risk factors, such as low levels of high-density lipoprotein (HDL) and hypertension. In addition, atherosclerosis is considered to represent a failure to resolve the inflammatory response instigated by the retention of LDL and other apoB-containing lipoproteins [1], which has focused attention on the roles of macrophages, dendritic cells, T cells, neutrophils and the chemokines/chemokine receptors involved in the plaque recruitment and homeostasis of these cells [2-4]. The known and yet to be discovered potential residual risk factors create the need for the discovery of novel therapeutic targets. An important issue that complicates the establishment of new cardiovascular drugs is the lack of valid surrogates and predictors for cardiovascular outcomes, efficacy and safety of new drugs in humans and the identification of potential therapy responders [5]. Therefore, novel biomarkers and imaging techniques to visualize plaque composition and phenotypes of the component cells are also needed both for diagnostic purposes and for following responses to therapies.
The finding that cardiovascular risk is associated with plasma levels of C-reactive protein (CRP) has strengthened the argument that CAD represents a state of unresolved inflammatory changes, though it still remains debatable if CRP resembles a reactive marker of the disease or a causal factor [6]. Currently, several drugs thought to have (sometimes vaguely defined) anti-inflammatory effects are being evaluated in clinical Phase II and III studies for the treatment of atherosclerosis. Targets of these drugs include lipid modifications (antioxidants and phospholipase inhibitors), inhibition of the leukotriene pathways to reduce leukocyte activity in plaques or inhibition of the CCL2–CCR2 pathway to prevent monocyte recruitment into plaques (for an excellent review about novel anti-inflammatory therapeutics, see [7]).
The ultimate treatment of atherosclerosis is not to halt further progression of the disease, but to achieve regression of existing plaques. To date various human and animal studies showed regression of atherosclerosis from early to advanced stages of atherosclerosis. In these studies, regression was achieved mostly by drastic changes of plasma lipids, especially levels of apoB-containing lipoproteins [8]. Vulnerable plaques, the kind most likely to rupture and cause a myocardial infarction, are typically less than 50% stenotic, usually have a thin fibrous cap and are mainly composed of intra- and extracellular lipids, activated macrophages (which secrete leukotrienes, cytokines and chemokines), plaque destabilizing proteases and a low content of smooth muscle cells [4,9]. Thus, the focus in plaque regression should reach beyond the actual change in lesion size toward the remodeling of plaque composition and changes in the activation state of the leukocytes.
In this review, we will discuss current concepts of atherosclerosis, animal models and designs of atherosclerosis studies, basic and advanced analytical methods of atherosclerotic lesions and the challenge to translate the findings from preclinical models to humans. Though all three major cell types in a plaque (endothelial cells, macrophages, smooth muscle cells) contribute to the pathology of the disease, we will focus on the role of the macrophage in this review, the cell type that is most responsible for the growth of the atheroma.
2. Animal models for an atherosclerosis study
Several animal models are used to study atherosclerosis including mouse, rabbits, pigs and nonhuman primates, with each one mimicking human atherosclerotic disease to different extents (for excellent review, see [10]). In modern atherosclerosis research, mice are the most commonly used animal model. Murine atherosclerosis models are well established due to the advantages including feasibility, the potential of extensive genetic modifications, small housing costs and the short generation and gestation times, all allowing the ready generation of high numbers of study animals. The small body size of mice also has the advantage that low amounts of test substances (often precious agents) are required. On the other hand, the small size of mice could limit the collection of sufficient amount of material such as cells, tissues and blood or the use of certain testing modalities.
Mice are in general highly resistant to atherosclerosis due to total cholesterol levels of < 100 mg/dl, which mainly occurs as HDL cholesterol. By dietary and genetic manipulations that lead to drastic increases in the non-HDL plasma lipoproteins, murine lines develop atherosclerotic lesions comparable with that in humans [8]. The two major murine atherosclerosis models, apolipoprotein E knockout (apoE−/−) and LDL receptor deficient (LDLr−/−) mice, are based on the increase in apoB-containing lipoproteins to atherogenic levels. Both animal models are widely accepted and can be further genetically manipulated to study any gene that might be involved in the pathophysiological mechanisms of atherosclerosis [11]. Mainly produced in the liver, ApoE on the surface of lipoproteins serves as a ligand for their clearance by primarily LDL receptors. Knocking out apoE, therefore, delays the clearance of lipoproteins and raises their levels. ApoE−/− mice spontaneously develop hyperlipidemia (total plasma cholesterol level of 300 – 400 mg/dl) and atherosclerosis on a low-fat diet [12]. Feeding a high-fat/cholesterol diet raises the plasma cholesterol levels (mainly that of very low-density lipoprotein (VLDL) and chylomicrons) even further (to well more than 1000 mg/dl) and leads to the accelerated development of advanced atherosclerotic lesions by 14 – 16 weeks [11]. Both diets are commonly used in apoE−/− mice for atherosclerosis studies, with most investigators favoring the high-fat/cholesterol diet because of its faster promotion of disease.
The discovery of the LDL receptor (LDLr) by Brown and Goldstein during studies of familial hypercholesterolemia was honored with the Nobel Prize in 1985. LDLr recognizes apoB100 on LDL and apoE on VLDL and chylomicrons (intestinal lipoproteins). In contrast to apoE−/− mice, LDLr−/− mice fed a standard low-fat chow diet do not develop significant atherosclerotic lesions [13]. However, on a high-fat diet (typically 40% of calories from saturated fat with 0.1 – 2% cholesterol by weight), total cholesterol levels (mainly VLDL, intermediate-density lipoproteins and LDL) increase four to five times and lead to the development of extensive atherosclerotic lesions comparable with apoE−/− mice [11].
On a high-fat diet, both mice models, LDLr−/− and apoE−/− mice, develop atherosclerosis from early ‘fatty streak’ lesions consisting mainly of lipid-laden macrophages (stages I – III based on the AHA-defined stages of human atherosclerotic lesions [14]) to advanced lesions containing necrotic cores and fibrous caps (stages IV – V). The types of lesions and the time on different diets to achieve various stages of atherosclerosis in LDLr−/− and apoE−/− mice are well documented and were excellently reviewed [15]. LDLr−/− and apoE−/− mice are usually on a C57BL6 background and have been extensively backcrossed. This is of importance as other genetic backgrounds have different susceptibility to atherosclerosis [16].
Mice models are indispensable to study atherosclerosis, but some limitations need to be considered when it comes to translate findings from mouse to humans: cholesterol levels in hyperlipidemic atherosclerosis mice extensively exceed the levels found in humans. Also mice naturally lack cholesteryl ester transfer protein (CETP) that transfers cholesteryl ester from HDL to apoB-containing lipoproteins in humans [17]. In the immune system, humans lack the clear T helper (Th) 1 and Th2 polarization found in mice, and markers for M1 and M2 macrophage phenotypes (see below) partially differ between both species [18]. Perhaps most notable is that mouse plaques are not prone to plaque vulnerability and plaque rupture. Despite various reports about plaque rupture, especially in the brachiocephalic artery of apoE−/− mice, there is still a lot of debate about whether this represents a pathology akin to what happens in humans [19]. The lack of rupture has been ascribed to differences in vessel size, location of plaques, thrombus formation and size and the fibrinolytic system [20].
3. Monocytes and macrophages in atherosclerosis
Macrophages are the major cell type in atherosclerotic plaques from the early fatty streak lesions to more advanced plaques. Macrophages derive from circulating monocytes that are recruited into the plaques, a step mediated by adhesion molecules on the endothelium, such as vascular cell adhesion molecule-1 (VCAM-1) and various chemokines secreted by endothelial cells and macrophages already in the plaque. Macrophages are heterogeneous cells, and a simple classification divides them into those predominately secreting pro- (M1 cells) or anti-inflammatory (M2 cells) mediators to regulate inflammatory processes within the plaque [21,22]. By the uptake of normal and modified LDL and other apoB-containing lipoproteins, macrophages in the arterial wall become lipid-laden foam cells and become progressively activated.
An expanding area of interest is the functional specialization of macrophage subtypes induced by particular microenvironments [21]. The M1 and M2 scheme mentioned above is based on Th1 and Th2 lymphocytes. The M1 state represents activated macrophages that are potent effector cells, producing primarily pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6 and IL-12, as well as reactive oxygen/nitrogen species and proteolytic enzymes. These factors promote inflammation, apoptosis and degradation of extracellular matrix components, which probably lead to plaque destabilization, increased risk of plaque rupture and thrombosis. By contrast, M2 macrophages produce a number of anti-inflammatory factors, such as IL-10, transforming growth factor-β (TGF-β) and IL-1 receptor antagonist (IL-1Ra) [23-25]. Recently, the M2 category has been expanded to include two overlapping subtypes, namely ‘wound healing’ (or, tissue remodeling) and ‘regulatory macrophages’ (which have high production of IL-10) [26]. M2 macrophages promote tissue repair by collagen synthesis, smooth muscle cell proliferation and clearance of apoptotic cells, which would be expected to result in enhanced plaque stability.
Besides the phenotypic state of macrophages, an important process to consider in plaque regression is a decrease in their number. Does this represent decreased recruitment into the plaque, apoptosis within the plaque and/or emigration out of the plaque? From mouse models, there is evidence to support all three possibilities [27-29]. It remains an important goal to further characterize each individual mechanism involved in macrophage and macrophage foam cell depletion during regression in different models, vascular sites and stages of disease.
Circulating monocytes that are recruited into the arterial vessel wall are the precursor of the lipid-laden plaque macrophage foam cells. Therefore, a particular interest lies on the role of different monocyte subsets in the initiation and progression of atherosclerosis in human and mice. Furthermore, monocyte subsets are currently evaluated as potential biomarkers to predict cardiovascular risk and outcome [30]. In mice two major monocyte subsets, Ly6Chigh (Gr1+/Ly6ChighCCR2+CX3CR1low) and Ly6Clow (Gr1−/Ly6ClowCCR2−CX3CR1high) have been identified. Both monocyte subtypes can differentiate into macrophages and dendritic cells. Ly6Chigh monocytes originally have been described as inflammatory monocyte subset, preferentially recruited to inflammatory sites, while the Ly6Clow subset were initially considered as resident monocytes [31].
In humans, three subtypes of monocytes are established that differ in phenotype and function: CD14++CD16+ (classical), CD14+CD16+ (intermediate) and CD14+CD16++ (nonclassical) monocytes. CD14++ monocytes represent the major monocyte population, show high phagocytic activity, express chemokine (C-C motif) receptor 2 (CCR2) and share similarities with Ly6Chigh monocytes in mice. By contrast, the CD16+ monocyte subsets have a weak phagocytic activity, preferentially taking up oxidized LDL, and secreting various inflammatory factors (for example, TNF-α, IL-1β). The human CD16+ monocyte subtypes are thought to be equivalent to the Ly6Clow monocytes in mice [22,30]. In apoE−/− mice, inhibition of chemokine (C–C motif) ligand 2 (CCL2), fractalkine receptor 1 (CX3CR1) and CCR5 led to attenuated atherosclerosis by abrogation of Ly6Chigh and Ly6Clow monocytosis with the highest lesion reduction after combined inhibition of all three pathways. Thereby, CCL2 affected both Ly6Chigh and Ly6Clow monocytosis, while CX3CR1 specifically affected numbers of Ly6Clow monocytes [32].
4. Atherosclerosis study designs
Atherosclerosis studies can be designed as a progression or regression study, or a combination of both, depending on the intended goal of the candidate factor or agent. In other words, it is possible to differentiate between slowing/halting of atherosclerosis progression from plaque regression (as defined by a decrease in lesion size and/or reduction in monocyte-derived cell content within in an atherosclerotic lesion).
Here we present three typical study designs to evaluate the effect of a candidate agent on atherosclerosis (what parameters are measured will be described in a later section):
To test the impact of a drug on progression of atherosclerosis
LDLr−/− or apoE−/− mice are typically weaned on to the high-fat diet at 4 weeks of age, at which time the agent is concurrently administered. Depending on the agent’s characteristics, administration can be by mixing in the chow, put in the drinking water, gavaged or injected (intravenous, subcutaneous, intraperitoneal). The diet is continued to the point of disease stage desired (e.g., 8 – 10 weeks for early fatty streaks, 12 – 14 for intermediate lesion and 16 – 20 weeks for advanced plaques) [12,15].
To test the ability of a drug to induce regression of atherosclerosis
A recent example was the test by our collaborators of an inhibitor of microRNA-33 (miR-33). This microRNA suppresses HDL formation and also the expression of cholesterol transporter ATP-binding cassette transporter 1 (ABCA1) [33], and it was hypothesized that inhibiting it by an antagomir (anti-miR-33) would promote atherosclerosis regression.
LDLr−/− mice were fed a high fat over 14 weeks to establish advanced plaques and then treated with anti-miR-33 over 4 weeks. As predicted, anti-miR-33 treatment led to increased reverse cholesterol transport through an increase in HDL levels and expression of ABCA1 in the liver and macrophages. Consistent with that, atherosclerotic lesions regressed by anti-miR-33 treatment as shown by significantly reduced plaque lesion size, lipid and macrophage content, increased collagen content and a diminished inflammatory state of the macrophages in the plaque [34].
To evaluate the effect of an agent during the regression of atherosclerosis
Established murine regression models include aortic arch transplantation model [35,36], Reversa mouse model [37,38], adenoviral gene transfer of known anti-atherosclerotic factors and infusion of ApoA-I (the major protein of HDL) and ApoA-I mimetics [8,28,39]. Independent of the underlying model it is a common finding that during regression of atherosclerosis, plaque macrophages and lipid content decrease, but plaque collagen content increases.
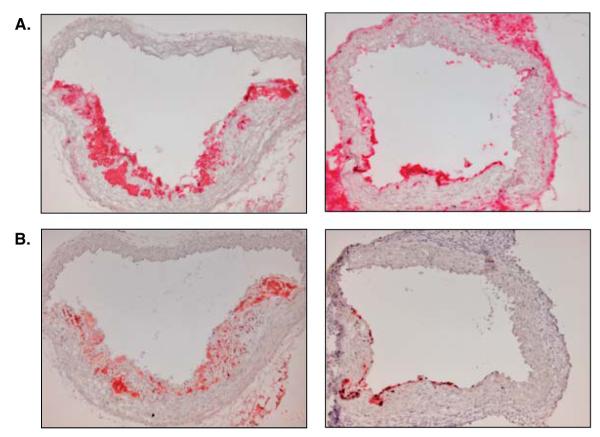
The hepatic overexpression of apoE in apoE−/− or LDL receptor in LDLr−/− mice are two major gene transfer strategies to induce regression. Both models are based on the normalization of atherogenic lipids [8]. A drawback of this method that has to be considered is a potential immune response of the host after the adenoviral gene transfer [40], though with the newer generation of vectors, this is less of a problem; the use of early vectors frequently complicated the interpretation of the inflammatory state of cells in the plaque. In the transplant model, the plaque-containing aortic arch from a donor mouse fed a high-fat diet for 16 – 20 weeks is interpositioned into the abdominal aorta of a normolipidemic recipient mouse [36,41]. Dramatic regression of the plaque in the transplanted arch occurs within a few days. Regression also occurs in the face of persistent non-HDL hyperlipidemia when the naturally low HDL levels of apoE−/− mice are normalized by transplanting the aortic apoE−/− mice into apoE−/− mice transgenic for human ApoA-I (Figure 1) [35,42]. Though not a high-throughput system, the transplant model can be used to test the effects of specific genes by the appropriate variation in the donor or recipient mice, or more conveniently, recipient mice can be treated with a potential agent at the time of transplantation to see whether it accelerates or retards the regression process.
Figure 1. Demonstration of changes in CD68-positive macrophages (A) and Oil Red O staining (B) of aortic arch plaques from apoE−/− mice during atherosclerosis regression.
Left panels show aortic arch lesions of an apoE−/− mouse after 16 weeks on a high-fat diet; right panels show regressing aortic arch lesions 5 days after transplantation of the aortic arch from the same type of apoE−/− mouse in the left panels into a transgenic human apolipoprotein A-I expressing apoE−/− mouse. Magnification: 10×.
The Reversa (Ldlr−/−Apob100/100Mttpfl/flMx1 Cre+/+) mouse is a nonsurgical regression model, based on the LDLr−/− ‘platform,’ in which the hyperlipidemia can be reversed at any time by inducing the conditional knock out of the microsomal triglyceride transfer protein (MTP) gene [38]. MTP is required for the proper assembly of VLDL, the precursor of LDL (reviewed by [43]). The reversal of hyperlipidemia by inactivation of MTP leads to regression of atherosclerosis accompanied with favorable changes in the composition of the atherosclerotic plaque and in the inflammatory state of the macrophages [37].
5. Differences in atherosusceptibility within the arterial system
The choice of the vascular site in an atherosclerosis study is an important factor for the study design, as atherosclerotic lesion development and responses to perturbations differ within the arterial tree due to interarterial differences in local hemodynamic environments and vascular endothelial cell biology [44,45]. Thus, drugs might even have opposite effects at different sites of the arterial system. Atheroprone regions in human are in particular the proximal left anterior descending coronary artery, the proximal part of the renal arteries, the aortic bifurcation, the major branches of the aortic arch and the carotid bifurcation [45,46]. Similar predilection sites for atherosclerosis are found in LDLr−/− and apoE−/− mice, with emphasis on the aortic root and arch (including the brachiocephalic artery) and the iliac bifurcation. To have the most confidence in the effects of any given agent, however, it is advised to assay responses at more than one arterial site.
6. Basic morphologic analyses of atherosclerotic plaques
Basic analyses of atherosclerotic plaques include plaque size, content of lipids, macrophages, collagen, and smooth muscle cells (Table 1 and Figure 1). A change in plaque size is the minimal information to be collected in an atherosclerosis study. There are two basic methods to quantify the extent of atherosclerosis in mice: en face analysis of the entire aorta and cross-sections of the aortic arch, the brachiocephalic artery or (most commonly used) of the aortic root. Atherosclerosis in the aortic root and the brachiocephalic artery progresses sooner than other sites of the aorta. The plaque size in the aortic root is usually evaluated by digitizing images of sequential cross-sections in the region were all three aortic valve leaflets are visible, followed by quantification by an image analysis software. The en face analysis is used to quantify the extent (percentage of lesion area) and distribution of atherosclerosis along the luminal surface of the entire aorta. Typically, flat preparations of the aorta from the aortic root to the iliac bifurcation are stained with Sudan-IV [47] or Oil Red O to detect lipid accumulations. En face analysis of the aorta lacks the three-dimensional component of cross-sections as the height of lesions is not measured. Furthermore, it does not give information about the complexity and composition of a plaque. This is especially important as the absence of significant changes in lesion area implies, for example, that a treatment has no impact on atherosclerosis, while potential beneficial compositional (e.g., more collagen) or phenotypic (less inflammation) changes in the lesions will be missed [15]. Both methods, en face and cross-section analysis, can be combined to get more accurate information about the extent of atherosclerosis in mice.
Table 1.
Basic analysis of atherosclerotic lesions in mice
| Parameter | Tissue | Staining |
|---|---|---|
| Lesion size | Cross-sections/ entire aorta for en face lesion analysis |
HE, (ORO for en face analysis) |
| Neutral lipids | Cross-sections | Oil Red O (ORO) |
| Collagen | Cross-sections | Sirius Red |
| Macrophages | Cross-sections | IHC (CD68 or MOMA-2 antibody) |
| Smooth muscle cells (SMC) |
Cross-sections | IHC (SMC α-actin antibody) |
IHC: Immunohistochemistry.
Collagen (predominantly type I) found in human atherosclerotic plaques is a marker of increased plaque stability. Collagen is stained with Sirius Red and the positive area needs to be analyzed under polarizing light. The necrotic core is a lipid-rich pool of cellular debris and extracellular lipids as a result of inefficient efferocytosis of dead cells in plaques. The necrotic core is a marker of plaque instability and can be quantified in cross-section of lesions [9,48]. Macrophages are typically identified by the marker CD68, and smooth muscle cells (SMC) by smooth muscle alpha-actin staining. Depending on the study, additional immunohistochemistry staining of the lesions for inflammatory markers such as monocyte chemotactic protein-1 (MCP-1), TNF-α or VCAM-1 may be added to the basic analysis. In all cases, digitized microscopic images are analyzed by image analysis software to compute either the absolute area positive or the relative area of a plaque that is positive.
7. Advanced analyses of atherosclerotic plaques
7.1 Laser capture microdissection
Advanced analysis of atherosclerotic plaques includes probing for cellular phenotypic changes within the plaque such as M1 and M2 macrophage polarization (e.g., [37]). The molecular analyses of specific cell populations in the plaque can focus on a small number of candidate factors or be approached more globally with microarrays. Such investigations are important for the understanding of disease pathogenesis and for the discovery of novel targets for therapies and interventions. Genetic information derived from cultured cells does not necessarily reflect the environment in the actual lesion, making analyses of the actual tissue of paramount importance going forward in the discovery process. Even with tissue, misleading results may be obtained if gene expression profiles are derived from homogenized aorta: data will reflect the cellular heterogeneity of arterial tissue including lymphocytes, SMC, endothelial cells, macrophages and adventitial fibroblasts. Not only will pathways in each cell type be obscured, changes per cell cannot be differentiated from changes in the number of a particular cell type.
These limitations were resolved with our establishment of the use of laser capture microdissection (LCM) in vascular biology [49]. With the use of a low-power infrared laser, single plaque cells from a frozen section are melted onto a cap containing a thermoplastic film. The DNA, RNA and protein of laser-captured cells remain intact and can be extracted from the cap for further analysis. This gives the opportunity to very selectively study changes in genes and their expressions at the RNA and protein levels of a specific cell type from atherosclerotic lesions. Extracted RNA can be used directly for qRT-PCR assays of a limited set of genes (probably up to 10) or amplified for microarrays.
We and others have applied LCM to analyze plaque macrophages, given their central importance in the pathophysiology of atherosclerosis, and the relative ease of identifying and selecting them. An example is our recent report on the effect of peroxisome proliferator-activated receptor- (PPAR-) gamma agonist pioglitazone on macrophages after plasma lipid levels were lowered in Reversa mice (to simulate giving this drug to patients already on a statin). Though pioglitazone had no additive effect to reducing plaque CD68+ cell content, treatment did polarize these cells to a more anti-inflammatory M2 state – an effect that would have been missed with a basic analysis of the plaques [37].
7.2 High-throughput screening techniques in atherosclerosis
After the success of the human and mouse genome projects, various new techniques emerged for the evaluation of transcriptional changes and regulatory genetic networks in biological processes [50]. High-throughput screening assays, such as DNA microarray, are efficient tools to assess responses to agents and to uncover mechanisms involved in atherosclerosis that may lead to the discovery of new drug targets. As mentioned earlier, however, profiling of atherosclerotic plaques is challenging due to the various cell types within a lesion and changes of cell composition and phenotypes during disease development or interventions. To provide maximal clarity in interpretation, LCM samples of specific cells types can be applied to microarray analysis to study the transcriptome. Another challenge lies in the interpretation of microarray data. Atherosclerosis is a very complex disease and conventional tools, such as gene set enrichment analyses, are probably too simplistic – more appropriate would be the evolving, sophisticated bioinformatics approaches of systems biology [51].
In cell biology, many changes and regulatory interactions occur at the protein, and not at the mRNA, level [50]. This has led to the development of chip detectors for proteins. High-throughput immunoblotting or Western array screening of tissue includes polyacrylamide gel electrophoresis and Western blotting followed by screening of the blots with an assortment of specific antibodies. Each monoclonal antibody identifies a unique target within the many proteins displayed on the Western blot, consequently, subnanogram quantities of proteins with altered expression can be detected [52]. Alternatively, proteomic and lipidomic analyses of plaques can also be conducted using mass spectrometry, either with whole aortic homogenates (with the same limitations as for gene expression analyses), selected cells and even in situ [53,54].
7.3 In vivo analysis of reverse cholesterol transport
As noted above, anti-miR-33 treatment of LDLr−/− mice led to increased reverse cholesterol transport (RCT) and the regression of atherosclerosis [34]. A number of approaches to increasing RCT are under study by academic and pharmaceutical investigators, with the hope that delayed progression or regression of atherosclerosis will result, as in the miR-33 study. This has identified a need for in vivo testing of RCT. Recently, a novel in vivo method for measuring cholesterol efflux from macrophages in mice was introduced by Daniel Rader and colleagues [55]. The assay traces the movement of radiolabeled cholesterol from cholesterolenriched macrophages injected into the peritoneum of a mouse into the bloodstream and the feces. In an extension of this method, by entrapping macrophages in semipermeable hollow fibers before implantation into the peritoneum, Weibl et al. were able to recover these cells at time points for the analysis of changes in cholesterol mass, a more rigorous test of cholesterol efflux [56]. Thus, it is now possible to test in vivo the effects of different genetic or pharmacological manipulations on cholesterol efflux from macrophages and the delivery of this cholesterol to the liver for elimination in the bile.
8. Conclusion
Atherosclerosis is the leading cause of death in the Western world. Despite huge advances in the understanding of the disease pathophysiology, current treatment is mostly based on ‘traditional’ risk factors, leaving a high residual risk for cardiovascular events and emphasizing the need for novel therapeutic targets. Mice are the most common used animal model in preclinical atherosclerosis studies. The information that can be gathered from atherosclerotic plaques of mice ranges from basic analysis (plaque size, morphology and (cell) composition) to more advanced analysis such as cellular phenotypic changes and gene expression profiles. Recent advances in genomics, proteomics, lipidomics and high-throughput screening techniques immensely help to uncover disease-related pathways and to discover potential treatment targets. Thereby, the ultimate treatment goal is not only to halt further progression of the disease, but also to achieve regression of existing plaques.
9. Expert opinion
The translation of findings derived from preclinical studies to humans is challenging, cost-intensive and requires many years and huge expenses until a beneficial outcome is established. At a minimum, if valid biomarkers and molecular imaging targets of atherosclerosis progression and regression can be gleaned from the types of studies we have described, these would have great adjunctive value in the clinic by allowing for individual risk assessment and response to therapy. More ambitious, of course, is to find factors that are promising therapeutic targets or to test new agents against established or newly discovered targets. Given the large (> 90%) overlap between the human and mouse genomes, the results of mouse studies will frequently be useful in ‘go/no go’ decisions in the pharmaceutical industry before conducting extensive clinical cardiovascular trials [7]. For example, the relevance of the two subsets of circulating monocytes to atherosclerosis in mice has provided catalytic insights to study the utility of the corresponding human subsets as predictive biomarkers in cardiovascular disease. Indeed, increased levels of CD14+CD16+ monocytes have been associated with the prevalence of CAD [56], and decreased CD14lowCD16+ monocytes have been found in CAD patients with positive family history and smoking [57]. By contrast, CD14++CD16− monocyte levels were increased with high risk and positive family history for CAD [57] and peak levels of CD14++CD16− monocyte counts were negatively correlated to left ventricular recovery outcome after acute myocardial infarction [58].
As alluded to above, imaging techniques to visualize plaque composition and inflammatory processes in human atherosclerotic plaques are hot topics. Current imaging techniques for the detection of atherosclerosis in human include invasive techniques (coronary angiography, intravascular ultrasound – IVUS), vascular ultrasound (including carotid-intimal-medial thickness – CIMT), computer tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET). Coronary angiography is the most validated imaging technique for the detection of coronary atherosclerosis, but it is two-dimensional. Even in combination with IVUS, it remains difficult to evaluate plaque composition and inflammatory processes [7]. A novel IVUS-derived technique, virtual histology (VH-IVUS), based on advanced radiofrequency analysis of ultrasound signals provides detailed analysis of plaque composition in the form of a reconstructed color-coded tissue map. VH-IVUS has been validated to differentiate among four basic tissue types: fibrous tissue, fibrofatty tissue, necrotic core and dense calcium. Although VH-IVUS already gives a more comprehensive assessment of plaque composition, inflammatory processes of the plaque still cannot be directly visualized [59]. In preclinical studies, nanoparticles carrying gadolinium have been shown to enhance the imaging of macrophage-rich plaques [60], and in human, fluoro-1-deoxyglucose PET-CT has shown promise to visualize activated macrophages in carotid plaques [61]. The dal-Plaque study is a recent example of using imaging techniques in clinical settings to assess the effect of a drug. The study evaluated the efficacy and safety of CETP inhibitor dalcetrapib in atherosclerosis by using a dual-imaging approach of MRI (for the assessment of morphological changes of the vessel wall) and PET-CT (for the assessment of vascular inflammation). One hundred and thirty patients with, or high risk for, CAD were randomized to either dalcetrapib or placebo treatment for 24 months. Both imaging techniques did not show evidence of a pathological effect of dalcetrapib on the arterial wall; actually MRI provided evidence for a beneficial vascular effect by a reduced enlargement of vessel area in patients given dalcetrapib [62].
It is important to continue and expand such studies to facilitate and expedite the translation of findings derived from preclinical studies to clinical application in atherosclerosis.
Article highlights.
Therapy of atherosclerosis is mainly based on treatment of traditional risk factors; processes that are based on disease causality such as inflammation should be targeted.
Basic analysis of atherosclerotic plaques includes information on lesion size and plaque composition such as content of lipids, macrophages, collagen and smooth muscle cells.
Advanced lesion analysis includes information on phenotypic properties of plaque cells such as M1 and M2 macrophages.
Valid biomarkers are needed to facilitate the translation of findings from animal studies to humans.
This box summarizes key points contained in the article.
Acknowledgments
B Hewing is supported by a grant from the German Research Foundation (DFG: HE 6092/1-1). His research on atherosclerosis is supported by NIH grants HL 084312 and HL 098055.
Footnotes
Declaration of interest E Fisher declares no conflict of interest and has received no payment in the preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–61. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–22. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–12. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 5.Plump AS, Lum PY. Genomics and cardiovascular drug development. J Am Coll Cardiol. 2009;53:1089–100. doi: 10.1016/j.jacc.2008.11.050. •• A review on the application of genomics to the development of novel atherosclerosis therapies.
- 6.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 7.Charo IF, Taub R. Anti-inflammatory therapeutics for the treatment of atherosclerosis. Nat Rev Drug Discov. 2011;10:365–76. doi: 10.1038/nrd3444. •• A review of current concepts of anti-inflammatory therapeutics in atherosclerosis.
- 8.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5:91–102. doi: 10.1038/ncpcardio1086. • A comprehensive review of human and animal studies of atherosclerosis regression.
- 9.Virmani R, Kolodgie FD, Burke AP, et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–75. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 10.Vilahur G, Padro T, Badimon L. Atherosclerosis and thrombosis: insights from large animal models. J Biomed Biotechnol. 2011;2011:907575. doi: 10.1155/2011/907575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breslow JL. Mouse models of atherosclerosis. Science. 1996;272:685–8. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 12.Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo e. Evaluation of lesional development and progression. Arterioscler Thromb J Vasc Biol Am Heart Assoc. 1994;14:141–7. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- 13.Teupser D, Persky AD, Breslow JL. Induction of atherosclerosis by low-fat, semisynthetic diets in ldl receptor-deficient c57bl/6j and fvb/nj mice: comparison of lesions of the aortic root, brachiocephalic artery, and whole aorta (en face measurement) Arterioscler Thromb Vasc Biol. 2003;23:1907–13. doi: 10.1161/01.ATV.0000090126.34881.B1. [DOI] [PubMed] [Google Scholar]
- 14.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, american heart association. Circulation. 1995;92:1355–74. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 15.Whitman SC. A practical approach to using mice in atherosclerosis research. Clin Biochem Rev Australian Assoc Clin Biochem. 2004;25:81–93. [PMC free article] [PubMed] [Google Scholar]
- 16.Allayee H, Ghazalpour A, Lusis AJ. Using mice to dissect genetic factors in atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1501–9. doi: 10.1161/01.ATV.0000090886.40027.DC. [DOI] [PubMed] [Google Scholar]
- 17.Tanigawa H, Billheimer JT, Tohyama J, et al. Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation. 2007;116:1267–73. doi: 10.1161/CIRCULATIONAHA.107.704254. [DOI] [PubMed] [Google Scholar]
- 18.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 19.Calara F, Silvestre M, Casanada F, et al. Spontaneous plaque rupture and secondary thrombosis in apolipoprotein e-deficient and ldl receptor-deficient mice. J Pathol. 2001;195:257–63. doi: 10.1002/path.915. [DOI] [PubMed] [Google Scholar]
- 20.Jackson CL, Bennett MR, Biessen EA, et al. Assessment of unstable atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2007;27:714–20. doi: 10.1161/01.ATV.0000261873.86623.e1. [DOI] [PubMed] [Google Scholar]
- 21.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. •• A review of monocyte and macrophage heterogeneity in human and animals.
- 22.Wilson HM. Macrophages heterogeneity in atherosclerosis - implications for therapy. J Cell Mol Med. 2010;14:2055–65. doi: 10.1111/j.1582-4934.2010.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–11. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 24.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–6. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trogan E, Feig JE, Dogan S, et al. Gene expression changes in foam cells and the role of chemokine receptor ccr7 during atherosclerosis regression in apoe-deficient mice. Proc Natl Acad Sci USA. 2006;103:3781–6. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potteaux S, Gautier EL, Hutchison SB, et al. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–36. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Gils JM, Derby MC, Fernandes LR, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–43. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hristov M, Weber C. Differential role of monocyte subsets in atherosclerosis. Thromb Haemost. 2011;106:757–62. doi: 10.1160/TH11-07-0500. • A review of monocyte subsets in human and mice.
- 31.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–16. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Combadiere C, Potteaux S, Rodero M, et al. Combined inhibition of ccl2, cx3cr1, and ccr5 abrogates ly6c(hi) and ly6c(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–57. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 33.Rayner KJ, Suarez Y, Davalos A, et al. Mir-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–3. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rayner KJ, Sheedy FJ, Esau CC, et al. Antagonism of mir-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–31. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feig JE, Rong JX, Shamir R, et al. Hdl promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci USA. 2011;108:7166–71. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chereshnev I, Trogan E, Omerhodzic S, et al. Mouse model of heterotopic aortic arch transplantation. J Surg Res. 2003;111:171–6. doi: 10.1016/s0022-4804(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 37.Feig JE, Parathath S, Rong JX, et al. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–98. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lieu HD, Withycombe SK, Walker Q, et al. Eliminating atherogenesis in mice by switching off hepatic lipoprotein secretion. Circulation. 2003;107:1315–21. doi: 10.1161/01.cir.0000054781.50889.0c. [DOI] [PubMed] [Google Scholar]
- 39.Van Craeyveld E, Gordts SC, Nefyodova E, et al. Regression and stabilization of advanced murine atherosclerotic lesions: a comparison of ldl lowering and hdl raising gene transfer strategies. J Mol Med. 2011;89:555–67. doi: 10.1007/s00109-011-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritter T, Lehmann M, Volk HD. Improvements in gene therapy: averting the immune response to adenoviral vectors. BioDrugs. 2002;16:3–10. doi: 10.2165/00063030-200216010-00001. [DOI] [PubMed] [Google Scholar]
- 41.Reis ED, Li J, Fayad ZA, et al. Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein e-deficient mouse in a novel transplantation model. J Vasc Surg. 2001;34:541–7. doi: 10.1067/mva.2001.115963. [DOI] [PubMed] [Google Scholar]
- 42.Rong JX, Li J, Reis ED, et al. Elevating high-density lipoprotein cholesterol in apolipoprotein e-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104:2447–52. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- 43.Brautbar A, Ballantyne CM. Pharmacological strategies for lowering ldl cholesterol: statins and beyond. Nat Rev Cardiol. 2011;8:253–65. doi: 10.1038/nrcardio.2011.2. [DOI] [PubMed] [Google Scholar]
- 44.Deng DX, Tsalenko A, Vailaya A, et al. Differences in vascular bed disease susceptibility reflect differences in gene expression response to atherogenic stimuli. Circ Res. 2006;98:200–8. doi: 10.1161/01.RES.0000200738.50997.f2. [DOI] [PubMed] [Google Scholar]
- 45.Burridge KA, Friedman MH. Environment and vascular bed origin influence differences in endothelial transcriptional profiles of coronary and iliac arteries. Am J physiol Heart Circ physiol. 2010;299:H837–46. doi: 10.1152/ajpheart.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeBakey ME, Lawrie GM, Glaeser DH. Patterns of atherosclerosis and their surgical significance. Ann Surg. 1985;201:115–31. doi: 10.1097/00000658-198502000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in ldl receptor-deficient and apolipoprotein e-deficient mice. J Lipid Res. 1995;36:2320–8. [PubMed] [Google Scholar]
- 48.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trogan E, Choudhury RP, Dansky HM, et al. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein e-deficient mice. Proc Natl Acad Sci USA. 2002;99:2234–9. doi: 10.1073/pnas.042683999. • The first article describing the use of laser capture microdissection in atherosclerosis.
- 50.Herrmann J. New kits on the blot–can we microarray the future of atherosclerosis? Cardiovasc Res. 2003;60:220–2. doi: 10.1016/s0008-6363(03)00542-x. [DOI] [PubMed] [Google Scholar]
- 51.Ramsey SA, Gold ES, Aderem A. A systems biology approach to understanding atherosclerosis. EMBO Molr Med. 2010;2:79–89. doi: 10.1002/emmm.201000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinet W. Western array analysis of human atherosclerotic plaques. Methods Mol Biol. 2007;357:165–78. doi: 10.1385/1-59745-214-9:165. [DOI] [PubMed] [Google Scholar]
- 53.Chaurand P, Norris JL, Cornett DS, et al. New developments in profiling and imaging of proteins from tissue sections by maldi mass spectrometry. J Proteome Res. 2006;5:2889–900. doi: 10.1021/pr060346u. [DOI] [PubMed] [Google Scholar]
- 54.Bowlus CL, Seeley EH, Roder J, et al. In situ mass spectrometry of autoimmune liver diseases. Cell Mol Immunol. 2011;8:237–42. doi: 10.1038/cmi.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Zanotti I, Reilly MP, et al. Overexpression of apolipoprotein a-i promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–3. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 56.Schlitt A, Heine GH, Blankenberg S, et al. Cd14+cd16+ monocytes in coronary artery disease and their relationship to serum tnf-alpha levels. Thromb Haemost. 2004;92:419–24. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 57.Hristov M, Leyendecker T, Schuhmann C, et al. Circulating monocyte subsets and cardiovascular risk factors in coronary artery disease. Thromb Haemost. 2010;104:412–14. doi: 10.1160/TH10-01-0069. [DOI] [PubMed] [Google Scholar]
- 58.Tsujioka H, Imanishi T, Ikejima H, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54:130–8. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 59.Konig A, Margolis MP, Virmani R, et al. Technology insight: in vivo coronary plaque classification by intravascular ultrasonography radiofrequency analysis. Nat Clin pract Cardiovasc med. 2008;5:219–29. doi: 10.1038/ncpcardio1123. [DOI] [PubMed] [Google Scholar]
- 60.Choudhury RP, Fisher EA. Molecular imaging in atherosclerosis, thrombosis, and vascular inflammation. Arterioscler Thromb Vasc Biol. 2009;29:983–91. doi: 10.1161/ATVBAHA.108.165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nahrendorf M, Zhang H, Hembrador S, et al. Nanoparticle pet-ct imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–87. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fayad ZA, Mani V, Woodward M, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-plaque): a randomised clinical trial. Lancet. 2011;378:1547–59. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]