Abstract
What you see depends not only on where you are looking but also on where you will look next. The pre-saccadic attention shift is an automatic enhancement of visual sensitivity at the target of the next saccade. We investigated whether and how perceptual factors independent of the oculomotor plan modulate pre-saccadic attention within and across trials. Observers made saccades to one (the target) of six patches of moving dots and discriminated a brief luminance pulse (the probe) that appeared at an unpredictable location. Sensitivity to the probe was always higher at the target’s location (spatial attention), and this attention effect was stronger if the previous probe appeared at the previous target’s location. Furthermore, sensitivity was higher for probes moving in directions similar to the target’s direction (feature-based attention), but only when the previous probe moved in the same direction as the previous target. Therefore, implicit cognitive processes permeate pre-saccadic attention, so that–contingent on recent experience–it flexibly distributes resources to potentially relevant locations and features.
Keywords: pre-saccadic attention, spatial attention, feature-based attention, eye movements, motion perception, intertrial effects
When observing a complex scene, we make many rapid eye movements (saccades) to view objects of interest with the high-resolution fovea. Curiously, we get better at seeing the next target object–and worse at seeing others–even before the eyes begin to move (e.g., Deubel & Schneider, 1996; Gersch et al., 2008, 2009; Gersch, Kowler & Dosher, 2004; Godijn & Theeuwes, 2003; Kowler et al., 1995). This “pre-saccadic attention shift” reveals a link between eye movement preparation and covert spatial attention, which can be deployed selectively to relevant locations even without directing our gaze to them (for a review on covert attention see Carrasco, 2011). Pre-saccadic changes in performance are accompanied by changes in appearance—the perceived intensity of the target increases shortly before a saccade (Rolfs & Carrasco, 2012). Pre-saccadic attention indeed changes the quality of visual representations (via signal enhancement and noise reduction; Zhao et al., in press). Correspondingly, physiological data demonstrate partial overlap between the neural circuits responsible for saccade preparation and for attentional enhancements in retinotopic visual cortex (reviewed by Awh, Armstrong & Moore, 2006).
What is the function of the pre-saccadic attention shift, and how strictly is it tied to the oculomotor plan? Some evidence suggests that it may be merely an obligatory stage of motor preparation that helps us acquire visual information necessary to move the eyes quickly and precisely. The deployment of pre-saccadic attention is closely locked in space to the target and in time to the saccade onset. Moreover, voluntary attention cannot override it: sensitivity is still highest at the saccade target even when observers know that a task-relevant stimulus is most likely to appear somewhere else (Deubel, 2008; Deubel & Schneider, 1996, 2003; Hoffman & Subramaniam, 1995). Attention to a relevant location in addition to the saccade target can improve sensitivity there (Montagnini & Castet, 2007), but doing so often comes at a cost to saccadic latency (Deubel, 2008; Hoffman & Subramaniam, 1995; Kowler et al 1995) and accuracy (Kowler et al 1995).
In addition to guiding motor preparation, pre-saccadic attention may function to improve the perception of potentially relevant stimuli. For instance, it could begin prioritized processing of the next target while the saccade is still being prepared (e.g., Henderson, Pollatsek & Rayner, 1989), and help integrate information about important objects across the different retinal images acquired before and after the movement (Mathot & Theeuwes, 2011; Rolfs et al., 2011). Usually, the saccade target is behaviorally relevant, which is why it has been selected for the next fixation and perhaps why it is covertly attended. But other stimuli may be simultaneously relevant to the observer’s ongoing goals, even if they are not relevant to the eye movement itself. The brain’s estimate of their relevance could depend on their visual features, and could be implicitly updated by recent experience.
Accordingly, we made the following predictions: if the only function of the pre-saccadic attention shift were to guide the eye movement or boost processing of the target, then every time an observer prepares a saccade to a particular location, sensitivity at each location should be modulated by the same magnitude. However, if pre-saccadic attention also took into account recent experience and the potential relevance of stimuli across the visual field, then sensitivity should depend on perceptual factors independent of the particular motor plan.
We investigated how two such factors influence the dynamics of visual sensitivity during saccade preparation, in the absence of any explicit task strategy. The first factor was the broader temporal context of the ongoing perceptual task while observers made a saccade to a particular location. In other experimental paradigms, events on recent trials implicitly bias the allocation of attention towards particular locations or features (e.g., Maljkovic & Nakayama, 1994; Found & Müller, 1996; Chun & Jiang, 1998; Kristjánnson, 2006), suggesting that the attentional system assumes that recent experience is predictive of future experience and adapts accordingly. We investigated unexplored intertrial effects that depend on the relations between locations or between features in previous trials. Specifically, we measured how the magnitude of the spatial attention shift driven by a particular eye movement depends on whether the locations of the task-relevant stimulus and the eye movement target had matched on the previous trial. If they had not, an adaptive mechanism may widen the distribution of pre-saccadic attention, to better perceive objects distant from the next saccade target.
The second factor we investigated deals with the visual features of the saccade target. Feature-based attention enhances sensitivity for particular feature values such as colors or directions of motion (e.g., Alais & Blake, 1999; Baldassi & Verghese, 2005; Liu, Stevens & Carrasco, 2007), and that enhancement spreads automatically across the visual field (e.g., Arman, Ciaramitaro, Boynton, 2006; Liu & Mance, 2011; Martinez-Trujillo & Treue, 2004; Serences & Boynton, 2007; White & Carrasco, 2011). Moreover, feature-based attention similarly affects perception and smooth pursuit eye movements (Spering & Carrasco, 2012). Does the shift of attention to the target of an impending saccade also result in an automatic attentional enhancement of the target’s features across the visual field? Perception could benefit from a global enhancement of target features just before the saccade shifts all stimuli to new locations on the retina, even without a voluntary top-down ‘task set’ for those features.
We investigated this issue by measuring sensitivity for stimuli far from the saccade target as a function of their feature similarity–in terms of motion direction–to the target. We also investigated a feature-based attention intertrial effect analogous to the spatial one described above: whether global feature-based enhancement on the current trial depends on the featural similarity between the previous saccade target and the previous task-relevant stimulus.
Observers had a simple dual task: to saccade to a cued location and discriminate a luminance change that could appear anywhere. Unbeknownst to them, we measured shifts of spatial and feature-based attention and how those developed from millisecond to millisecond and trial to trial. The display consisted of six patches of moving dots, one of which was the saccade target and one of which underwent a brief contrast increment (the probe) during saccade preparation (Fig. 1). To measure spatial attention we evaluated sensitivity to the probe as a function of its distance from the saccade target. To measure feature-based attention we evaluated sensitivity to the probe as a function of its similarity in motion direction to the saccade target, which was irrelevant to the task. In addition, the time between the saccade cue and the probe was variable, as was the time between the probe and the saccade onset. This allowed us to measure the temporal dynamics of the attentional effects, and to examine the stages of saccade preparation during which the intertrial context and target features influence attention and perception.
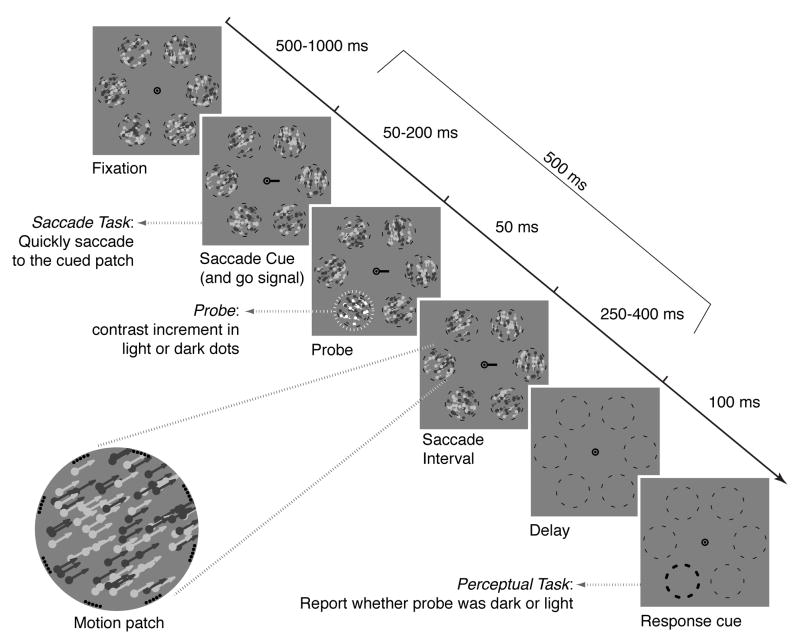
Figure 1.
Example trial sequence in which the saccade target is horizontally to the right of fixation and a white probe appears down and to the left. The total time between cue onset and dots offset was always 500 ms, and in all trials analyzed the saccade began between the probe offset and the dots offset. Arrowheads on dots and white highlight around probe location were not present in the experiment. Not to scale.
Methods
Observers
Eight observers (ages 22–29; 4 female) with normal visual acuity participated in the experiment. All but one (author A.W.) were naïve as to the goals of the study, and all gave informed consent. The NYU Institutional Review Board approved the study.
Experimental Setup and Stimuli
Observers sat in a silent and dimly lit room with their heads positioned on a chin rest 57 cm from a gamma-linearized 22″ Sony GDM-F520 screen (1280×960 pixels, 100 Hz refresh rate). We recorded the right eye’s gaze position using an EyeLink 1000 Desktop Mount (SR Research, Ontario, Canada). An Apple iMac computer running MATLAB (MathWorks, Massachusetts, USA) with the Psychophysics and Eyelink toolboxes (Brainard, 1997; Pelli, 1997; Cornelissen et al., 2002) controlled stimulus presentation and response collection.
The stimulus array (Fig. 1) consisted of six groups of moving dots on a gray background (60.4 cd/m2). Each group was constrained to a 4° diameter circular aperture (with a dashed black outline) centered on an imaginary ring 6° from the central fixation point (a 0.3° diameter circle). The 50 dots (0.06° diameter) in each group moved coherently at 5°/s in a direction randomly selected from a set of six (30–330°) with the constraint that on every trial exactly two groups moved in the same direction. Dots reaching the edge of the aperture moved to the opposite edge, randomly displaced orthogonal to their motion. Within each group, half of the dots were light (82.5 cd/m2) and half dark (34.2 cd/m2).
Procedure and task
Each trial began with the dots moving for 500–1000 ms while the observer fixated centrally [Fig. 1]. The eye movement cue, a 0.4° black line, then appeared at the fixation mark pointing towards a randomly selected dot group (the saccade target). We instructed observers to saccade as quickly as possible to the target center upon seeing the cue. 50–200 ms after cue onset, either the light or dark dots in an independently selected dot group underwent a 50-ms contrast increment (the probe), whose magnitude we individually adjusted in pretests (see below). For observers whose saccade latencies tended to be short, causing probes on many trials to appear after saccade onset, we reduced the maximum cue-probe delay (≥130 ms). The dots remained visible until 500 ms after the cue onset, when they and the movement cue disappeared, leaving only the fixation mark and the dashed outlines.
After 100 ms, the probe’s location outline became thicker. This response cue prompted observers to report with a keypress whether the contrast increment at that location had occurred in the light or dark dots. A tone indicated response correctness. We instructed observers that, on any given trial, the probe could appear at any location with equal probability, coinciding with the saccade target on only 1/6 of trials. The dots’ motion was irrelevant to the task.
Trials were immediately aborted and repeated later in the block if gaze shifted >2° from the fixation mark during the interval before the cue, or if gaze did not land <3° from the target center 500 ms after movement cue onset and remain there for ≥30 ms.
The magnitude of the probe’s contrast pulse was determined for each observer in pre-tests, in which observers maintained fixation and a ring appeared around the fixation mark instead of the movement cue. Observers reported whether the probe was on the left or right half of the screen, rather than its polarity. Over two blocks of 60 trials, a Quest staircase (Watson & Pelli, 1983) adjusted the magnitude of the luminance change separately for the light and dark dots and converged on 80% correct thresholds, which were used in the main experiment. Pretests were repeated between blocks if needed to keep overall performance between 70% and 90% correct.
Observers completed 7–9 one-hour-long sessions of 8–12 blocks, each composed of 72 trials, for ~5,570 total trials per observer. We calibrated the eye-tracker with a standard 9-point routine at the start of each or every other block, as necessary.
Data analysis
Eye movements
Using low-pass filtered eye position data, we detected saccades offline based on their 2D velocity distribution in each trial (Engbert & Mergenthaler, 2006). To do this, we computed smoothed eye velocities using a moving average over five subsequent eye position samples (5 ms). Saccades were events that exceeded the median velocity by 5 SDs for at least 8 ms. We merged events separated by 10 ms or less into a single saccade, avoiding the detection of saccadic overshoots as separate saccades. The response saccade was the first saccade that left a circular fixation region and landed inside a target area, each having a radius of 3°. We rejected trials with blinks, saccades larger than 1° before a response saccade, saccades that lasted more than 100 ms from onset to landing, and trials in which the response saccade did not start between 70 and 500 ms after the cue onset. Finally, we also excluded trials in which a return saccade to the central fixation mark began less than 250 ms after the landing of the saccade to the target. Although such trials were rare (<1%), this was done in order to avoid contamination of performance by attention shifts related to planning return saccades at the end of the trial.
Visual sensitivity
To analyze behavioral performance, for each condition of interest we computed the observer’s sensitivity for discriminating the contrast polarity of the probe:
where z is the inverse of the normal cumulative density function, prespond light | light is the proportion of correct responses to probes in light dots (the analogue of the hit rate if this had been a detection task with light probes only), and prespond light | dark is the propability of an incorrect response to probes in dark dots (the analogue of the false alarm rate).
All analyses of sensitivity reported below only included trials in which the probe offset occurred before the saccade onset. This condition, combined with the other offline rejection criteria applied (see above), resulted in an average of 26% of trials being excluded from the analysis. Repeated-measures ANOVAs were performed using the Huynh-Felt correction of the degrees of freedom. Follow-up t-tests between pairs of conditions were corrected for multiple comparisons using the Dunn-Šidák procedure. Error bars on plots are +/− 1 SEM computed after removing each observer’s global mean across conditions, and then scaled by J/(J−1), where J is the number of within-subject conditions in the analysis, following Morey (2008).
Temporal dynamics
To analyze the temporal dynamics of pre-saccadic attention, we measured how performance in each condition (e.g., probe-target distance) changed both with time between the cue and probe onsets (cue-locked analysis) and between the probe offset and the saccade onset (saccade-locked analysis). To do so, for each observer and relative position we computed d′ at each millisecond by convolving hit and false alarm rates with a bisquare filter w(t), resulting in temporal smoothing:
Here, t is time in milliseconds (between cue and probe or between probe and saccade), t0 is the filter center, and k controls the width of the entire filter. k was set to 30 ms. Only timepoints t for which the sum of w(t) exceeded 40 trials for each observer were included in this analysis.
To evaluate when sensitivity at different locations (or directions) differed from each other, and how those differences evolved over time, we bootstrapped d′ values at each time point. On each boostrap repetition we resampled with replacement from all 8 observers, and for each observer generated new hit and false alarm rates at each time point by drawing from binomial distributions with the original means and trial numbers. These rates were temporally smoothed to compute d′ at each time point as described above. We subtracted the resampled observer’s overall mean (across timepoints) from his bootstrapped data, and then averaged across the 8 resampled observers to create a bootstrapped timecourse. Then, after 10,000 repetitions, we computed 95% confidence intervals of the bootstrapped d′ distributions at each timepoint (plotted in Figure 3).
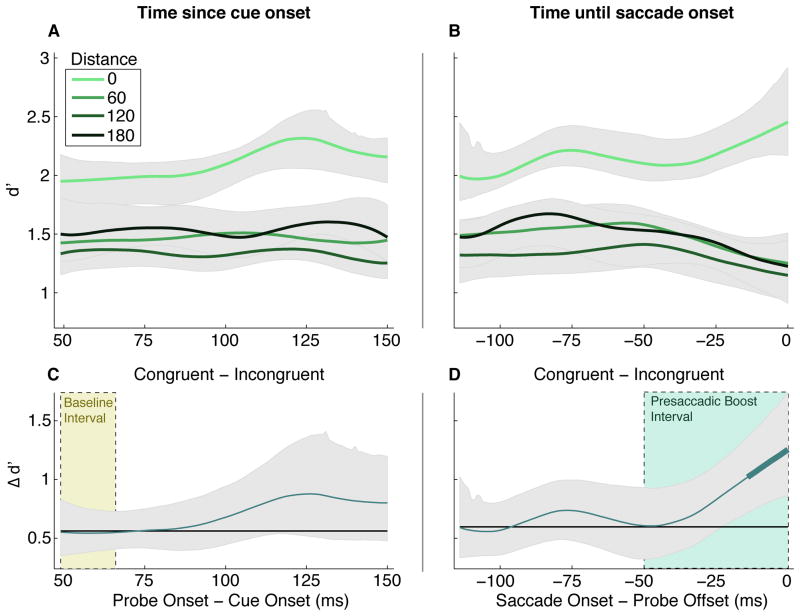
Figure 3.
Temporal dynamics of pre-saccadic spatial attention. Left Panels (A and C): Sensitivity as a function of time between cue onset and probe onset. Right Panels (B and D): Sensitivity as a function of time between probe offset and saccade onset. Top panels (A and B): Each solid line represents one probe-target distance, indicated by the legend on the left. Shaded regions are 95% confidence intervals from bootstrapping. Bottom panels (C and D): The difference between performance at the saccade target (congruent) and elsewhere (incongruent), relative to the magnitude of that difference (black line) in the Baseline Interval containing the earliest 100 trials. Thicker points indicate significant change from the early baseline.
To compare d′ across pairs of conditions, on each bootstrap repetition for each resampled observer we computed the difference of resampled d′ across conditions. From this we generated 95% confidence intervals of the difference at each timepoint. To compute the statistical signifiance of the differences, we estimated a p-value for the null hypothesis that the mean difference was not different from 0 by estimating what fraction of the bootstrapped distribution lay beyond 0. To correct for multiple comparisons we constrained the False Discovery Rate to 0.05 using the procedure of Benjamini and Hochberg (1995).
We also measured how the differences between conditions developed over time (e.g., if the relative benefit for probes at the saccade target vs elsewhere increased with time after the cue). To do this, we first computed the magnitude of the difference in an early interval to be used as a baseline. The start of that interval was the earliest possible probe time. To define the end of that interval, for each observer we sorted all trials by the time of the probe, from earliest to latest, and averaged across observers the time of the 100th trial in that list. The baseline intervals were 50 to 66 ms for the cue-locked analysis and −330 to −115 ms for the saccade-locked analysis. When bootstrapping, the mean difference across time was compared to this early baseline d′ value, and the statistical significance of deviations from that baseline was determined using the False Discovery Rate procedure.
Results
Overall attentional effects
Spatial attention
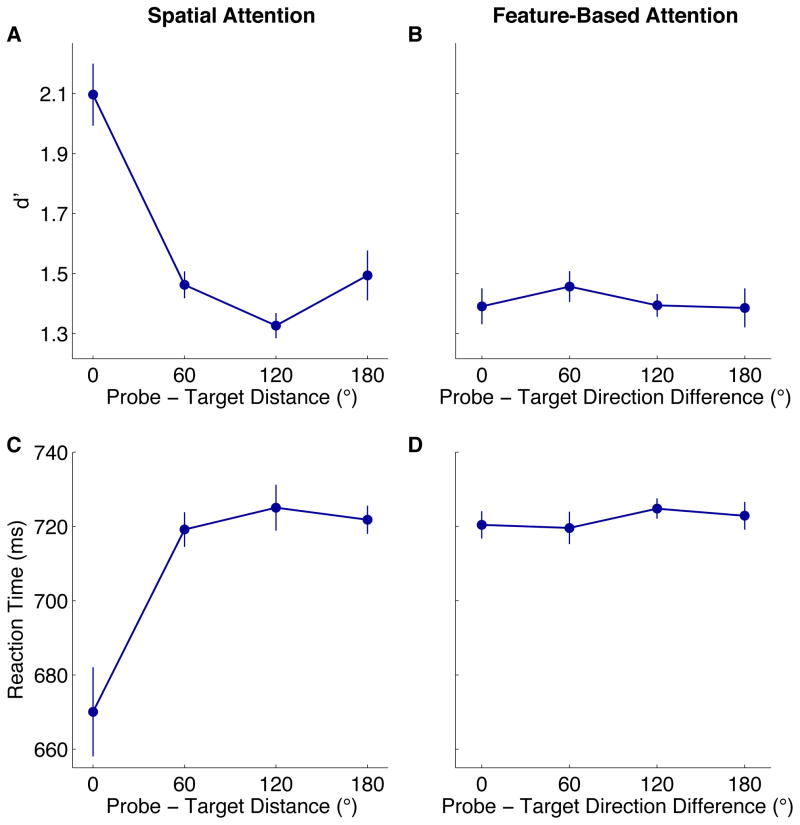
Observer’s ability to report the contrast polarity of the probe depended strongly on where it occurred relative to the saccade target [Fig. 2A], despite the equal distance of all dot groups from fixation and the equal likelihood of the probe appearing at any of them (F(1.9, 13.5)=29.4, p<0.001). Sensitivity was higher at the saccade target than at any other location (all ps<0.05). No other pairwise comparisons were significant.
Figure 2.
Spatial and feature-based attention. Effect of distance between the probe and saccade target on sensitivity (A) and manual reaction time (C). Effect of the difference in motion directions of probe and saccade target on sensitivity (B) and manual reaction time (D) (excluding trials with the probe at the saccade target). Error bars are ±1 SEM across observers.
The pattern of manual response times (computed from the onset of the probe) mirrored those of sensitivity [Fig. 2C]: probe-target distance significantly affected response time (F(1.3,9.0)=16.6, p<0.01); responses to probes at the target location were significantly faster than all others (p<0.05, corrected).
Saccade landing errors (unsigned distance between landing position and target center; mean=1.38° visual angle) were also affected by the probe’s location (F(3.0,21.0)=8.3, p<0.01). Errors were lowest when the probe was at the saccade target and highest for probes 60° of polar angle away (specifically, 0.08° higher, p<0.05). Saccade latencies (mean=233 ms) lengthened with increasing probe distance from the saccade target (F(2.2,15.2)=8.2, p<0.05), but only by 8 ms when the probe was 180° away (p=0.07). Thus, pre-saccadic probes at non-target locations interfered only slightly with the saccade.
Feature-based attention
We measured performance for probes that were not at the saccade target as a function of the difference between the probe and target’s motion directions. Overall, direction difference had no effect on sensitivity [Fig. 2B], response times [Fig. 2D], saccade landing errors, or saccadic latencies (all Fs<1). Feature-based attention, however, played a significant role across trials; see below.
Temporal dynamics
Spatial attention
We assessed how the profile of spatial attention shifted over time since the movement cue appeared [Fig. 3A, 3C]. At all cue-probe stimulus onset asynchronies (SOAs) that we tested (50–154 ms), sensitivity was significantly (p<0.05 from bootstrapping) higher at the saccade target (“congruent”) than all other locations (“incongruent”). There was no significant change over time in the difference between sensitivity at the saccade target and other locations relative to its early baseline [Fig. 3C].
We also analyzed sensitivity as a function of the time between probe offset and saccade onset [Fig. 3B, 3D]. Note that in this analysis, each time-point contains a distribution of cue-probe SOAs, dependent on the observers’ saccade latency distributions. The benefit at the saccade target relative to other locations was significant at all probe-saccade timepoints we tested (−114–0 ms). A strong temporal pattern also emerged: the difference between congruent and incongruent probes increased relative to its early baseline. Becoming significant in the final 14 ms, this monotonic increase in the attention effect began ~50–0ms before the saccade onset [Fig. 3D]. This suggests a link between the final stages of saccade preparation and the diversion of processing resources towards the target. We will refer henceforth to the interval 50–0 ms before the saccade as the “pre-saccadic boost interval.”
Feature-based attention
No effect of the difference in motion direction between the probe and the target emerged at any time after the cue or before the saccade.
Intertrial effects
Spatial attention
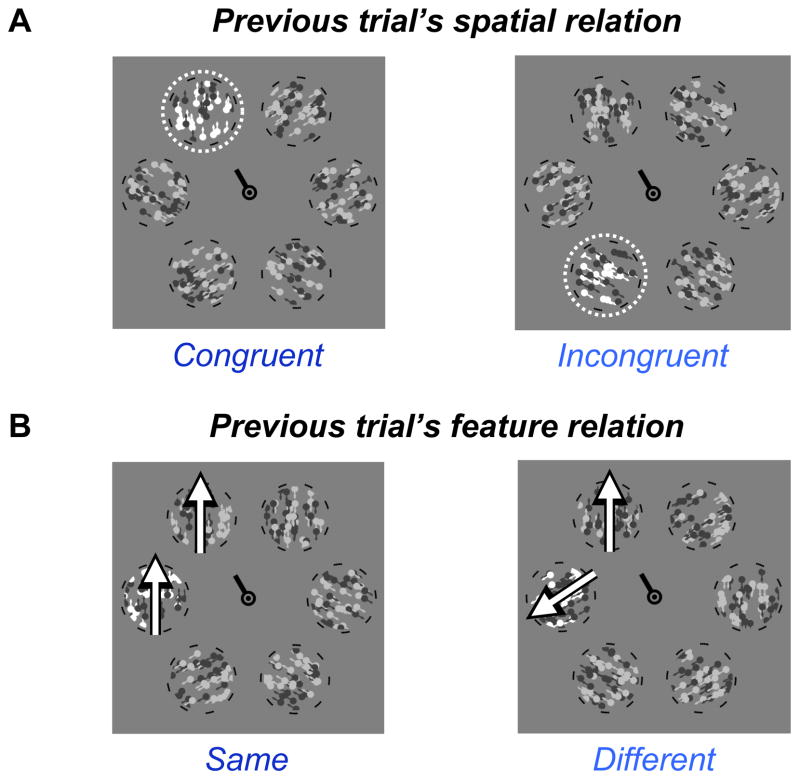
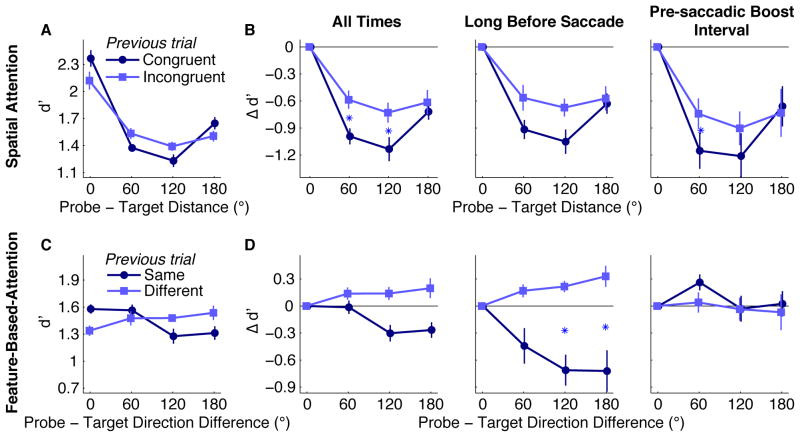
To examine whether the temporal context of the perceptual task modulates pre-saccadic attention, we categorized each trial n into one of two conditions depending on the spatial relation in the previous trial, n-1 (illustrated in Fig. 4A). Trial n-1 could have been “congruent”, meaning that the probe had appeared at the saccade target, or “incongruent,” meaning that the probe had appeared anywhere other than the saccade target. For both of these conditions we then measured the distribution of spatial attention relative to the current saccade target, wherever it happened to be. In this analysis we only included trials n in which the observer’s response on n-1 had been correct (77%), to ensure that observers were attentive enough to have seen the probes and been influenced by their location. We obtained the same pattern of results when error trials were included.
Figure 4.
Diagram of the previous trial conditions used in the intertrial analyses for spatial attention (A) and feature-based attention (B). The saccade target is indicated by the cue line at fixation. The probe is illustrated by the dashed outline in A, and the probe and target’s motion directions are illustrated by the arrows in B.
The previous trial’s spatial relation modulated the spatial profile of pre-saccadic attention (Fig. 5A). A 2×4 repeated-measures ANOVA revealed a main effect of probe-target distance (F(2.4,16.8)=50.14, p<0.001), but no main effect of the previous trial’s condition (F<1). Importantly, the interaction between the previous trial’s condition and the current trial’s probe-target distance was significant: F(3,21)=10.15, p<0.001. The significant interaction indicates that the effect of probe-target distance on the current trial (i.e., the spatial profile of attention) depended on whether the previous trial had been congruent or incongruent.
Figure 5.
Intertrial effects. A: Spatial Attention: The effect of probe-target distance, depending on whether the previous probe was at the previous saccade target (congruent) or not (incongruent). B: Same data as in A but plotted as differences from performance at the saccade target (“All Times”) and then divided by the time of the probe’s offset relative to saccade onset on the current trial (“Long Before Saccade” vs “Pre-saccadic Boost Interval”). C: Feature-Based Attention: The effect of probe-target direction differences in the current trial, depending on whether the previous probe and previous target moved in the same or different directions. D: Same data as in C but divided as in B. Asterisks are between points that differ across intertrial conditions. Error bars are ±1 SEM across observers.
To explore this interaction we compared the magnitude of the spatial attention effects under both intertrial conditions, previous trial congruent and previous trial incongruent, by computing the difference from d′ at the saccade target for each probe-target distance [Fig. 5B, left]. The effect of attention was greater following congruent than incongruent trials (F(1,7)=11.69, p<0.05), particularly at probe-target distances of 60° and 120° (p<0.05, corrected).1 Manual reaction times qualitatively mirrored the d′ results, showing no sign of a speed-accuracy tradeoff, but there was no significant interaction of distance and the previous trial’s spatial relation.
Does the previous trial modulate attention during all stages of saccade preparation? To find out, we tested whether the intertrial effect depends on how soon before the saccade the probe appeared on trial n; specifically, whether it is as strong in the pre-saccadic boost interval as long before the saccade [Fig. 5B, right and middle]. We found no sign of a 3-way interaction among time period, previous trial congruency, and probe-target distance (F<1), suggesting that the attention shift just before the saccade onset [Fig. 3] is not exclusively shaped by oculomotor preparation, but also influenced by recent experience.
Crucially, saccade latencies and landing errors were not affected by the previous trial’s congruency, nor was there any interaction with probe-target distance on the current trial (all Fs<1). Thus, the context of a perceptual task can affect the deployment of pre-saccadic attention independently of the particular eye movement being prepared, even in the last moments before the eyes begin to move.
In perception and cognition, the influence of previous trials most often shows up as priming: faciliation of responses for stimuli (or features of stimuli) that repeat. However, the intertrial effect on sensitivity reported here does not reflect that type of priming, which may have increased sensitivity for probes appearing at locations that had been relevant on the previous trial. In fact, there was no overall effect of the distance between the current probe and the previous probe, or between the current probe and the previous saccade target (Fs<1). Thus, the intertrial effect reported above is not priming in the traditional sense, but rather a flexible scaling of the attention shift depending on the spatial relations between the saccade target and the task-relevant stimulus in the previous trial.
Feature-based attention
Although we found no overall effect of feature-based attention, we investigated whether a global enhancement of the saccade target’s motion direction is contingent upon the similarity of probe and target within the previous trial. The results of this analysis, which is analogous to the spatial attention intertrial effect reported above, are plotted in Figure 5C. We computed sensitivity to non-target probes on trial n as a function of their direction difference from the target on trial n, divided by the feature relation on the previous trial, n-1 (illustrated in Fig. 4B). Trial n-1 could have been a “same” trial, meaning that the probe on that trial moved in the same direction as the target on that trial, or a “different” trial, meaning that the probe and target did not move in the same direction as each other.
A 2×4 repeated-measures ANOVA revealed that the main effect of the previous trial’s feature relation was not significant (F<1); nor was there a main effect of the current trial’s probe-target direction difference (F(2.8,19.6)=1.93, p>0.1). Importantly, however, the interaction between the previous trial’s feature relation and the current trial’s probe-target direction difference was significant: F(3,1)=5.91, p<0.01.
To explore this interaction we evaluated the effect of the current trial’s probe-target direction difference separately within each intertrial condition. Following a “different” trial, there was no such feature-based effect (F(3,21)=1.84, p>0.1). In contrast, after a “same” trial, sensitivity significantly decreased with increasing direction difference (F(3,21)=6.03, p<0.01).
This effect demonstrates that only when the previous probe and previous target had moved in the same direction as each other, the next target’s motion direction was enhanced across the display. Indeed, in that condition sensitivity to probes moving in the same direction as the target was significantly higher than to probes with directions 120° different (p<0.05). In Figure 5D (left) these data are plotted as the magnitude of the feature-based attention effect under both previous trial conditions, “same” and “different.” These values were computed at each direction difference by subtracting the d′ level for the current saccade target’s motion direction in that condition.
The previous trial did not significantly modulate manual reaction times (main effects: F<1, interaction: F(3,21)=1.46, p>0.2), and there was no sign of a speed-accuracy trade-off.
Does the feature-based intertrial effect on sensitivity change during the course of saccade preparation? We tested how the influence of the previous trial depended on whether the probe on trial n appeared long before the saccade or in the pre-saccadic boost interval. A three-way interaction among time period, previous trial’s feature relation, and direction difference was significant (F(3,21)=6.08, p<0.01). To interpret this complex pattern, we measured the two-way interactions of previous trial and motion direction difference separately within each time period.
Long before the saccade [Fig. 5D, middle], the previous trial’s condition significantly interacted with relative direction (F(2.23,15.9)=9.74, p=0.001). After a “same” trial, sensitivity to early probes decreased with direction difference (F(2.9,20.1)=7.44, p<0.01), whereas after a “different” trial sensitivity increased with direction difference (F(2.9,20.3)=4.56, p=0.01). In other words, long before the saccade, the current target’s motion direction was enhanced if the previous probe and previous target’s directions had matched, but relatively suppressed if the previous directions had not matched.
In the pre-saccadic boost interval, however, the intertrial effect collapsed and no effects of a two-way ANOVA of previous trial condition and relative direction were significant [Fig. 5D, right]. Therefore, the previous trial has no evident influence on motion sensitivity; feature-based attention plays no role soon before saccade onset. Rather, spatial attention dominates perceptual processing just before the eyes begin to move (Figs. 3B, 5B).
For saccade latencies and landing errors, there were no significant main effects nor interactions of the previous trial condition and relative direction (F<1). Therefore, these feature-based effects obtained during saccade preparation were independent of oculomotor performance.
Finally, the feature-based intertrial effect on sensitivity does not reflect priming of particular motion directions. Simple priming would have increased sensitivity for repeated directions. We found no effect of the direction difference between the current probe and the previous probe, nor of the direction difference between the current probe and the previous target (Fs<1). Therefore, analogous to the intertrial effect on spatial attention reported above, this feature-based modulation is not classical priming either, but rather a potentiation of feature-based attention to the current saccade target’s direction depending on the feature relations in the previous trial. If on a given trial, probe detection would have benefitted from attending to dots moving similarly to the target, then on the next trial the target’s direction was enhanced.
Intertrial effects: Looking two trials back
Spatial attention
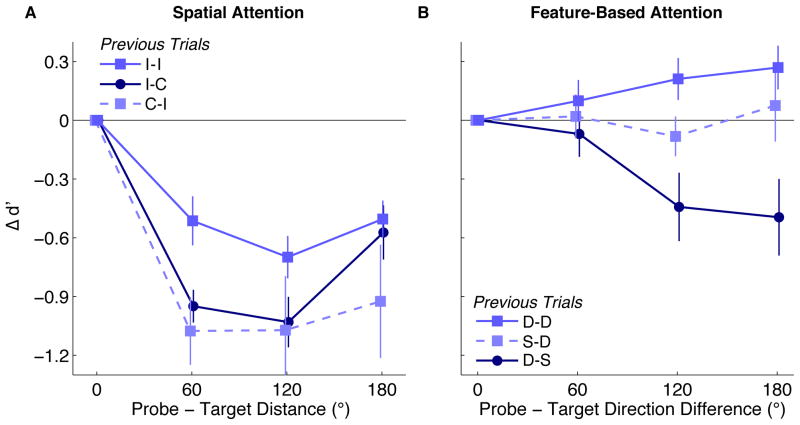
How long do the intertrial repetition effects last? Conversely, how many trials with probes different from the saccade target are required to divert attention away from the next saccade target? To investigate these questions for spatial attention, we divided the data further by whether the previous two trials (n-1 and n-2) had been congruent (probe at saccade target) or incongruent (probe not at saccade target). Specifically, we examined three conditions: when trial n-2 was incongruent but n-1 was congruent (“I-C”); vice versa (“C-I”); and when both trials n-1 and n-2 were incongruent (“I-I”). Figure 6A plots the effects of pre-saccadic spatial attention in each of these conditions, computed as differences in sensitivity (relative to d′ at the saccade target) as a function of probe-target distance on trial n. (There were too few trials (< 20 per distance) for a meaningful analysis of the condition in which the last two trials were both congruent).
Figure 6.
Intertrial effects of two previous trials. A: Spatial attention effect plotted as difference from d′ at the saccade target. “I-C” indicates that only the previous trial’s probe was at the saccade target (congruent), and the trial before that was incongruent. “C-I” is the opposite condition. “I-I” indicates that both of the previous two sequential trials were incongruent. B: Feature-based attention effect plotted as difference from d′ at 0° difference. Condition labels as in A, but for S (“Same”, probe direction = target direction) and D (“Different”, probe direction ~= target direction). Error bars are ±1 SEM across observers.
The “I-C” condition contains the same data as the congruent condition in Figure 5B, except it excludes the few trials in which trial n-2 was also congruent. Comparing that condition to “C-I” and “I-I” shows that the spatial profile of attention is only flattened out after two incongruent trials in a row. This could be because it takes at least two incongruent probes to widen the distribution of attention, or because the effect of a congruent probe to sharpen the distribution of attention lingers for longer than just the succeeding trial. These patterns were confirmed by repeated-measures ANOVAs, which revealed that only “I-C” and “I-I” differed significantly (main effect of previous trials: F(1,7)=7.07, p<0.05; interaction with distance: F(2,14)=3.95, p<0.05),
Feature-based attention
A similar pattern emerged for the feature-based intertrial effect. We analyzed three conditions looking two trials back for which we had sufficient data: when the probe and target directions on trial n-2 were the same but on trial n-1 were different (“S-D”); vice versa (“D-S”); and when the directions were different on both n-1 and n-2 (“D-D”).
For each of those conditions, we measured d′ as a function of the difference between the probe and target’s diretion on trial n, plotted as feature-based attention effects normalized to d′ for 0° difference (Figure 6B). Repeated-measures ANOVAs revealed that the only significant difference was between “D-S” and “D-D” (main effect of previous trials: F(1,7)=7.0, p<0.05; interaction with direction differences: F(2,14)=6.9, p<0.01).
Thus, it took a repetition of two trials with probes different in motion from the target for feature-based attention to shift from the current saccade target’s direction to other directions. Alternatively, the effect of a probe moving in the same direction as the target slowly decays over more than the duration of just a single trial.
Discussion
In the moments immediately preceding a saccade, the distribution of visual processing resources is complex and dynamic. The present results show that it depends not only on the parameters of the oculomotor plan, but also on recent experience with the spatial and featural similarity between saccade targets and behaviorally relevant stimuli.
We measured visual sensitivity to a brief contrast pulse presented while observers prepared a saccade to one of 6 patches of moving dots. The probe’s location coincided with the saccade target on a random 1/6 of trials. Nonetheless, sensitivity was ~50% higher for probes at the saccade target than elsewhere [Fig. 2A]. The benefit at the saccade target emerged very early, by 50 ms after cue onset [Fig. 3A], and sharpened just before the saccade onset [Fig. 3D]. This late modulation seems strongly related to the impending motor plan (consistent with Deubel, 2008, and Rolfs & Carrasco, 2012).
However, recent experience can divert more or fewer resources to the saccade target, without any changes to the task or to saccade latencies and precision. The difference between sensitivity at the saccade target and other locations was greater if the previous trial’s probe and target had overlapped than if they had not [Fig. 5B]. That is, if a task-relevant stimulus had recently appeared where the eyes were about to move, then attention more readily shifted to the next saccade target. But if that task-relevant stimulus had recently appeared far from where the eyes were moving, attentional resources were more evenly spread across space while preparing the next movement. Surprisingly, this intertrial effect remained in the pre-saccadic boost interval, when perceptual benefits became most pronounced at the movement goal.
Recent experience can also elicit a feature-based attentional enhancement of the saccade target’s motion direction across the visual field [Fig. 5C]. Although the similarity between the probe and target had no effect on sensitivity overall [Fig. 2B], the effect of global feature-based attention (e.g., Martinez-Trujillo & Treue, 2004; Serences & Boynton, 2007; White & Carrasco, 2011) was contingent upon events in previous trials. This could be an adaptive process that maximizes sensitivity for potentially relevant stimuli–which are likely to have similar features–distant from the target of an eye movement. If recent probes moved in the same direction as the recent saccade targets, then attention globally enhances the motion direction of the next saccade target; otherwise the next saccade target’s direction may if anything be suppressed[Fig. 5D]. Unlike the spatial attention intertrial effect, this priming of featural relations was strikingly present long before the saccade but absent in the pre-saccadic boost interval [Fig. 5D]. This finding suggests that in the final moments before the saccade, attention focuses resources primarily on the target’s location, while the target’s direction is no longer attended.
In our experiment, any given trial was not predictive of the next, so intertrial modulations of attention were not, strictly speaking, adaptive or beneficial. However, they could be adaptive in reliably dynamic environments. When important objects cannot all be fixated quickly enough, processing resources should be more evenly distributed to encompass relevant stimuli not at the next saccade target. This is the case while playing basketball, for instance, but not while reading text on a printed page, when the most relevant stimulus is the next word to be fixated. Similarly, when many simultaneously relevant stimuli share a feature, such as team players wearing a particular color, they should all receive a boost even while moving gaze to just one of them. The results suggest that the attentional control system tracks spatial and featural relations between behaviorally relevant stimuli and saccade targets. The tighter the relation has been, the more selective the distribution of attention towards the next gaze position and the target’s features. The analysis of trial n-2, in addition to n-1, suggests that these statistics are accumulated over multiple independent events lasting several seconds.
Both the overall spatial attention effect and the intertrial effects are most likely automatic. We say “most likely” because we cannot be absolutely certain that observers did not adopt an explicit strategy to remember the relation between probe and target and on the next trial willfully attend to dot patches with the same relationship to the target. We find this highly unlikely, though, because observers’ only instructions were to make a quick saccade and judge a brief flash that could occur anywhere. All stimulus parameters were varied randomly and independently across trials, and observers were reminded that the probe and the target were independent of one another. Therefore, such a complex strategy would have been futile and inherently difficult, given that only 50 – 200 ms elapsed between the onset of the cue, which identified the saccade target, and the onset of the probe.
The feature-based attentional modulation of motion directions, contingent on previous trials, is especially unlikely to be strategy-based given that motion was entirely irrelevant to the task. This distinguishes the present study from most others in the literature on feature-based attention, in which the feature value of the attended stimulus is explicitly cued. Moreover, the present study is the first to measure pre-saccadic effects of feature-based attention independently of any consistent differences in the physical display and in the (likely) absence of a voluntary top-down ‘task set’ for a particular feature value. Some previous work, however, has investigated the role of visual features during saccade preparation. While making sequences of saccades along a path of green discs in a field of red discs, observers’ ability to see and remember a stimulus is relatively enhanced if it appears within a green disc that had been already fixated (Gersch et al., 2008, 2009). Therefore, the distribution of parafoveal sensitivity during saccade preparation depends on both salient task-relevant visual features (Gersch et al., 2008, 2009), as well as on the implicit attentional effects we report here.
In sum, visual sensitivity before saccades is shaped by recent experience, rapidly adapting to the short-term history of the location and features of eye movement targets and other task-relevant stimuli. Our results complement findings that covert attention speeds saccades to the attended location only if on recent trials the saccade and the attended locations had also matched (Belopolsky & Theeuwes, 2009). That study also suggested that the maintenance, but not the shifting, of covert attention can be dissociated from saccade planning. Our findings go further to show that the link between shifts of attention and saccade planning has some flexibility.
A recent review by Awh, Belopolksy and Theeuwes (2012) provides a potentially useful framework for interpreting these results. Those authors posit that three dissociable classes of factors influence attentional selection: bottom-up stimulus salience; the observer’s current goals; and the recent history of selection and reward. Our intertrial effects fall into the third category with respect to selection. Events in previous trials were irrelevant to the observers’ goals during any particular trial, and were independent of the bottom-up stimulus salience.
It should be noted that most of the literature on selection history reviewed by Awh et al. (2012) demonstrates that repetition of particular locations, feature values, or feature dimensions facilitates the deployment of attention to the next target (e.g., Maljkovic & Nakayama, 1994; Found & Müller, 1996; Chun & Jiang, 1998). In contrast, we found no effects of the repetition of particular locations or directions of motion. The absence of priming could be due to the fact that the task-relevant feature of the probe–luminance contrast–was independent of both space and motion. It is possible that with a localization or motion discrimination task such priming effects would emerge. Instead, we found that priming of spatial congruency and feature similarity—relations between probes and targets implicitly—potentiated the deployment of covert attention to the particular attributes of the next saccade target. The present study thus contributes to mounting evidence (e.g., Belopolsky & Theeuwes, 2009, Kristjánsson, 2006) that relational history effects modulate attentional selection, and that trials should not be analyzed only in isolation.
Conclusion
The ability to extract meaningful information from the barrage of scattered light in a scene depends largely on eye movements. We show that the automatic shifts of attention driven by an impending eye movement do not merely assist motor control or a strictly selective boost in processing of the next saccade target. Implicit cognitive processes permeate pre-saccadic attention, so that it flexibly distributes resources to potentially relevant locations and features just before the saccade disrupts vision. An implicit record of the relevance of visual information at the targets of recent saccades shapes this distribution of attention and—as we move our eyes one-hundred thousand times each day—our experience of the world.
Highlights.
We measured implicit attentional effects on sensitivity during saccade preparation
Shifts of spatial and feature-based attention depended on relations between stimuli in previous trials
Eye movements themselves were not affected by the previous trial
Pre-saccadic attention is flexibly distributed to potentially relevant stimuli
Acknowledgments
Funded by NIH Grant RO1 EY016200 to MC and Marie Curie fellowship (EU Grant PIOF-GA-2009-235625) to MR. We thank the Carrasco Lab members for helpful comments.
Footnotes
In addition, following congruent but not incongruent trials, the location 180° away from the target improved relative to 120° (p<0.05, corrected).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alais D, Blake R. Neural strength of visual attention gauged by motion adaptation. Nature Neuroscience. 1999;2:1015–1018. doi: 10.1038/14814. [DOI] [PubMed] [Google Scholar]
- Arman AC, Ciaramitaro VM, Boynton GM. Effects of feature-based attention on the motion aftereffect at remote locations. Vision Research. 2006;46:2968–2976. doi: 10.1016/j.visres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends in Cognitive Sciences. 2006;10:124–30. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Awh E, Belopolsky AV, Theeuwes J. Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends in cognitive sciences. 2012;16:437–443. doi: 10.1016/j.tics.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassi S, Verghese P. Attention to locations and features: Different top-down modulation of detector weights. Journal of Vision. 2005:556–570. doi: 10.1167/5.6.7. [DOI] [PubMed] [Google Scholar]
- Belopolsky AV, Theeuwes J. When are attention and saccade preparation dissociated? Psychological Science. 2009;20:1340–1347. doi: 10.1111/j.1467-9280.2009.02445.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological) 1995;90:289–300. [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:443–446. [PubMed] [Google Scholar]
- Carrasco M. Visual attention: The past 25 years. Vision research. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Jiang Y. Contextual cueing: implicit learning and memory of visual context guides spatial attention. Cognitive Psychology. 1998;36:28–71. doi: 10.1006/cogp.1998.0681. [DOI] [PubMed] [Google Scholar]
- Cornelissen FW, Peters EM, Palmer J. The eyelink toolbox: Eye tracking with MATLAB and the psychophysics toolbox. Behavior Research Methods, Instruments, & Computers. 2002;34:613–617. doi: 10.3758/bf03195489. [DOI] [PubMed] [Google Scholar]
- Deubel H. The time course of presaccadic attention shifts. Psychological Research. 2008;72:630–640. doi: 10.1007/s00426-008-0165-3. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vision Research. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Delayed Saccades, but Not Delayed Manual Aiming Movements, Require Visual Attention Shifts. Annals of the New York Academy of Sciences. 2003;1004:289–296. doi: 10.1196/annals.1303.026. [DOI] [PubMed] [Google Scholar]
- Engbert R, Mergenthaler K. Microsaccades are triggered by low retinal image slip. Proceedings of the National Academy of Sciences. 2006;103:7192–7197. doi: 10.1073/pnas.0509557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Found A, Müller HJ. Searching for unknown feature targets on more than one dimension: investigating a —dimension-weighting account. Perception & Psychophysics. 1996;58:88–101. doi: 10.3758/bf03205479. [DOI] [PubMed] [Google Scholar]
- Gersch TM, Kowler E, Dosher BA. Dynamic allocation of visual attention during the execution of sequences of saccades. Vision Research. 2004;44:1469–83. doi: 10.1016/j.visres.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Gersch TM, Kowler E, Schnitzer BS, Dosher BA. Visual memory during pauses between successive saccades. Journal of Vision. 2008;8(16):15, 1–18. doi: 10.1167/8.16.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersch TM, Kowler E, Schnitzer BS, Dosher BA. Attention during sequences of saccades along marked and memorized paths. Vision Research. 2009;49:1256–1266. doi: 10.1016/j.visres.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godijn R, Theeuwes J. Parallel allocation of attention prior to the execution of saccade sequences. Journal of Experimental Psychology: Human Perception and Performance. 2003;29:882–896. doi: 10.1037/0096-1523.29.5.882. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Pollatsek A, Rayner K. Covert visual attention and extrafoveal information use during object identification. Perception & Psychophysics. 1989;45:196–208. doi: 10.3758/bf03210697. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Perception & Psychophysics. 1995;57:787–95. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher BA. The role of attention in the programming of saccades. Vision Research. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Kristjánsson Á. Rapid learning in attention shifts: A review. Visual Cognition. 2006;13:324–362. [Google Scholar]
- Liu T, Mance I. Constant spread of feature-based attention across the visual field. Vision Research. 2011;51:26–33. doi: 10.1016/j.visres.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Liu T, Stevens ST, Carrasco M. Comparing the time course and efficacy of spatial and feature-based attention. Vision Research. 2007;47:108–113. doi: 10.1016/j.visres.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Maljkovic V, Nakayama K. Priming of pop-out: I. Role of features. Memory & Cognition. 1994;22:657–72. doi: 10.3758/bf03209251. [DOI] [PubMed] [Google Scholar]
- Martínez Trujillo JC, Treue S. Feature-based attention increases the selectivity of population responses in primate visual cortex. Current Biology. 2004;14:744–751. doi: 10.1016/j.cub.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Mathôt S, Theeuwes J. Visual attention and stability. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2011;366:516–27. doi: 10.1098/rstb.2010.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnini A, Castet E. Spatiotemporal dynamics of visual attention during saccade preparation: Independence and coupling between attention and movement planning. Journal of Vision. 2007;7(14):8, 1–16. doi: 10.1167/7.14.8. [DOI] [PubMed] [Google Scholar]
- Morey RD. Confidence intervals from normalized data: A correction to Cousineau (2005) Tutorial in Quantitative Methods for Psychology. 2008;4:61–64. [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Rolfs M, Carrasco M. Rapid simultaneous enhancement of visual sensitivity and perceived contrast during saccade preparation. Journal of Neuroscience. 2012;32:13744–13752. doi: 10.1523/JNEUROSCI.2676-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfs M, Jonikaitis D, Deubel H, Cavanagh P. Predictive remapping of attention across eye movements. Nature Neuroscience. 2011;14:252–256. doi: 10.1038/nn.2711. [DOI] [PubMed] [Google Scholar]
- Serences J, Boynton GM. Feature-based attentional modulations in the absence of direct visual stimulation. Neuron. 2007;55:301–312. doi: 10.1016/j.neuron.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Spering M, Carrasco M. Similar effects of feature-based attention on motion perception and pursuit eye movements at different levels of awareness. Journal of Neuroscience. 2012;32(22):7594–7601. doi: 10.1523/JNEUROSCI.0355-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Perception & Psychophysics. 1983;33:113–20. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- White AL, Carrasco M. Feature-based attention involuntarily and simultaneously improves visual performance across locations. Journal of Vision. 2011;11(6):15, 1–10. doi: 10.1167/11.6.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Gersch TM, Schnitzer BS, Dosher BA, Kowler E. Eye movements and attention: The role of pre-saccadic shifts of attention in perception, memory and the control of saccades. Vision Research. 2012 doi: 10.1016/j.visres.2012.06.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]