Abstract
Mitochondria are a class of dynamic organelles that constantly undergo fission and fusion. Mitochondrial dynamics is governed by a complex molecular machinery and finely tuned by regulatory proteins. During cell injury or stress, the dynamics is shifted to fission, resulting in mitochondrial fragmentation, which contributes to mitochondrial damage and consequent cell injury and death. Emerging evidence has suggested a role of mitochondrial fragmentation in the pathogenesis of renal diseases including acute kidney injury and diabetic nephropathy. A better understanding of the regulation of mitochondrial dynamics and its pathogenic changes may unveil novel therapeutic strategies.
Introduction
Mitochondria are the main energy-producing organelles in mammalian cells, but they also play a central role in cell injury and death signaling. The dysfunction of mitochondria induces the loss of cellular homeostasis that contributes to sublethal injury. Severe damage of mitochondria, in the form of permeabilization of inner and/or outer membranes of the organelles, can further lead to cell death. Research during the last few years has revealed a striking morphological change of mitochondria during cell injury, i.e., mitochondrial fragmentation. Importantly, mitochondrial fragmentation is not just a morphological change, but also an early and critical process contributing to mitochondrial membrane leakage and consequent cell death. In kidney cells and tissues, mitochondrial fragmentation has been demonstrated in the experimental models of acute kidney injury as well as diabetic nephropathy. In this review, we elucidate the regulation of mitochondrial dynamics and how its changes result in mitochondrial fragmentation during cell injury and stress. We present the evidence for an emerging role of mitochondrial fragmentation in the pathogenesis of kidney diseases. The mechanism whereby mitochondrial fragmentation contributes to cell death is also discussed. We propose that maintenance of mitochondrial dynamics may lead to the preservation of mitochondrial integrity and prevention of cell death and tissue damage, resulting in a novel therapy for relevant diseases.
Mitochondrial dynamics
In traditional textbooks, mitochondria were depicted as a class of cellular organelles with mixed morphologies, ‘mitos’ for threads and ‘chondron’ for grains in Greek. However, it has been recognized recently that mitochondria are highly dynamic, constantly undergoing fission and fusion. Fission results in the production of short mitochondrial rods or spheres. On the contrary, fusion promotes a long, filamentous morphology of mitochondria (Figure 1).1, 2 Under physiological conditions, the frequencies of fusion and fission events are finely tuned and balanced to maintain mitochondrial homeostasis. Under intra- or extra- cellular stresses, this balance is disrupted, resulting in the cleavage of mitochondria from an elongated network into small spheres or short rods, i.e., mitochondrial fragmentation.3 The intensive research during the last decade has gained significant insights into the molecular basis of mitochondrial dynamics as well as their involvement in many biological processes such as apoptosis, autophagy, metabolism, development and ageing. 3–6 It is now recognized that the dynamic nature of the double membrane-bound organelles is crucial not only for the maintenance of mitochondrial shape but also for their function, inheritance and quality control. 1, 7
Figure 1.

(A) Diagram of mitochondrial fusion-fission. Mitochondria are dynamic organelles that constantly undergo fusion and fission processes to maintain an interconnected network in healthy cells. Mitochondrial fusion is mediated by Mfn1, Mfn2, and OPA1, while fission mainly involves Drp1 and Fis1. (B, C) Mitochondrial morphology in human kidney tubular epithelial (HK-2) cells. A control HK2 cell shows filamentous mitochondria (B), whereas mitochondria become fragmented in high glucose treated cells (C). Red: mitochondria; Blue: Nuclei.
The molecular machinery governing mitochondrial dynamics was initially discovered in budding yeasts and Drosophila.8, 9, 10 Subsequent work identified the mammalian orthologs of the key proteins of mitochondrial dynamics, indicating a highly conserved mechanism.11, 12 These include the fission mediators (Fis1 and Drp1: Dnm1 in yeast) and the fusion proteins (Mfn1, Mfn2 and OPA1: Fzo1 and Mgm1in yeast) (Figure 1). Defects in these proteins lead to severely altered mitochondrial morphology as well as impaired mitochondrial function, loss of mitochondrial DNA (mtDNA) integrity and eventually cell death. In mammals, including humans, defects in either mitochondrial fission or fusion proteins causes developmental defects and neurological anomalies or neurodegenerative diseases.13, 14
Mitochondrial fission machinery
Drp1 is a large GTPase of the dynamin superfamily that plays an essential role in mitochondrial fission in mammalian cells. Structurally, Drp1 contains an N-terminal GTPase domain, a central domain and a c-terminal GTPase effector domain (Figure 2A).15 In un-stressed cells, Drp1 is mainly cytosolic. When activated, Drp1 accumulates to mitochondria, localizing to the fission sites of mitochondrial outer membrane (MOM), where they oligomerize to form spirals to drive MOM constriction and cleavage (Figure 2B).16, 17 Interestingly, after mitochondrial fission, Drp1 returns to the cytosol and as a result, it cycles between the cytosol and its target membrane.18 The activation and translocation of Drp1 as well as its shuttling between cytosol and mitochondria are regulated by mechanisms of post-translational modifications including phosphorylation, ubiquitylation and sumoylation.19 In addition, recruitment of Drp1 to mitochondria may also involve its interaction with Fis1.19
Figure 2. Schematic diagrams of mitochondrial fission machinery.
(A) Drp1 consists of an N-terminal GTPase, a central domain and a C-terminal GTPase effector domain (GED). Fis1 includes two central tandem tetratricopeptide repeats (TPRs) and a single C-terminal transmembrane (TM) domain, which facilitate Fis1 anchoring on the OMM. (B) Upon stimulation, Drp1 is activated and translocates to the scission sites of OMM through interaction with Fis1, where they oligomerize and form spirals to constrict OMM through GTP hydrolysis, resulting in mitochondrial fission.
Blockade of Drp1 by dominant-negative mutants or by siRNA-mediated down-regulation can suppress mitochondrial fission, leading to elongated mitochondria; conversely, overexpression of Drp1 results in mitochondrial fragmentation, indicating the requisite of Drp1 in mitochondrial fission. 18, 20 The physiological significance of Drp1 in mammals and humans is clearly established by a recent report that a dominant-negative mutation in Drp1 was associated with defects in the fission in both mitochondria and peroxisomes resulting in abnormal brain development, optic atrophy and premature death of a newborn girl.14 In Huntington’s disease, the mutant huntingtin protein may interact with Drp1 to enhance its enzymatic activity, resulting in mitochondrial fragmentation, which subsequently impairs the transport of mitochondria and reduces neuronal survival.21 In mice, ablation of Drp1 led to defects in embryonic development and synapse formation, particularly in the forebrain.22
Fis1 is another key component of the mitochondrial fission machinery in mammalian cells. As a small membrane protein, Fis1 is evenly anchored on the outer membrane of mitochondria through a single C-terminal transmembrane domain with its N-terminal region facing the cytosol (Figure 2). The cytosolic domain of Fis1 forms a six-helix bundle that includes two central tandem tetratricopeptide repeats (TPRs), which may facilitate specific protein–protein interactions.23, 24 Overexpression of Fis1 promoted mitochondrial fission resulting in mitochondrial fragmentation, followed by the release of cytochrome c and ultimately apoptosis.25 Knockdown of Fis1 via siRNA induced mitochondrial fusion and inhibited apoptosis. 26 It has been suggested that Fis1 may act as a receptor protein for Drp1 on the outer membrane of mitochondria (Figure 2B). 19
In addition to Drp1 and Fis1, recent work has suggested the involvement of several accessory proteins in mitochondrial fission. For example, Bif-1 or endophilin B1 was originally identified as a Bax interacting protein that facilitated Bax activation and the release of cytochrome c release during apoptosis. 27, 28 However, Bif-1 contains a BAR domain with homology to endophilins and translocates to mitochondria during mitochondrial fission.27, 29, 30 Moreover, knockdown of Bif-1 induced a dissociation of the outer membrane from the inner membrane in mitochondria and the formation of an aberrant mitochondrial morphology, suggesting a role of Bif-1 in mitochondrial fission. 30 In addition, MTP18, a novel nuclear-encoded mitochondrial membrane protein, has been implicated in controlling mitochondrial fission through recruitment of Drp1 to mitochondrial outer membrane.31 Future work should investigate how these proteins participate in mitochondrial fission and whether their involvement is direct or indirect.
Mitochondrial fusion machinery
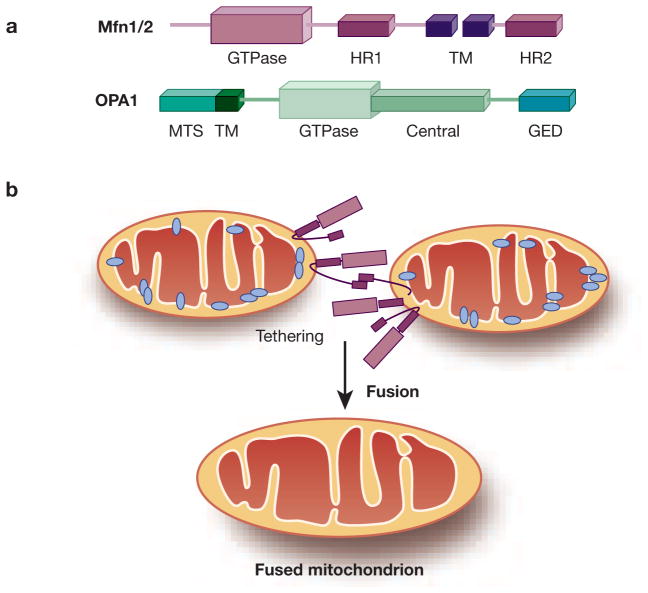
The basic components of the mitochondrial fusion machinery in mammalian cells include mitofusin 1 (Mfn1), mitofusin 2 (Mfn2) and Optic Atrophy 1 (OPA1). Similar to Drp1, these three fusion proteins are all large GTPases that belong to the dynamin superfamily (Figure 3A). Mfn1 and Mfn2 are localized to mitochondrial outer membrane (MOM) to mediate MOM tethering of adjacent mitochondria for fusion. On the contrary, OPA1 is localized on mitochondrial inner membrane (MIM) to act as a key to MIM fusion (Figure 3B). Successful mitochondrial fusion requires a coordinated action of Mfns and OPA1, although it is unclear how the molecular events at MOM are co-ordinated with those at MIM. 32
Figure 3. Schematic diagrams of mitochondrial fusion machinery.
(A) Mfn 1 and 2 contain an N-terminal GTPase domain, two transmembrane (TM) domains and two separating heptad repeat regions (HR1 and HR2). OPA1 comprises an N-terminal mitochondrial targeting sequence (MTS), GED at the C-terminus and other functional domains inbetween. (B) Mfn1 and 2 mediate OMM tethering through interaction of adjacent C-terminal HR2 regions, which leads to subsequent OMM fusion by GTP hydrolysis via Mfn. OPA1 is required in IMM fusion and the maintenance of cristae structure.
The mitochondrial fusion function of Mfns was first revealed in mammals in 2001.33 Mfn1 and 2 share a similar molecular structure that contains an N-terminal GTPase domain, two transmembrane domains spanning the MOM and separating two heptad repeat regions (HR1 and HR2, Figure 3A).33 The C-terminal HR2 functions in mitochondrial tethering. Specifically when Mfn1 and 2 form homo- or hetero-dimers to tether the outer membranes of adjacent mitochondria together, the HR2 region mediates mitofusin oligomerization by assembling a dimeric, antiparallel coiled coil (Figure 3B). 34 The GTPase domain of mitofusins performs an imperative action in the subsequent fusion process.35 It is noteworthy that Mfn1 exhibits much higher GTPase activity than Mfn2 during GTP-dependent membrane tethering. 36 In addition, a genetic analysis has suggested that OPA1 functionally requires Mfn1 (but not Mfn2) to regulate mitochondrial fusion.32 The studies indicate that Mfn1 and 2 may have distinct functions in mitochondrial fusion. It has been suggested that Mfn1 is the main tethering protein while Mfn2 may play a regulatory role. 3, 37
Mfn1 or 2-deficient cells are defective in mitochondrial fusion, which is accompanied by severe cellular defects including widespread heterogeneity of mitochondrial membrane potential, decreased cellular respiration, and poor cell growth and proliferation.38 Germline knockout of Mfn1 or 2 in mice results in embryonic lethality, indicating a role of the genes in development.39 In human, mutations in Mfn2 cause Charcot-Marie-Tooth type 2A, an autosomal dominant hereditary disease characterized by axonal neuropathy and neurodegeneration.40 In mice, conditional knockout of Mfn2 from neurons results in massive Purkinje cell degeneration and defects in postnatal development of the cerebellum, suggesting an essential role of Mfn2 in mitochondrial distribution and outgrowth of dendritic spines in Purkinje cells. Further in vitro analysis showed a variety of defects in Mfn2-null Purkinje cells including the electron transport chain dysfunction, lack of mtDNA nucleoids as well as disturbance of mitochondrial ultrastructure.41 These studies have thus provided insight into the cellular mechanisms through which impaired mitochondrial fusion links to severe defects in cell respiration in neurodegenerative disorders. More recently, conditional knockout studies further demonstrated that the Mfn-mediated fusion event is required to maintain mtDNA stability and fidelity in skeletal muscle as well as enable cells to tolerate high levels of mtDNA mutations. It is concluded that a proper mitochondrial dynamics may be a protective factor in human disorders associated with mtDNA mutations.42, 43
While Mfns are known as mitochondrial fusion proteins, they may have pleiotropic effects. For example, it has been shown that Mfn2 may function as a cell proliferation suppressor which is independent of its mitochondrial fusion activity, and down-regulation of Mfn2 results in vascular proliferative disorders such as atherosclerosis.44 Mfn2 is also enriched at the endoplasmic reticulum (ER)-mitochondria interface, where it tethers ER to mitochondria and facilitate their interactions for efficient mitochondrial Ca(2+) uptake.45 More recently, Hailey et al. showed that disruption of mitochondria/ER connections by Mfn2 depletion dramatically impairs starvation-induced autophagy. 46 In this regard, Mfn2 tethering activity is therefore necessary for autophagosome formation during starvation, in addition to the inter-organelle communication during Ca2+ signaling. It is noteworthy that some of the described pleitropic effects may be secondary to Mfn-mediated mitochondrial fusion.
The complexity of mitochondrial dynamics, including both fission and fusion, lies in part on the double-membrane structure of the organelles. It has been suggested that mitochondrial fusion starts from the outer membrane tethering and fusion, followed by inner membrane fusion. While outer membrane fusion involves Mfns as discussed above, the fusion of inner membrane is mediated by OPA1.47 OPA1 was discovered in 2000 as the gene mutated in autosomal dominant optic atrophy, the most common cause of hereditary optic neuropathy.48, 49 Structurally, OPA1 contains a mitochondrial targeting sequence at the N-terminus, a GTPase effector domain at the C-terminus, and other functional regions inbetween (Figure 3A). The human OPA1 gene yields eight mRNA isoforms as a result of extensive alternative splicing at the N-terminal portion.50 These spliced transcript variants are further processed into long and short (L and S) forms. Both L- and S-OPA1 participate in mitochondrial fusion and as a result, the proteolysis or cleavage of L-OPA1 induces fusion arrest during mitochondrial fragmentation.51 In addition to its pro-fusion activity, OPA1 is critical to the maintenance of the cristae and preservation of cytochrome c in mitochondria. During apoptosis, the proteolysis of L-OPA1 induces the remodeling of mitochondrial cristae to release cytochrome c from the cristae into the intermembrane space for subsequent leakage through the outer membrane.52 Presenilin-associated rhomboid-like (PARL), an inner mitochondrial membrane rhomboid, was suggested to be responsible for the proteolytic cleavage of OPA1 under certain conditions of mitochondrial stress or apoptosis.53 However, more recent studies have established a crucial role of OMA-1 in OPA1 cleavage during apoptosis. 54–57 In addition to mitochondrial regulation in cell death, OPA1 contributes to the maintenance of mitochondrial electron transport chain58 and mtDNA stability.59, 60 These physiological functions of OPA1 may be related to its activity in mitochondrial fusion.
Mechanisms leading to mitochondrial fragmentation in cell injury
The mechanism of mitochondrial fragmentation during cell injury and apoptosis is being actively investigated. It is generally understood that the pathological fragmentation may be a combined result of the activation of fission and suppression of fusion. However, the signaling pathways leading to the changes of mitochondrial dynamics are largely unclear and may vary according to the cellular context. In this aspect, recent studies have implied the involvement of Bcl2 family proteins, Drp1, and Ca2+/calcineurin-mediated dephosphorylation. Reactive oxygen species (ROS) and mitochondrial permeability transition (MPT) may also contribute to the alterations of mitochondrial dynamics in pathological conditions (Figure 4).
Figure 4. Mechanisms of mitochondrial fragmentation in cell death.
Upon cellular stress, Drp1 is activated and translocates to mitochondria to initiate mitochondrial fission by form a spiral constriction ring. Meanwhile, Bak is activated resulting in a shift of binding to Mfn1 and arrest of mitochondrial fusion. Together, the activation of mitochondrial fission and the arrest of mitochondrial fusion result in mitochondrial fragmentation. Fragmented mitochondria are sensitized to Bax insertion and oligomerization to induce the formation of pathological pores, i.e. MOMP, to trigger cell death. Drp1 is regulated by multiple post-translational modifications that may be induced by the increases of intracellular Ca2+ and ROS. In addition, Ca2+ and ROS can trigger MPT to induce MOMP or, directly, necrosis. As a cytoprotective mechanism, autophagy may remove fragmented mitochondria via mitophagy to suppress cell death.
Bcl-2 family proteins
Characterized by the presence of Bcl-2 homology (BH) domain(s), Bcl-2 family proteins are divided into three groups: a) multi-BH domain anti-apoptotic proteins such as Bcl-2 and Bcl-xL; b) multi-BH domain proapoptotic proteins including Bax and Bak; and c) BH3-only proteins like Bid and Bad that contains only one BH domain, the BH3.61, 62 Bcl-2 family proteins are recognized as the central regulators of mitochondrial integrity during cell injury and death. In this regard, Bax and Bak provide the requisite gateway of MOMP, which is inhibited by Bcl-2/Bcl-xL under normal conditions and activated by BH3-only proteins during apoptosis. Interestingly, recent research has revealed the regulation of mitochondrial dynamics by Bcl-2 family proteins. Especially, Bax and Bak regulate mitochondrial dynamics under both physiological and pathological conditions.
In 2002, Karbowski et al. revealed that Bax coalesces with Drp1 and Mfn2 into discrete foci at the mitochondrial fission sites during apoptosis, suggesting a possible role of Bax in the regulation of these proteins and mitochondrial dynamics. 63 It was shown later that Bax and Bak are required for mitochondrial morphogenesis in normal healthy cells. Specifically they may promote mitochondrial fusion by regulating Mfn2 complex assembly, membrane mobility and distribution on the outer membrane.64 During apoptosis, however Bax and Bak were suggested to promote mitochondrial fragmentation by stimulating the sumoylation of Drp1and its association of Drp1 with mitochondrial membranes.17 In 2007, we reported a unique role of Bak in promoting mitochondrial fragmentation during apoptosis.65 In a variety of apoptotic models, mitochondrial fragmentation was shown to be attenuated in Bak-knockout cells, but not in Bax-knockout cells. Reconstitution of Bak into Bak/Bax-double knockout cells could restore a rapid mitochondrial fragmentation, whereas reconstitution of Bax was much less effective. In addition, Bcl-2 and Bcl-XL could partially, but significantly suppress mitochondrial fragmentation, and their inhibitory effects required the presence of Bak. Mechanistically, Bak was shown to interact with both Mfn1 and 2 in unstressed cells. During apoptosis, Bak dissociated from Mfn2 to increase the association with Mfn1 resulting in the inhibition of mitochondrial fusion (Figure 5). Notably, mutation of Bak in its BH3 domain prevented Bak dissociation from Mfn2 and diminished its mitochondrial fragmentation activity.65 Our latest work further demonstrated that mitochondrial fragmentation induced by renal ischemia is diminished in Bak-knockout mice (Wei and Dong, unpublished). Thus Bcl-2 family proteins participate in the regulation of mitochondrial dynamics, which is centered on Bax and Bak and their interactions with the key proteins in the mitochondrial fission-fusion machinery.
Figure 5. Hypothetic model for Bax/Bak regulation of mitochondrial dynamics during apoptosis.
In unstressed cells, Bak interacts with both Mfn1 and 2 to maintain a filamentous mitochondrial network, while Bax is in an inactive form in the cytosol. Following apoptotic stress, Bak dissociates from Mfn2 and increases its association with Mfn1, resulting in the suppression of mitochondrial fusion and mitochondrial fragmentation (Hit 1). Meanwhile, Bax is activated and translocates to mitochondria. Fragmented mitochondria are sensitized to Bax insertion and oligomerization (Hit 2), leading to the formation of pathological pores for the release of apoptogenic factors such as cytochrome c.
Drp1 activation
The role of Drp1 in mitochondrial fission or fragmentation has been well established. For example, Drp1 is activated rapidly following acute kidney cell injury and translocates to mitochondria leading to mitochondrial fragmentation.66 The research in the last few years has gained important insight into the regulatory mechanisms of Drp1. Especially, posttranslational modifications, including phosphorylation67–70, dephosphorylation71, 72, ubiquitination73, sumoylation17, 74 and S-nitrosylation 75, have been implicated in Drp1 regulation under various physiological and pathological conditions (Figure 4).
Taguchi et al. demonstrated the phosphorylation of Drp1 by Cdk1/cyclin B on Ser616, which promotes mitochondrial fission during mitosis. 68 The cell cycle-dependent activation of Drp1 is important for mitochondrial division and subsequent distribution to daughter cells. While the phosphorylation at Ser616 is physiological, Drp1 phosphorylation at Ser637 (on human Drp1 variant 1) appears to be more pathological. 67–70 Interestingly, Drp1 phosphorylation at Ser637 can be mediated by distinct protein kinases. Even more puzzling is that Drp1 phosphorylation at Ser637 by different protein kinases results in completely different or opposite effects. Protein Kinase A (PKA)-mediated phosphorylation of Drp1 leads to the suppression of mitochondrial fission culminating in a filamentous morphology and resistance to apoptosis, 67, 69 whereas phosphorylation of Drp1 by Ca2+/calmodulin-dependent protein kinase alpha (CaMKI) activates mitochondrial fission.70 More recently, Danesh and colleagues demonstrated that Rho-associated coiled coil-containing protein kinase 1 (ROCK1) phosphorylates Drp1 under high-glucose stimulation, resulting in Drp1 translocation and mitochondrial fission.76 The basis of these obvious discrepancies is still unclear. Nevertheless, dephosphorylation of Drp1 Ser637 by calcineurin, a Ca2+-activated phosphatase, promotes Drp1 translocation to mitochondria and mitochondrial fragmentation, sensitizing cells to apoptosis.69, 71 In line with these observations, we recently detected Drp1 dephosphoylation at Ser637 during ATP-depletion in renal tubular cells and ischemia-reperfusion in kidney tissues.72 Moreover, inhibitors of calcineurin prevented Drp1 dephosphorylation, accompanied by that the inhibition of mitochondrial fragmentation, MOMP, and apoptosis. Thus, Drp1 dephosphorylation may contribute significantly to mitochondrial fragmentation during renal tubular cell apoptosis. In renal tubular cells, Nowak et al. recently reported PKC-ε-mediated cell death, in which Drp1 was shown to translocate to mitochondria prior to mitochondrial fragmentation, but it is unclear whether Drp1 is phosphorylated directly by PKC-ε.77 On the other hand, in neurons PKCδ can phosphorylates Drp1 at Ser579 inducing mitochondrial fragmentation to contribute to cell death under oxidative stress.78 Thus, depending on the experimental condition Drp1 may be phosphorylated at multiple sites by different protein kinases, resulting in either inhibition or activation of mitochondrial fission.
In addition to phosphorylation, ubiquitination of Drp1 by MARCH5, a mitochondrial E3 ubiquitin ligase, enhances the subcellular trafficking of Drp1 to mitochondrial scission sites resulting in mitochondrial fission.73 Sumoylation of Drp1 has also been reported to stabilize Drp1 to induce mitochondrial fragmentation and apoptosis.17, 79 More recently, S-nitrosylation has been shown to increase Drp1 dimer formation and GTPase activity, leading to mitochondrial fission or fragmentation during nitric oxide-induced neuronal synaptic injury and death, suggesting a novel pathogenic mechanism of neurodegeneration.75 Whether these post-translational modifications regulate Drp1 in kidney cells and tissues have yet to be investigated.
Ca2+/calcineurin
Perturbation of Ca2+ homeostasis plays a crucial role in cell injury and death.80 Physiologically, mitochondria are involved in the maintenance of Ca2+ homeostasis by buffering cytosolic Ca2+ when it rises to high levels. However, excessive Ca2+ overload in mitochondria may lead to mitochondrial damage, resulting in cell injury and death.80 While previous research emphasized Ca2+ overload-induced mitochondrial disruption, recent emerging evidence has illustrated a regulation of mitochondrial dynamics by Ca2+ and calcineurin, a Ca2+-activated phophatase. In 2008, Cereghetti et al. demonstrated that sustained Ca2+ rise in cytosol can induce mitochondrial fragmentation in a Drp1-dependent manner.71 Further detailed investigation revealed an Drp1/calcineurin interaction and importantly, high cytosolic Ca2+ was shown to activate calcineurin to directly dephosphorylate Drp1 at Ser637, leading to Drp1 activation and translocation to mitochondria to induce mitochondrial fission and fragmentation.69, 71 In renal tubular cells and tissues, Drp1 dephosphorylation at Ser637 has been demonstrated during ATP-depletion and renal ischemia-reperfusion.72 Notably, two dissimilar calcineurin inhibitors, cyclosporine A and FK506, can suppress Drp1 dephosphorylation and prevent mitochondrial fragmentation, Bax accumulation, cytochrome c release, and apoptosis following ATP depletion in renal tubular cells.72 Thus calcineurin may promote apoptosis through at least two distinct mechanisms, i.e. dephosphorylation of the proapoptotic Bcl-2 family protein Bad 81 and dephosphorylation of the mitochondrial fission protein Drp1. The latest study by Wang et al. has further suggested an interesting regulation of mitochondrial dynamics by microRNA-499, which represses calcineurin to block Drp1 activation, mitochondrial fragmentation and apoptosis in cardiomyocytes. 82
Reactive Oxygen Species
Mitochondria are known to be a major intracellular source of ROS. Under pathological conditions, uncoupling of oxidative phosphorylation and loss of mitochondrial membrane integrity induce excessive ROS production from the respiratory chain especially at the Complex I and III. On the other hand, mitochondria are also a critical target of the damaging effects of ROS. Oxidative damage leads to mitochondrial dysfunction and disruption, triggering mitochondrial permeability transition and/or the release of pro-apoptotic proteins like cytochrome c to induce cell death.83 Recent studies have determined the relationship between ROS production and mitochondrial dynamics in several experimental models. In neurons exposed to oxidative stress and diabetic coronary endothelial cells, increased ROS production was shown to be accompanied by the disturbance of mitochondrial dynamics, resulting in mitochondrial fragmentation and cell death.84, 85 Importantly, mitochondrial fragmentation in these experimental models was suppressed by ROS scavengers, indicating that ROS might serve as a direct initiator of the mitochondrial change.84, 86 It was suggested that ROS may regulate mitochondrial fission–fusion proteins to result in mitochondrial fragmentation.86 In line with this idea, Qi et al. recently demonstrated that PKC-δ was activated under oxidative stress and phosphorylates Drp1, leading to Drp1 activation and mitochondrial fragmentation78. Notably, in some of these studies, tilting of mitochondrial dynamics (overexpression of Drp1 or Mfn2) did not affect ROS generation, negating a role of mitochondrial fragmentation in ROS production. 84, 85
Conversely, Yu et al. reported an important role of the disturbance of mitochondrial dynamics in ROS production. They showed that in cells cultured with high glucose, mitochondria underwent rapid fragmentation, which induced ROS production. Importantly, inhibition of mitochondrial fragmentation prevented ROS production under this condition, supporting a critical role for the alterations of mitochondrial dynamics in ROS production.87, 88 These findings were recently extended by Shenouda et al, showing that in diabetic ambience, down-regulation of Fis1 or Drp1 with siRNA (inhibiting mitochondrial fission) could inhibit ROS production and nitric oxide synthase activation in human endothelial cells 89. In renal tubular cells, Nowak et al. demonstrated that Protein kinase C-ε activation induced mitochondrial fragmentation and ROS production and, the attenuation of ROS with antioxidants did not prevent mitochondrial fragmentation, suggesting that ROS is not the triggering mechanism of mitochondrial fragmentation in this model.77 This is consistent with the viewpoint that increased mitochondrial ROS is a consequence, not the cause of mitochondrial fragmentation. 89
Thus there is a complex interaction between ROS and mitochondrial dynamics. In some experimental models, ROS may be the triggering event of mitochondrial fragmentation, but in others mitochondrial fragmentation may lead to mitochondrial dysfunction and ROS production. It is also conceivable that under certain conditions, these two events may exacerbate each other to form a vicious cycle ultimately resulting in cell injury and death.
Mitochondrial Permeability Transition
Mitochondrial permeability transition (MPT) is characterized by the opening of the MPT pore in the inner mitochondrial membrane, which allows for solutes of less than 1.5 KD to pass. MPT is recognized as a key event in cell death, especially necrosis. MPT can be triggered by multiple factors such as elevated mitochondrial Ca2+, increased ROS and a variety of lipid metabolites. Persistent MPT leads to the loss of mitochondrial membrane potential, mitochondrial swelling, outer membrane rupture and consequent release of apoptogenic factors.90, 91 The exact composition of the MPT pore complex remains ambiguous but it is generally accepted that the basic unit of the pore contains the adenine nucleotide translocator (ANT), voltage-dependent anion channel (VDAC), and cyclophilin D (CypD), a prolyl isomerase located within the mitochondrial matrix. It is believed that upon stimulation, these proteins form a complex in the inner membrane resulting in the opening of the MPT pore.83, 92
MPT has been shown to be involved in renal cell injury and death under numerous forms of insults including ischemia-reperfusion injury, oxidative stress, nephrotoxicity, Ca2+ overload, etc. Notably, CypD is widely accepted as a critical mediator of MPT in cell death, especially in necrosis.90, 93–95 Transgenic mice overexpressing CypD undergo mitochondrial swelling and spontaneous cell death, whereas CypD knockout mice are developmentally normal but are protected from ischemia-reperfusion injury in heart, brain and kidneys90, 93–96. Using the model of hypoxic injury in renal tubules, Weinberg and colleagues recently revealed a new and important regulatory mechanism of MPT via nonesterified fatty acids. It was suggested that nonesterified fatty acids accumulated during kidney injury sensitize mitochondria to MPT.96, 97
Despite the recognized role of MPT in mitochondrial disruption, whether and how it is related to alterations of mitochondrial dynamics is unknown. Decades ago, it was reported that Ca2+ uptake stimulated drastic changes in mitochondrial morphology and functional activity owing to the opening of MPT pore, implying that MPT may disturb mitochondrial dynamics.83, 98 On the other hand, it is conceivable that pro-longed changes of mitochondrial dynamics may trigger irreversible mitochondrial dysfunction and disruption including MPT. It is noteworthy that MPT mainly occurs as an inner membrane event, whereas the current research of mitochondrial dynamics is largely limited to outer membrane. It is thus important to understand the dynamic regulation of inner membrane, which is more proximate to MPT.
Mechanisms whereby mitochondrial dynamics or its disruption contributes to mitochondrial damage and cell death
Outer membrane permeabilization-MOMP
Despite some controversies, it is now generally accepted that mitochondrial fragmentation plays a crucial role in mitochondrial injury during cell death, especially in outer membrane permeabilization (MOMP). It has been puzzling how the fragmentation, a seemingly morphological change, contributes to MOMP and loss of mitochondrial integrity. In this regard, the research in the last few years has gained significant insights.
In 2007, when a role of Bak (and not Bax) in mitochondrial fragmentation was unveiled, we postulated that these two proteins may function at two different yet connected stages of mitochondrial injury.65 Specifically, we proposed a “two-hit” hypothesis, in which Bak triggers the first hit, i.e. mitochondrial fragmentation, followed by the second hit that involves Bax activation and formation of pathological pores in mitochondrial outer membrane (Figure 4).65, 99 In this hypothesis, mitochondrial fragmentation is postulated as a likely process that facilitates Bax activation in mitochondrial membrane. Moreover, it emphasizes a collaborative action of Bak and Bax in promoting MOMP. In line with this notion, in a variety of apoptotic models neither Bak nor Bax alone is sufficient to induce maximal cytochrome c release (indicative of MOMP), which requires both molecules.65, 100 While this hypothesis proposed non-redundant, collaborative roles of Bax and Bak in the development of MOMP, it was then not clear at all how mitochondrial fragmentation contributes to Bax regulation and MOMP. Our latest study 101 demonstrated that preservation of filamentous mitochondria by either blocking mitochondrial fission or enhancing mitochondrial fusion can suppress Bax insertion and oligomerization in mitochondrial membrane. Moreover, mitofusin-null cells with fragmented mitochondria are highly sensitive to Bax insertion and oligomerization in mitochondrial membrane resulting in increased MOMP and apoptosis. Notably, mitochondrial morphology does not affect Bax accumulation in mitochondria under these conditions. Together, these results suggest that the alteration of mitochondrial dynamics and consequent mitochondrial fragmentation contribute to MOMP at least partially by facilitating Bax insertion and activation in mitochondrial membrane.101 Consistently, Gall and colleagues showed very recently that kidney epithelial cells isolated from conditional Mfn2-knockout mice have fragmented mitochondria and are more sensitive to Bax activation, MOMP and apoptosis during metabolic stress.102 While these studies did not elucidate why fragmented mitochondria are sensitized to Bax activation, we postulated that the changes in biophysical and biochemical properties of mitochondrial membrane during mitochondrial fission or fragmentation may result in changes of membrane curvature as well as the related proteins and lipids that may facilitate Bax insertion and oligomerization.65, 99 Such a scenario is supported by the recent study from Montessuit et al. 103, which specifically delineated the mechanism whereby Drp1 contributes to MOMP during apoptosis. Using an in vitro liposome reconstitution system, this study revealed that Drp1 promotes Bax oligomerization by triggering membrane tethering and hemifusion. Importantly, during apoptosis-associated mitochondrial fragmentation, Drp1 may constrict the mitochondrial outer membrane resulting in the formation of similar intermediate membrane structures to induce Bax oligomerization. Indeed, expression of a Drp1 mutant lacking the membrane remodeling activity decreased Bax oligomerization, MOMP, and apoptosis. Thus, the changes of membrane property induced by mitochondrial fragmentation, rather than mitochondrial fission-fusion proteins (eg. Drp1) per se, play a critical role in Bax activation and MOMP, a conclusion supported by our latest work.99
Cristae remodeling
In addition to MOMP, the release of apoptogenic factors from mitochondria also depends on the remodeling of mitochondrial cristae, the inner membrane structure.52, 104 This is particularly true for cytochrome c because ~85% of the molecule is sequestered within the mitochondrial cristae and as a result, its release requires the opening of the cristae. Indeed remodeling of mitochondrial cristae has been documented in several apoptotic models, which is characterized by the fusion of individual cristae and the widening of cristae junctions resulting in the opening into the intermembrane space for cytochrome c release.104–106 Recent studies have documented mitochondrial cristae remodeling during apoptosis by mitochondrial dynamics proteins. 52, 105
In 2006, two groups independently reported the role of OPA1 in cristae remodeling during apoptosis 52, 53. It was shown that OPA1, the profusion protein on the inner membrane, existed as both long and short forms, which interacted and oligomerized to tighten the cristae junctions. Down-regulation of OPA1 or disruption of OPA1 oligomers not only caused mitochondrial fragmentation but also disrupted normal cristae structure. Interestingly, lack of PARL, the enzyme for OPA1 processing, did not affect mitochondrial fusion but accelerated OPA1-dependent cristae remodeling and cytochrome c release during apoptosis. Thus, OPA1 may govern the remodeling of mitochondrial cristae independently from its regulation of mitochondrial fusion. 52, 53, 107
Somewhat surprisingly, Drp1, the outer mitochondrial membrane fission protein, has also been implicated in the remodeling and opening of mitochondrial cristae.105 Suppression of Drp1 by expressing its dominant-negative mutant was shown to block mitochondrial cristae remodeling induced by Bik, whereas complete release of cytochrome c was induced by the combination of cristae remodeling, mitochondrial fragmentation, and Bax activation.105 It is unclear how Drp1 affect the remodeling of cristae. However, Estaquier et al. 108 suggested that inhibition of mitochondrial fission may block the release of soluble OPA1, which is required for cristae remodeling and cytochrome c release from mitochondria. In essence, Drp1 may contribute to cristae remodeling indirectly via OPA1.
These findings suggest that mitochondrial fragmentation during apoptosis may affect cristae remodeling.109, 110 We speculate that cristae remodeling is a key event induced by mitochondrial fragmentation for the release of apoptogenic factors, such as cytochrome c. Apparently, the molecular basis linking these structural changes is largely unknown and deserves further in-depth investigation.
Respiration and ATP production
Mitochondrial dynamics is critical to the maintenance of mitochondrial homeostasis and function. As such, disruption of mitochondrial dynamics may induce acute mitochondrial damage, such as MOMP and cristae structural damage as discussed above, resulting in cell death. When the insult is not severe enough to kill mitochondria and the cell immediately, an alteration in mitochondrial dynamics is often accompanied by respiration defects, decreased cellular ATP, and increased mitochondrial ROS production followed by oxidative damage.111
In 2003, Bach and colleagues reported that knock-down of Mfn2 led to the reduction of glucose oxidation, mitochondrial membrane potential and cell respiration, resulting in less oxidative phosphorylation and increased dependency on anaerobic glycolysis.112 Two years later, Chan and colleagues generated Mfn-null cells that showed a widespread heterogeneity of mitochondrial membrane potential and decreased cellular respiration, accompanied by poor cell growth. Consistently, knockdown of OPA1 also suppressed mitochondrial fusion and resulted in similar cellular defects.38 In vivo, conditional knockout of Mfns induces mitochondrial fragmentation, defects in mitochondrial respiration, instability in mitochondrial DNA, and cell death or tissue degeneration in brain, skeletal muscle and heart.41, 42, 113 Collectively, these studies have demonstrated convincing evidence that lack of mitochondrial fusion promotes mitochondrial fragmentation, resulting in functional defects in respiration and ATP production.
Somewhat surprisingly, suppression of mitochondrial fission induces functional defects in mitochondria similar to those caused by the lack of fusion. In this regard, Rossignol and colleagues showed that knockdown of Drp1 resulted in an extensively branching, filamentous mitochondrial network in HeLa cells, which nevertheless had significantly lower respiration and energy production.114 In PTEN-induced kinase 1(Pink1)-knockout flies, mitochondria showed a significantly lower capacity of respiration and ATP synthesis due to decreases in electron transport chain complex I and IV activity and notably, the bioenergetic defect was corrected by expressing Drp1. It was suggested that lack of mitochondrial fission may cause defective assembly of the ETC complexes, leading to bioenergetics deficiency.115 Consistently, Sesaki and Colleagues recently demonstrated that ablation of Drp1 from cerebellar Purkinje cells in mice led to abnormally elongated mitochondrial tubules, which became large spheres due to oxidative damage and lost respiratory function.116, 117 Thus, suppression of either fusion or fission results in mitochondrial dysfunction, manifested by a reduction in respiration and ATP production. Conversely, a dynamic balance, and not fission or fusion alone, is important to the maintenance of mitochondrial function and long-term health.
Changes of mitochondrial dynamics and role in renal pathophysiology
Renal diseases, including acute kidney injury (AKI) and chronic kidney disease (CKD), are characterized by renal tissue injury and declines of renal function. Both AKI and CKD involve complex pathogenic mechanisms, in which mitochondrial pathology has been implicated.118–121 Notably, recent work has demonstrated emerging evidence for the alterations of mitochondrial dynamics in acute and progressive renal disease models, including renal ischemia-reperfusion, nephrotoxicity as well as hyperglycemia-induced kidney injury. 66, 122 Under these conditions, normal filamentous mitochondria in renal cells become fragmented and importantly, mitochondrial fragmentation has been demonstrated to contribute to mitochondrial damage and consequent cell death, suggesting a pathogenic role of mitochondrial fragmentation in relevant renal diseases.
Disruption of mitochondrial dynamics in AKI
AKI is a major kidney disease characterized by an abrupt decline in renal function, which is associated with significant in-hospital morbidity and mortality. Recent basic and epidemiological research further indicates that AKI contributes significantly to the development of progressive CKD.123–126 Depending on the primary causes, AKI is generally categorized as prerenal, renal or intrinsic, and postrenal AKI. Intrinsic AKI is the most common and severe subtype of AKI in hospitalized patients that is mainly induced by renal ischemia-reperfusion, sepsis and nephrotoxicity. With a complex, multiphasic and multifactorial pathogenesis, AKI is nevertheless precipitated by renal tubular cell injury and death. 118, 119, 127–129
For decades, mitochondrial damage has been recognized in renal tubular cell injury and death in AKI. 130, 131 While these earlier studies showed mitochondria damage as a key injurious event in tubular cell death, recent research has further established an active role of mitochondria in initiating and mediating cell death. This is well exemplified by apoptosis where mitochondria release apoptogenic factors to activate caspases for the execution of cell demise. Notably, such mitochondrial pathology has been detected in human kidneys.132 Does mitochondrial dynamics play a role in mitochondrial damage and tubular cell apoptosis in AKI? To address this question, our recent work66 monitored mitochondrial morphology in renal tubular cells during ischemic and cisplatin nephrotoxic AKI. The analysis revealed a rapid mitochondrial fragmentation upon AKI (Figure 6). In cultured renal tubular cells subjected to ATP depletion or cisplatin treatment, mitochondrial fragmentation preceded MOMP as shown by cytochrome c release. Notably, the mitochondrial fission protein Drp1 was detected to be activated and translocated to the mitochondria early during tubular cell injury, and inhibition of Drp1 via a dominant-negative mutant or siRNA could block mitochondrial fragmentation as well as MOMP and apoptosis. Furthermore, in mouse models of AKI mitochondrial fragmentation was revealed by using 2D and 3D electron microscopy. Thirty minutes of renal ischemia followed by brief reperfusion induced mitochondrial fragmentation in 30–40% of proximal tubule cells. Importantly, mdivi-1, a pharmacologic inhibitor of Drp1, prevented mitochondrial fragmentation and protected kidneys against both ischemic and cisplatin nephrotoxic AKI 66. This study has therefore provided compelling evidence for the role and regulation of mitochondrial dynamics in tubular cell injury and death in AKI, suggesting a novel strategy for the prevention and treatment of this kidney disease. The role of mitochondrial fragmentation in acute tissue damage has been confirmed recently by Ong et al. using an ischemic model of myocardial infarction.133 Notably, Gall et al.102 have recently established a conditional gene knockout model in which Mfn2 was specifically deleted from kidney epithelial cells. The mice from this model had 20% fewer nephrons, but were relatively normal in renal function. Primary proximal tubular cells isolated from these mice showed significant mitochondrial fragmentation and were highly sensitive to Bax activation, cytochrome c release and apoptosis following ATP-depletion. These findings provide further support for the involvement of mitochondrial dynamics in AKI.
Figure 6. Mitochondrial fragmentation in AKI.

(A) A cultured renal tubular cell under control conditions shows a filamentous morphology of mitochondria, which become fragmented upon ATP-depletion. (B) Images of electron microscopy showing elongated mitochondria in a proximal tubular cell in kidney tissues and fragmented mitochondria in ischemically injured tubular cells. The images are adopted from Brooks et al. J Clin Invest. 119:1275–85, 2009.
Alteration of mitochondrial dynamics in chronic renal diseases
Despite recent research on the role and regulation of mitochondrial dynamics in other chronic diseases like neurodegeneration or coronary heart disease, very limited is known about its effect on chronic kidney diseases. Diabetic nephropathy is a leading cause of end stage renal disease, in which chronic hyperglycemia induces dysfunction in glomerular, tubular and interstitial cells of the kidney and ultimate progression to chronic renal failure.121 Notably, renal cell apoptosis is frequently observed in kidney biopsies of diabetic patients, especially in tubular cells.134 Our earlier work122 demonstrated a drastically altered mitochondrial morphology along with cristae remodeling during tubular cell apoptosis in diabetic mouse kidneys by electron microscopy. Consistently, confocal microscopy revealed condensed mitochondrial fragments followed by cytochrome c release and apoptosis in high-glucose treated tubular cells, implicating the change of mitochondrial dynamics and associated apoptosis in the development of diabetic nephropathy.122 The latest study by Danesh and colleagues76 has further demonstrated mitochondrial fragmentation during diabetic nephropathy. Remarkably, using elegant conditional gene knock-out and knock-in mouse models this study has unveiled a role of ROCK1 in Drp1 phosphorylation and activation, leading to mitochondrial fission and fragmentation in kidney cells.76 Mitochondrial fragmentation has also been detected in kidney biopsies of diabetic human patients (Sun et al, unpublished), further implicating a pathogenic role of the alterations in mitochondrial dynamics.
Mechanisms whereby mitochondrial fragmentation contributes to kidney diseases
Obviously, one pathogenic mechanism of mitochondrial fragmentation is by inducing damages in the outer and/or inner membranes of the organelles, resulting in cell death. As discussed above, in both ischemic and nephrotoxic models of AKI, mitochondrial fragmentation plays a critical role in the permeabilization of mitochondrial outer membrane with ensuing tubular cell apoptosis. Mechanistically, fragmented mitochondria are sensitized to Bax insertion, a key event in mitochondrial outer membrane permeabilization during apoptosis.101
While cell killing is the predominant mechanism in acute, severe injury conditions, it is important to recognize that mitochondrial fragmentation may also have less dramatic yet chronic effects. Especially, as eluded earlier mitochondrial fragmentation may reduce respiratory chain activity and ATP production, culminating in dysfunction in cells and tissues. The decrease of mitochondrial function is known in a variety of kidney diseases.135–137 For example, respiration complex dysfunctions have been widely documented in experimental models of ischemic and nephrotoxic AKI, leading to ATP depletion, ROS production, and cellular damage.77, 138 More recent work by Schnellmann and colleagues further demonstrated a persistent disruption of mitochondrial homeostasis after AKI, as indicated by the loss of mitochondrial proteins.139 In diabetic nephropathy, high glucose induced mitochondrial superoxide generation, a decline in cell ATP, and Complex I and III inactivation and deficiency.140, 141 It remains unclear whether and to what extents mitochondrial fragmentation contributes to the mitochondrial dysfunction and cellular energetic deficits in these experimental models. However, we speculate that disruption of mitochondrial dynamics has a role in the pathogenesis of both acute and chronic kidney diseases. In the presence of severe injury, mitochondrial fragmentation directly participates in tubular cell death by triggering mitochondrial membrane permeabilization, while in the presence of moderate insult, mitochondrial fragmentation results in suboptimal respiration, declines in cell ATP, and tissue dysfunction.
Maintaining mitochondrial dynamics as a therapeutic strategy
The demonstration of mitochondrial fragmentation and its role in cell death suggests that maintaining or restoring mitochondrial dynamics may offer a novel strategy for the prevention and/or treatment of relevant diseases. In 2009, we tested this possibility in mouse models of AKI induced by renal ischemia-reperfusion and cisplatin nephrotoxicity. Upon AKI, Drp1 showed a rapid accumulation in mitochondria in renal tubular cells, accompanied by mitochondrial fragmentation. Mdivi-1, a pharmacological inhibitor of Drp1, had a partial inhibitory effect on mitochondrial fragmentation in these models. Importantly, mdivi-1 could partially, but significantly, protect against ischemic and nephrotoxic kidney injury as attested by the preservation of renal function and the inhibition of renal tissue damage and apoptosis. Consistently, in mice subject to coronary artery occlusion and reperfusion Ong et al. demonstrated that mdivi-1 inhibited mitochondrial injury and reduced cell death resulting in a marked decrease in myocardial infarct size.133 Significant beneficial effects of Mdivi-1 were also shown during ischemic injury of mouse retina. 142 Collectively, these studies have not only provided in vivo evidence for mitochondrial fragmentation in disease conditions but also suggested a new therapeutic strategy. Of note, these studies were all conducted in acute injury models. Although pathological alternations of mitochondrial dynamics occur in chronic disease conditions such as diabetes, whether blockade of mitochondrial fragmentation is beneficial is largely unknown. Especially it may be concerning that a dynamic fission-fusion balance is essential to cellular homeostasis and thus disruption of fission in a pro-longed fashion may be toxic by inducing mitochondrial dysfunction, cell injury and tissue damage. Nevertheless, the latest work by Jheng et al. showed that inhibition of mitochondrial fragmentation with mdivi-1 could improve insulin signaling in skeletal muscles and systemic insulin sensitivity of obese mice.143 Mdivi-1 was also effective in ameliorating neuropathic pain in several animal models. 144 In a rat model of diabetic nephropathy, the expression of Mfn2 was shown to decrease.145 Although mitochondrial morphology was not examined in detail and the underlying mechanism remains unknown, adenovirus-mediated gene transfer of Mfn2 suppressed diabetic nephropathy-associated proteinuria and pathological changes in this study. 145 In addition, intensive research has been initiated to test the therapeutic potential of modulating mitochondrial dynamics for the treatment of neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease.146 More preclinical studies are expected to test the strategy of targeting mitochondrial dynamics for therapy in a variety of disease models. In addition, more efficacious and specific mitochondrial dynamics-modulating reagents are being investigated.147 Together, these efforts may lead to the development of new and effective therapies.
Conclusions and Perspectives
Mitochondria are highly dynamic organelles that constantly undergo fission and fusion to maintain the homeostasis of the cell. The dynamics is orchestrated by a class of mitochondrial shaping proteins on the outer and inner membranes. During cell injury or stress, the dynamics is altered resulting in mitochondrial fragmentation, which contributes to MOMP at the outer membrane and cristae remodeling in the inner membrane resulting in the release of apoptogenic factors and cell death. The research in the last few years has shed significant new light on the regulation of mitochondrial dynamics by discovering the role of Bcl-2 family proteins and implicating the involvement of ROS, Ca2+/calcineurins, and related signaling pathways. Despite these findings, important and exciting new areas remain to be explored. For example, little is known about inner membrane fission during mitochondrial fragmentation in mammalian cells. Also it remains poorly understood how the fragmentation events at the outer and inner membranes are coordinated. In addition, the fate of fragmented mitochondria remains to be investigated. While mitochondria fragmentation is temporally reversible, consequent membrane permeabilization dictates “the point of no return”. These mitochondria may be engulfed by autophogasomes via the process called mitophagy. The mechanistic connection and functional relationship between the regulation of mitochondrial dynamics and mitophagy remains to be delineated (Figure 6).
Emerging evidence has suggested a pathogenic role of mitochondrial fragmentation in kidney diseases such as acute kidney injury and diabetic nephropathy. The mechanism responsible for the change of mitochondrial dynamics resulting in mitochondrial fragmentation under these disease conditions is largely unknown. Moreover, it is unclear how mitochondrial fragmentation interacts with other mitochondrial and cellular damage mechanisms to culminate in tissue pathology in these diseases. In ischemic and cisplatin nephrotoxic AKI models, blockade of mitochondrial fragmentation offers a renoprotective effect.66 It remains to be tested whether similar approaches are effective in other kidney diseases. Further investigation of mitochondrial dynamics and its changes in kidney diseases may identify more specific and effective treatments.
Acknowledgments
Sources of Support: National Natural Science Foundation of China (30971379; L. Sun). Doctoral Fund of Ministry of Education of China (20110162110012; L. Sun). Furong Scholars Fund from Education Department of Hunan Province of China (L. Sun). National Institutes of Health (Z. Dong) and Department of Veterans Affairs of U.S.A. (Z. Dong).
References
- 1.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 2.Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 3.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 4.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 2010;123:2533–2542. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet. 2005;39:503–536. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- 9.Osteryoung KW, Nunnari J. The division of endosymbiotic organelles. Science. 2003;302:1698–1704. doi: 10.1126/science.1082192. [DOI] [PubMed] [Google Scholar]
- 10.Hales KG, Fuller MT. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell. 1997;90:121–129. doi: 10.1016/s0092-8674(00)80319-0. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Chan DC. Mitochondrial dynamics in mammals. Curr Top Dev Biol. 2004;59:119–144. doi: 10.1016/S0070-2153(04)59005-1. [DOI] [PubMed] [Google Scholar]
- 12.Meeusen S, DeVay R, Block J, Cassidy-Stone A, Wayson S, McCaffery JM, Nunnari J. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006;127:383–395. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet. 2009;18:R169–176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 15.Pitts KR, McNiven MA, Yoon Y. Mitochondria-specific function of the dynamin family protein DLP1 is mediated by its C-terminal domains. J Biol Chem. 2004;279:50286–50294. doi: 10.1074/jbc.M405531200. [DOI] [PubMed] [Google Scholar]
- 16.Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari J. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song W, Chen J, Petrilli A, Liot G, Klinglmayr E, Zhou Y, Poquiz P, Tjong J, Pouladi MA, Hayden MR, Masliah E, Ellisman M, Rouiller I, Schwarzenbacher R, Bossy B, Perkins G, Bossy-Wetzel E. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med. 2011;17:377–382. doi: 10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 23.Dohm JA, Lee SJ, Hardwick JM, Hill RB, Gittis AG. Cytosolic domain of the human mitochondrial fission protein fis1 adopts a TPR fold. Proteins. 2004;54:153–156. doi: 10.1002/prot.10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki M, Neutzner A, Tjandra N, Youle RJ. Novel structure of the N terminus in yeast Fis1 correlates with a specialized function in mitochondrial fission. J Biol Chem. 2005;280:21444–21452. doi: 10.1074/jbc.M414092200. [DOI] [PubMed] [Google Scholar]
- 25.James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 26.Stojanovski D, Koutsopoulos OS, Okamoto K, Ryan MT. Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J Cell Sci. 2004;117:1201–1210. doi: 10.1242/jcs.01058. [DOI] [PubMed] [Google Scholar]
- 27.Cuddeback SM, Yamaguchi H, Komatsu K, Miyashita T, Yamada M, Wu C, Singh S, Wang HG. Molecular cloning and characterization of Bif-1. A novel Src homology 3 domain-containing protein that associates with Bax. J Biol Chem. 2001;276:20559–20565. doi: 10.1074/jbc.M101527200. [DOI] [PubMed] [Google Scholar]
- 28.Rostovtseva TK, Boukari H, Antignani A, Shiu B, Banerjee S, Neutzner A, Youle RJ. Bax activates endophilin B1 oligomerization and lipid membrane vesiculation. J Biol Chem. 2009;284:34390–34399. doi: 10.1074/jbc.M109.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 30.Karbowski M, Jeong SY, Youle RJ. Endophilin B1 is required for the maintenance of mitochondrial morphology. J Cell Biol. 2004;166:1027–1039. doi: 10.1083/jcb.200407046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tondera D, Czauderna F, Paulick K, Schwarzer R, Kaufmann J, Santel A. The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells. J Cell Sci. 2005;118:3049–3059. doi: 10.1242/jcs.02415. [DOI] [PubMed] [Google Scholar]
- 32.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114:867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- 34.Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- 35.Meeusen S, McCaffery JM, Nunnari J. Mitochondrial fusion intermediates revealed in vitro. Science. 2004;305:1747–1752. doi: 10.1126/science.1100612. [DOI] [PubMed] [Google Scholar]
- 36.Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- 37.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, Parman Y, Evgrafov O, Jonghe PD, Takahashi Y, Tsuji S, Pericak-Vance MA, Quattrone A, Battaloglu E, Polyakov AV, Timmerman V, Schroder JM, Vance JM. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 41.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 42.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumann K. Organelle dynamics: Fusing for stability. Nat Rev Mol Cell Biol. 2010;11:391. doi: 10.1038/nrm2910. [DOI] [PubMed] [Google Scholar]
- 44.Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D, Li P, Qiu X, Wen S, Xiao RP, Tang J. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol. 2004;6:872–883. doi: 10.1038/ncb1161. [DOI] [PubMed] [Google Scholar]
- 45.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 46.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141:656–667. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon Y, Galloway CA, Jhun BS, Yu T. Mitochondrial dynamics in diabetes. Antioxid Redox Signal. 2011;14:439–457. doi: 10.1089/ars.2010.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 49.Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 50.Delettre C, Griffoin JM, Kaplan J, Dollfus H, Lorenz B, Faivre L, Lenaers G, Belenguer P, Hamel CP. Mutation spectrum and splicing variants in the OPA1 gene. Hum Genet. 2001;109:584–591. doi: 10.1007/s00439-001-0633-y. [DOI] [PubMed] [Google Scholar]
- 51.Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. Embo J. 2006;25:2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 53.Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D’Adamio L, Derks C, Dejaegere T, Pellegrini L, D’Hooge R, Scorrano L, De Strooper B. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 54.Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou JC, Westermann B, Rugarli EI, Langer T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187:1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol. 2009;187:959–966. doi: 10.1083/jcb.200906083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McBride H, Soubannier V. Mitochondrial function: OMA1 and OPA1, the grandmasters of mitochondrial health. Curr Biol. 2010;20:R274–276. doi: 10.1016/j.cub.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 57.Quiros PM, Ramsay AJ, Sala D, Fernandez-Vizarra E, Rodriguez F, Peinado JR, Fernandez-Garcia MS, Vega JA, Enriquez JA, Zorzano A, Lopez-Otin C. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. Embo J. 2012;31:2117–2133. doi: 10.1038/emboj.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Z, Wakabayashi N, Wakabayashi J, Tamura Y, Song WJ, Sereda S, Clerc P, Polster BM, Aja SM, Pletnikov MV, Kensler TW, Shirihai OS, Iijima M, Hussain MA, Sesaki H. The Dynamin-related GTPase Opa1 Is Required for Glucose-stimulated ATP Production in Pancreatic Beta Cells. Mol Biol Cell. 2011 doi: 10.1091/mbc.E10-12-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amati-Bonneau P, Valentino ML, Reynier P, Gallardo ME, Bornstein B, Boissiere A, Campos Y, Rivera H, de la Aleja JG, Carroccia R, Iommarini L, Labauge P, Figarella-Branger D, Marcorelles P, Furby A, Beauvais K, Letournel F, Liguori R, La Morgia C, Montagna P, Liguori M, Zanna C, Rugolo M, Cossarizza A, Wissinger B, Verny C, Schwarzenbacher R, Martin MA, Arenas J, Ayuso C, Garesse R, Lenaers G, Bonneau D, Carelli V. OPA1 mutations induce mitochondrial DNA instability and optic atrophy ‘plus’ phenotypes. Brain. 2008;131:338–351. doi: 10.1093/brain/awm298. [DOI] [PubMed] [Google Scholar]
- 60.Yu-Wai-Man P, Sitarz KS, Samuels DC, Griffiths PG, Reeve AK, Bindoff LA, Horvath R, Chinnery PF. OPA1 mutations cause cytochrome c oxidase deficiency due to loss of wild-type mtDNA molecules. Hum Mol Genet. 2010;19:3043–3052. doi: 10.1093/hmg/ddq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 62.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- 65.Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci U S A. 2007;104:11649–11654. doi: 10.1073/pnas.0703976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. The Journal of clinical investigation. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 68.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 69.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han XJ, Lu YF, Li SA, Kaitsuka T, Sato Y, Tomizawa K, Nairn AC, Takei K, Matsui H, Matsushita M. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol. 2008;182:573–585. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cho SG, Du Q, Huang S, Dong Z. Drp1 dephosphorylation in ATP depletion-induced mitochondrial injury and tubular cell apoptosis. Am J Physiol Renal Physiol. 2010;299:F199–206. doi: 10.1152/ajprenal.00716.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karbowski M, Neutzner A, Youle RJ. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol. 2007;178:71–84. doi: 10.1083/jcb.200611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, McBride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci. 2007;120:1178–1188. doi: 10.1242/jcs.03418. [DOI] [PubMed] [Google Scholar]
- 75.Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, Chang BH, Schumacker PT, Danesh FR. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell metabolism. 2012;15:186–200. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nowak G, Bakajsova D, Samarel AM. Protein kinase C-epsilon activation induces mitochondrial dysfunction and fragmentation in renal proximal tubules. Am J Physiol Renal Physiol. 2011;301:F197–208. doi: 10.1152/ajprenal.00364.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qi X, Disatnik MH, Shen N, Sobel RA, Mochly-Rosen D. Aberrant mitochondrial fission in neurons induced by protein kinase C{delta} under oxidative stress conditions in vivo. Mol Biol Cell. 2011;22:256–265. doi: 10.1091/mbc.E10-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol. 2004;14:340–345. doi: 10.1016/j.cub.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 80.Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Calcium in cell injury and death. Annu Rev Pathol. 2006;1:405–434. doi: 10.1146/annurev.pathol.1.110304.100218. [DOI] [PubMed] [Google Scholar]
- 81.Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 82.Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, Li YR, Li PF. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 83.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 84.Makino A, Scott BT, Dillmann WH. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia. 2010;53:1783–1794. doi: 10.1007/s00125-010-1770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grohm J, Plesnila N, Culmsee C. Bid mediates fission, membrane permeabilization and peri-nuclear accumulation of mitochondria as a prerequisite for oxidative neuronal cell death. Brain Behav Immun. 2010;24:831–838. doi: 10.1016/j.bbi.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 86.Wu S, Zhou F, Zhang Z, Xing D. Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission-fusion proteins. Febs J. 2011;278:941–954. doi: 10.1111/j.1742-4658.2011.08010.x. [DOI] [PubMed] [Google Scholar]
- 87.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, Vita JA. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 91.Rasola A, Sciacovelli M, Pantic B, Bernardi P. Signal transduction to the permeability transition pore. FEBS Lett. 2010;584:1989–1996. doi: 10.1016/j.febslet.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta. 2009;1787:1402–1415. doi: 10.1016/j.bbabio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 93.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 94.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Devalaraja-Narashimha K, Diener AM, Padanilam BJ. Cyclophilin D gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol. 2009;297:F749–759. doi: 10.1152/ajprenal.00239.2009. [DOI] [PubMed] [Google Scholar]
- 96.Park JS, Pasupulati R, Feldkamp T, Roeser NF, Weinberg JM. Cyclophilin D and the mitochondrial permeability transition in kidney proximal tubules after hypoxic and ischemic injury. Am J Physiol Renal Physiol. 2011;301:F134–150. doi: 10.1152/ajprenal.00033.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feldkamp T, Park JS, Pasupulati R, Amora D, Roeser NF, Venkatachalam MA, Weinberg JM. Regulation of the mitochondrial permeability transition in kidney proximal tubules and its alteration during hypoxia-reoxygenation. Am J Physiol Renal Physiol. 2009;297:F1632–1646. doi: 10.1152/ajprenal.00422.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria, I The protective mechanisms. Arch Biochem Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 99.Brooks C, Dong Z. Regulation of mitochondrial morphological dynamics during apoptosis by Bcl-2 family proteins: a key in Bak? Cell Cycle. 2007;6:3043–3047. doi: 10.4161/cc.6.24.5115. [DOI] [PubMed] [Google Scholar]
- 100.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brooks C, Cho SG, Wang CY, Yang T, Dong Z. Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am J Physiol Cell Physiol. 2011;300:C447–455. doi: 10.1152/ajpcell.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gall JM, Wang Z, Liesa M, Molina A, Havasi A, Schwartz JH, Shirihai O, Borkan SC, Bonegio RG. Role of mitofusin 2 in the renal stress response. PloS one. 7:e31074. doi: 10.1371/journal.pone.0031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basanez G, Meda P, Martinou JC. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 105.Germain M, Mathai JP, McBride HM, Shore GC. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. Embo J. 2005;24:1546–1556. doi: 10.1038/sj.emboj.7600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ulivieri C. Cell death: insights into the ultrastructure of mitochondria. Tissue Cell. 2010;42:339–347. doi: 10.1016/j.tice.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 107.Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278:7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 108.Estaquier J, Arnoult D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ. 2007;14:1086–1094. doi: 10.1038/sj.cdd.4402107. [DOI] [PubMed] [Google Scholar]
- 109.Gottlieb E. OPA1 and PARL keep a lid on apoptosis. Cell. 2006;126:27–29. doi: 10.1016/j.cell.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 110.Scorrano L. Opening the doors to cytochrome c: changes in mitochondrial shape and apoptosis. Int J Biochem Cell Biol. 2009;41:1875–1883. doi: 10.1016/j.biocel.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 111.Palorini R, De Rasmo D, Gaviraghi M, Danna LS, Signorile A, Cirulli C, Chiaradonna F, Alberghina L, Papa S. Oncogenic K-ras expression is associated with derangement of the cAMP/PKA pathway and forskolin-reversible alterations of mitochondrial dynamics and respiration. Oncogene. 2012 doi: 10.1038/onc.2012.50. [DOI] [PubMed] [Google Scholar]
- 112.Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, Rabasa-Lhoret R, Wallberg-Henriksson H, Laville M, Palacin M, Vidal H, Rivera F, Brand M, Zorzano A. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism, A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- 113.Chen Y, Liu Y, Dorn GW., 2nd Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109:1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120:838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 115.Liu W, Acin-Perez R, Geghman KD, Manfredi G, Lu B, Li C. Pink1 regulates the oxidative phosphorylation machinery via mitochondrial fission. Proc Natl Acad Sci U S A. 2011;108:12920–12924. doi: 10.1073/pnas.1107332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kageyama Y, Zhang Z, Roda R, Fukaya M, Wakabayashi J, Wakabayashi N, Kensler TW, Reddy PH, Iijima M, Sesaki H. Mitochondrial division ensures the survival of postmitotic neurons by suppressing oxidative damage. J Cell Biol. 2012;197:535–551. doi: 10.1083/jcb.201110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Z, Kageyama Y, Sesaki H. Mitochondrial division prevents neurodegeneration. Autophagy. 2012;8 doi: 10.4161/auto.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. The Journal of clinical investigation. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 120.Weinberg JM. Mitochondrial biogenesis in kidney disease. J Am Soc Nephrol. 2011;22:431–436. doi: 10.1681/ASN.2010060643. [DOI] [PubMed] [Google Scholar]
- 121.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun L, Xie P, Wada J, Kashihara N, Liu FY, Zhao Y, Kumar D, Chugh SS, Danesh FR, Kanwar YS. Rap1b GTPase ameliorates glucose-induced mitochondrial dysfunction. J Am Soc Nephrol. 2008;19:2293–2301. doi: 10.1681/ASN.2008030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Hsu CY. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010;21:345–352. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010 doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sanoff S, Okusa MD. Impact of acute kidney injury on chronic kidney disease and its progression. Contrib Nephrol. 2011;171:213–217. doi: 10.1159/000327332. [DOI] [PubMed] [Google Scholar]
- 127.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 128.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011 doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pabla N, Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 130.Weinberg JM, Harding PG, Humes HD. Mitochondrial bioenergetics during the initiation of mercuric chloride-induced renal injury, I Direct effects of in vitro mercuric chloride on renal mitochondrial function. J Biol Chem. 1982;257:60–67. [PubMed] [Google Scholar]
- 131.Weinberg JM, Harding PG, Humes HD. Mitochondrial bioenergetics during the initiation of mercuric chloride-induced renal injury, II Functional alterations of renal cortical mitochondria isolated after mercuric chloride treatment. J Biol Chem. 1982;257:68–74. [PubMed] [Google Scholar]
- 132.Castaneda MP, Swiatecka-Urban A, Mitsnefes MM, Feuerstein D, Kaskel FJ, Tellis V, Devarajan P. Activation of mitochondrial apoptotic pathways in human renal allografts after ischemiareperfusion injury. Transplantation. 2003;76:50–54. doi: 10.1097/01.TP.0000069835.95442.9F. [DOI] [PubMed] [Google Scholar]
- 133.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 134.Kumar D, Robertson S, Burns KD. Evidence of apoptosis in human diabetic kidney. Molecular and cellular biochemistry. 2004;259:67–70. doi: 10.1023/b:mcbi.0000021346.03260.7e. [DOI] [PubMed] [Google Scholar]
- 135.Hall AM, Unwin RJ. The not so ‘mighty chondrion’: emergence of renal diseases due to mitochondrial dysfunction. Nephron Physiol. 2007;105:p1–10. doi: 10.1159/000096860. [DOI] [PubMed] [Google Scholar]
- 136.Zorov DB. Amelioration of aminoglycoside nephrotoxicity requires protection of renal mitochondria. Kidney Int. 2010;77:841–843. doi: 10.1038/ki.2010.20. [DOI] [PubMed] [Google Scholar]
- 137.Casalena G, Daehn I, Bottinger E. Transforming Growth Factor-beta, Bioenergetics, and Mitochondria in Renal Disease. Semin Nephrol. 2012;32:295–303. doi: 10.1016/j.semnephrol.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Feldkamp T, Kribben A, Roeser NF, Senter RA, Kemner S, Venkatachalam MA, Nissim I, Weinberg JM. Preservation of complex I function during hypoxia-reoxygenation-induced mitochondrial injury in proximal tubules. Am J Physiol Renal Physiol. 2004;286:F749–759. doi: 10.1152/ajprenal.00276.2003. [DOI] [PubMed] [Google Scholar]
- 139.Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol. 2012;302:F853–864. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, Tan AL, Fukami K, Thallas-Bonke V, Nawroth PP, Brownlee M, Bierhaus A, Cooper ME, Forbes JM. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742–752. doi: 10.1681/ASN.2008050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Munusamy S, MacMillan-Crow LA. Mitochondrial superoxide plays a crucial role in the development of mitochondrial dysfunction during high glucose exposure in rat renal proximal tubular cells. Free Radic Biol Med. 2009;46:1149–1157. doi: 10.1016/j.freeradbiomed.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 142.Park SW, Kim KY, Lindsey JD, Dai Y, Heo H, Nguyen DH, Ellisman MH, Weinreb RN, Ju WK. A selective inhibitor of drp1, mdivi-1, increases retinal ganglion cell survival in acute ischemic mouse retina. Invest Ophthalmol Vis Sci. 2011;52:2837–2843. doi: 10.1167/iovs.09-5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, Chang CR, Tsai YS. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 2012;32:309–319. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ferrari LF, Chum A, Bogen O, Reichling DB, Levine JD. Role of Drp1, a key mitochondrial fission protein, in neuropathic pain. J Neurosci. 2011;31:11404–11410. doi: 10.1523/JNEUROSCI.2223-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tang WX, Wu WH, Zeng XX, Bo H, Huang SM. Early protective effect of mitofusion 2 overexpression in STZ-induced diabetic rat kidney. Endocrine. 2011 doi: 10.1007/s12020-011-9555-1. [DOI] [PubMed] [Google Scholar]
- 146.Oettinghaus B, Licci M, Scorrano L, Frank S. Less than perfect divorces: dysregulated mitochondrial fission and neurodegeneration. Acta Neuropathol. 2012;123:189–203. doi: 10.1007/s00401-011-0930-z. [DOI] [PubMed] [Google Scholar]
- 147.Lackner LL, Nunnari J. Small molecule inhibitors of mitochondrial division: tools that translate basic biological research into medicine. Chem Biol. 2010;17:578–583. doi: 10.1016/j.chembiol.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]