Abstract
Adrenocorticotropic hormone (ACTH) has a renoprotective effect in chronic kidney disease; however, its effect on acute kidney injury (AKI) remains unknown. In a rat model of tumor necrosis factor (TNF)–induced AKI, we found that ACTH gel prevented kidney injury, corrected acute renal dysfunction, and improved survival. Morphologically, ACTH gel ameliorated TNF-induced acute tubular necrosis, associated with a reduction in tubular apoptosis. While the steroidogenic response to ACTH gel plateaued, the kidney-protective effect continued to increase at even higher doses, suggesting steroid-independent mechanisms. Of note, ACTH also acts as a key agonist of the melanocortin system, with its cognate melanocortin 1 receptor (MC1R) abundantly expressed in renal tubules. In TNF-injured tubular epithelial cells in vitro, ACTH reinstated cellular viability and eliminated apoptosis. This beneficial effect was blunted in MC1R-silenced cells, suggesting that this receptor mediates the anti-apoptotic signaling of ACTH. Moreover, ACTH gel protected mice against cecal ligation puncture–induced septic AKI better than α-melanocyte-stimulating hormone: a protein equal in biological activity to ACTH except for steroidogenesis. Thus, ACTH has additive renoprotective actions achieved by both steroid-dependent mechanisms and MC1R-directed anti-apoptosis. ACTH may represent a novel therapeutic strategy to prevent or treat AKI.
Keywords: acute kidney injury, adrenocorticotropic hormone, apoptosis, melanocortin, melanocortin receptor, rodent
Acute kidney injury (AKI) is a complex and heterogeneous disease entity characterized by an abrupt loss of kidney function that could be ascribed to numerous causes. In the past 30 years, although progress is indeed being made in identifying biomarkers for early diagnosis of AKI,1, 2, 3 management of AKI is still largely limited to general supportive measures;4, 5 no specific intervention that either prevents or treats the injured kidney or improves survival has been successfully developed.6 It is imperative to develop a novel, effective, and pragmatic approach to prevent kidney injury or to promote kidney repair and regeneration.7
Adrenocorticotropic hormone (ACTH) is an important component of the hypothalamic–pituitary–adrenal axis.8, 9 In the 1950s and the early 1960s, ACTH was widely used for the treatment of lipoid nephrosis.9, 10, 11 Recent clinical and experimental evidence suggests that ACTH has a renoprotective effect in chronic kidney diseases.9, 11, 12 Besides governing steroidogenesis, ACTH is also an important physiological agonist of the melanocortin system.9, 13 This system comprises multiple components, including five class A guanine nucleotide–binding protein (G protein)–coupled melanocortin receptors (MCRs) MC1R∼MC5R; endogenous antagonists; and peptide agonists derived from the anterior pituitary gland, including α-melanocyte-stimulating hormone (MSH), β-MSH, γ-MSH, and ACTH.9, 13 Accumulating data demonstrate that melanocortins,14 in particular α-MSH15 and its synthetic mimetics,16 possess a potent protective activity in acute injuries in multiple organ systems,17, 18, 19 including the kidney.15 Nevertheless, although α-MSH has similar MCR agonizing potency as its parent molecule ACTH,9, 13 the effect of ACTH on AKI has been barely investigated. This study examined the effect of ACTH gel (HP Acthar Gel, Questcor Pharmaceuticals, Hayward, CA), which is an FDA-approved formulation of natural porcine ACTH, on a rat model of AKI induced by tumor necrosis factor (TNF)20, 21, 22 and a murine model of septic AKI induced by cecal ligation puncture (CLP).16, 23, 24 We found that ACTH has a kidney-protective effect on AKI that is mediated by both steroid-dependent and -independent mechanisms.
RESULTS
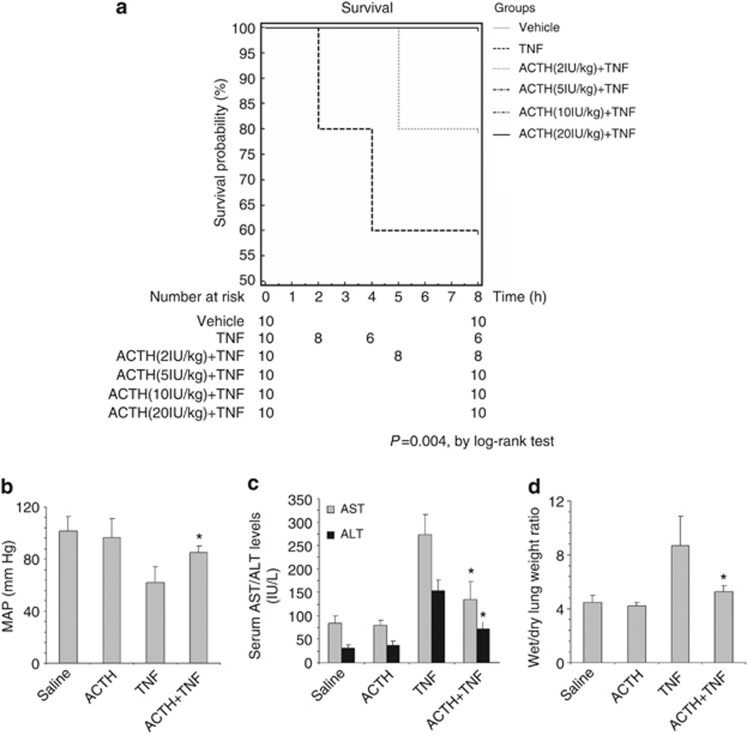
ACTH gel improves survival of rats with TNF-induced systemic organ injury
TNF is a pleiotropic prodeath and proinflammatory cytokine involved in the pathogenesis of multiple organ dysfunction and systemic inflammatory response syndrome.20, 21 A single injection of exogenous recombinant TNF immediately disturbed systemic homeostasis and resulted in 40% mortality within 8 h (Figure 1a). ACTH pretreatment reduced mortality and markedly improved general survival in a dose-dependent manner: even at low doses (2 IU/kg), pretreatment with ACTH increased survival to 80% at higher doses, ACTH treatment resulted in 100% survival from TNF stimulation (Figure 1a), suggesting a general beneficial effect of ACTH. To determine whether ACTH gel therapy has a systemic beneficial action, mean arterial pressure (MAP) was measured and the injury of the liver and lung was assessed. TNF injection induced a profound drop in MAP and caused a hypotensive shock (Figure 1b). Furthermore, TNF elicited acute liver injury as evidenced by the elevated serum levels of hepatic enzymes including aspartate aminotransferase and alanine aminotransferase. TNF also augmented wet to dry lung weight ratios, in agreement with the formation of acute pulmonary edema. ACTH gel treatment (10 IU/kg) significantly corrected the blood pressure, lowered serum aspartate aminotransferase and alanine aminotransferase levels (Figure 1c), and normalized wet to dry lung weight ratios (Figure 1d), signifying a general improvement in hemodynamics and systemic organ injury.
Figure 1.
Adrenocorticotropic hormone (ACTH) gel treatment significantly improves survival and ameliorates systemic organ injury in tumor necrosis factor (TNF)–injured rats. (a) Rats were pretreated subcutaneously with saline or ACTH gel at indicated doses 60 min before intravenous bolus injection of recombinant rat TNF (2 mg/kg wt). Rats were evaluated hourly and survival was recorded. Survival rate was plotted against the time course. The survival probability of rats receiving different treatment was determined by Kaplan–Meier survival analysis. P=0.0040 among the groups by log-rank test. (b–d) Rats were pretreated subcutaneously with saline or ACTH gel (10 IU/kg) 60 min before intravenous bolus injection of recombinant rat TNF (2 mg/kg wt). (b) Mean arterial pressure (MAP) was measured and rats were killed 8 h later. (c) Blood was collected and serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured to assess acute liver injury. (d) Lung was excised and wet to dry lung weight ratio was determined to assess acute lung injury. *P<0.05 versus TNF-alone-treated group (n=5).
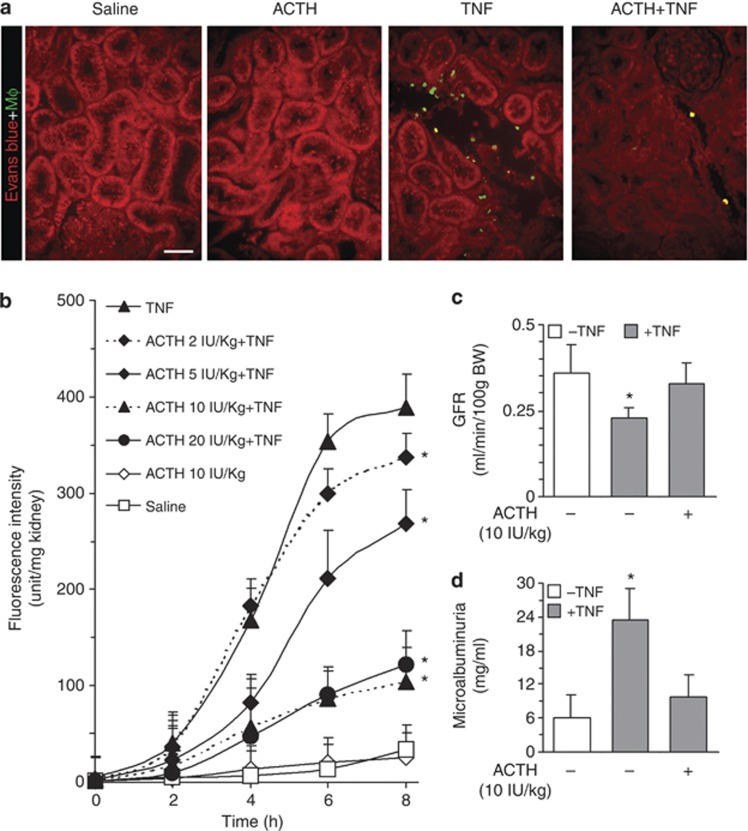
ACTH attenuates kidney injury and dysfunction in TNF-injured rats
To examine the effect of ACTH on TNF-induced kidney injury and to determine the minimal dose of ACTH gel that can maximally protect the kidney, fluorescence-labeled viable rat alveolar macrophages (RAM) were infused into the carotid artery of some animals 60 min before they were killed.22 Tissues were processed for fluorescent microscopic analysis. As shown in Figure 2a, many fluorescent RAM cells were sequestered in kidneys of TNF-injured animals, most prominently in the interstitium of the juxtamedullary cortex. Very few were found in kidneys from rats treated with saline or ACTH gel alone. ACTH gel treatment remarkably prevented the sequestration of RAM in the kidney, denoting a kidney-protective and anti-inflammatory effect. The amount of RAM infiltration was quantified by fluorometric analysis of homogenized kidney tissues, which revealed a dose-dependent inhibitory effect of ACTH gel on fluorescent RAM sequestration in TNF-injured kidney, consistent with the morphologic observations (Figure 2b). Because no further reduction in macrophage sequestration in the kidney was noted in animals treated with ACTH gel at doses higher than 10 IU/kg, the dose of 10 IU/kg was thus adopted as the optimal dose of ACTH gel for treatment of kidney injury in the following studies. At this dose, ACTH gel treatment also remarkably prevented the TNF-induced sequestration of RAM in the liver and lung shown by fluorometric analysis of liver and lung homogenates (Supplementary Figure 1a online). This was further corroborated by direct fluorescent microscopy of lung and liver specimens (Supplementary Figure 1b online), consistent with a systemic protective effect of ACTH. To further validate the renoprotective action of ACTH gel, kidney function was estimated by the glomerular filtration rates measured by inulin clearance assay and demonstrated that ACTH gel significantly improved acute kidney dysfunction (Figure 2c). Moreover, ACTH gel treatment also prominently diminished microalbuminuria induced by TNF (Figure 2d).
Figure 2.
Adrenocorticotropic hormone (ACTH) gel prevents acute renal injury in a dose-dependent manner and prominently improves kidney dysfunction at an optimal dose. Rats were treated with ACTH gel and tumor necrosis factor (TNF) as described in Figure 1 and were killed at the indicated time. To estimate an optimal kidney-protective dose of ACTH gel, fluorescence-labeled rat alveolar macrophage (RAM) cells were infused into the carotid artery 60 min before killing. Kidney sections were counterstained with Evans blue (red) to visualize and evaluate RAM sequestration (green; a), which was further quantified by (b) fluorometric analysis of kidney homogenates; *P<0.05 versus TNF-alone-treated group (n=5); bar=100 μm in a. (c) At 10 IU/kg, ACTH gel significantly attenuates TNF-impaired glomerular filtration rate (GFR) as estimated by inulin clearance; *P<0.05 versus other groups (n=5). (d) Albumin levels in the collected ureteral urine were measured by enzyme-linked immunosorbent assay and demonstrated that ACTH gel at 10 IU/kg markedly abrogates TNF-induced microalbuminuria. *P<0.05 versus other groups (n=5). BW, body weight.
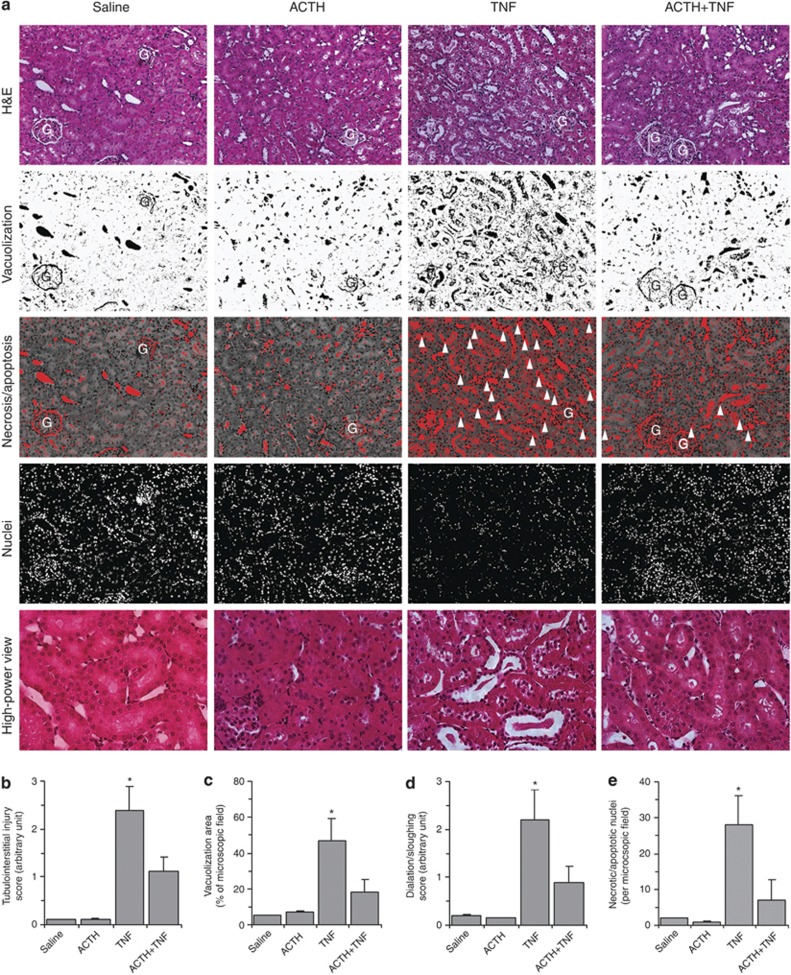
ACTH gel ameliorates histological AKI induced by TNF
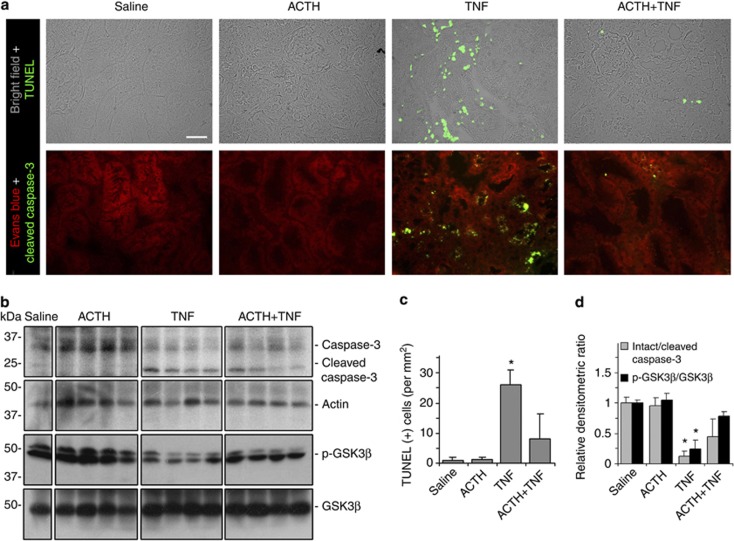
Morphologically, TNF induced a typical pattern of acute tubular necrosis, characterized by epithelial simplification, vacuolization of proximal tubular epithelium, luminal ectasia, epithelial necrosis, sloughing of tubular cell into lumen, loss of brush border, nuclear enlargement and pleomorphism, nuclear pyknosis and karyolysis, and prominent inflammatory infiltration, concomitant with tubular apoptosis (Figure 3a). ACTH gel at a dose of 10 IU/kg remarkably ameliorated these histological lesions evoked by TNF (Figure 3a). Semiquantitative morphometric analysis confirmed and quantified the morphologic findings and revealed that ACTH gel treatment significantly reduced the tubulointerstitial injury score and the dilation sloughing score (Figure 3b and d). Computerized morphometric analysis demonstrated that the area of vacuolization, as well as the number of necroapoptotic nuclei in TNF-injured kidneys, was remarkably diminished after ACTH gel treatment (Figure 3c and e). TNF is one of the prototypes of prodeath cytokines that could promote cell apoptosis.21 In support of this, TNF injection elicited massive cellular apoptosis in the kidney, as indicated by TdT-mediated dUTP nick-end labeling (TUNEL) staining, mainly located to the renal tubules in the juxtamedullary cortex (Figure 4a). ACTH gel treatment drastically abrogated the proapoptotic effect of TNF and greatly diminished the TUNEL positivity in the kidney (Figure 4a). Absolute counting of TUNEL-positive cell number corroborated the morphologic findings (Figure 4c). To examine whether the change in tubular cell apoptosis is associated with the change in the activity of proapoptotic signaling pathway, kidney specimens were stained for cleaved or active form of caspase-3, a pivotal transducer of the apoptotic-signaling pathway. Shown in Figure 4a, prominent expression of cleaved caspase-3 was induced predominantly in tubules in the juxtamedullary cortex of TNF-injured kidneys, and this could be markedly blunted by ACTH gel treatment. Moreover, immunoblot analysis of kidney homogenates indicated that ACTH gel treatment markedly attenuated the TNF-induced reduction in the ratios of intact/cleaved caspase-3 and phosphorylated glycogen synthase kinase (GSK)3β/GSK3β (Figure 4b and d), denoting that ACTH abrogated caspase-3-directed cell death and blunted GSK3β activation, which has been shown to be proapoptotic25 and has a detrimental role in AKI.26
Figure 3.
Adrenocorticotropic hormone (ACTH) gel therapy ameliorates histological acute kidney injury in tumor necrosis factor (TNF)–injured rats. Rats were pretreated subcutaneously with saline or ACTH gel (10 IU/kg) 60 min before intravenous bolus injection of recombinant rat TNF (2 mg/kg wt). (a) Rats were killed 8 h later and kidney sections were prepared for hematoxylin and eosin (H&E) staining to evaluate histological injury; original magnification, × 200 (high-power view, x400); necroapoptotic nuclei highlighted by arrowheads. G, glomerulus. Semiquantitative ((b) tubulointerstitial injury score and (d) tubular dilation/sloughing score), as well as computerized ((c) vacuolization area and (e) necroapoptotic nuclei), morphometric analysis was used to estimate kidney injury. *P<0.05 versus other groups (n=5).
Figure 4.
Adrenocorticotropic hormone (ACTH) gel prevents renal tubular cell apoptosis in tumor necrosis factor (TNF)–injured rats. Rats were pretreated subcutaneously with saline or ACTH gel (10 IU/kg) 60 min before intravenous bolus injection of recombinant rat TNF (2 mg/kg wt). (a) Rats were killed 8 h later and kidney sections were prepared for TdT-mediated dUTP nick-end labeling (TUNEL) staining (green) or immunofluorescent staining of cleaved caspase-3 (green) to evaluate renal cell apoptosis; bar=100 μm. (b) Immunoblot analysis of kidney homogenates for intact or cleaved caspase-3, phosphorylated GSK3β or GSK3β, and actin. (c) Absolute counting of TUNEL-positive cells in the kidney sections indicates an anti-apoptotic effect by ACTH treatment. *P<0.05 versus other groups. (d) Arbitrary units of intact/cleaved caspase-3 ratios or p-GSK3β/GSK3β ratios expressed as immunoblot densitometric ratios of the molecules as folds of the saline-treated group. *P<0.05 versus other groups (n=5).
Steroid-independent mechanism contributes to kidney protection by ACTH
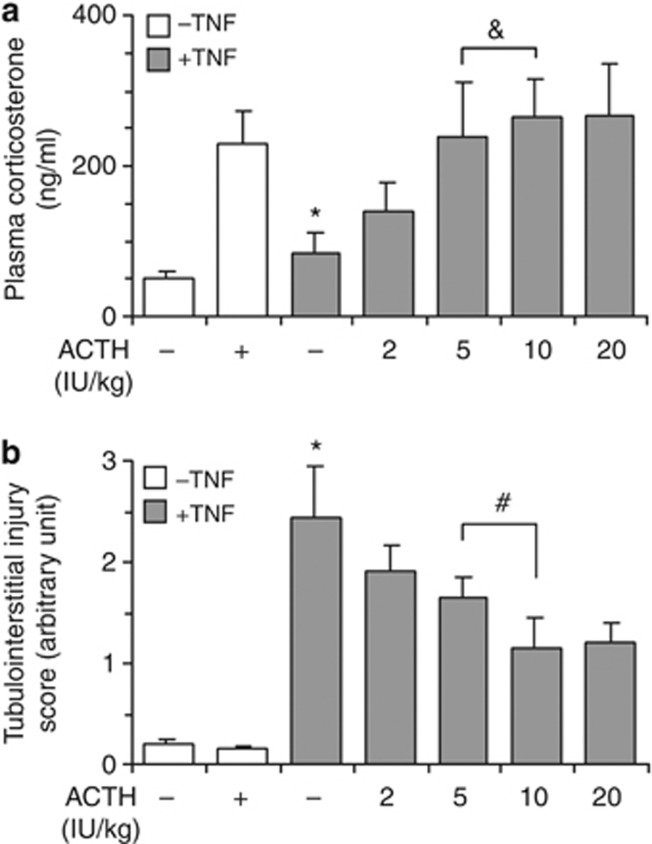
ACTH possesses a canonical steroidogenic activity, through which ACTH directs the adrenal release of glucocorticoid, a typical immunomodulatory and anti-inflammatory hormone that could attenuate renal inflammation and thus might mediate the renoprotective effects of ACTH.9, 13 Indeed, in TNF-injured animals, the tubulointerstitial injury score (Figure 5b) inversely correlated with steroidogenic response to ACTH gel as determined by the plasma levels of corticosterone (Figure 5a) at low doses. However, although the steroidogenic response to ACTH gel above a dose of 5 IU/kg reached a plateau, the renoprotective effect continued to increase with even higher doses (10 IU/kg, 20 IU/kg), suggesting that steroid-independent mechanisms also contribute.
Figure 5.
Adrenocorticotropic hormone (ACTH) gel therapy attenuates acute kidney injury in tumor necrosis factor (TNF)–injured rats via, at least in part, a steroid-independent mechanism. Rats were pretreated with saline or ACTH gel subcutaneously at indicated doses 60 min before intravenous bolus injection of recombinant rat TNF (2 mg/kg wt). Rats were killed 8 h later and plasma and kidney specimens were collected. (a) Plasma corticosterone levels were determined by enzyme-linked immunosorbent assay and suggested that the steroidogenic response to ACTH above a dose of 5 IU/kg reaches a plateau; *P<0.05 versus other groups (n=5); ¬ significant. (b) Severity of tubulointerstitial injury was scored as stated in Figure 3 and revealed that the renoprotective effect of ACTH continues to increase with even higher doses than 5 IU/kg, suggesting that steroid-independent mechanisms also contribute to kidney protection; *P<0.05 versus other groups (n=5); #P<0.05.
Melanocortin 1 receptor is the major intrarenal MCR that is predominantly expressed on renal tubular cells
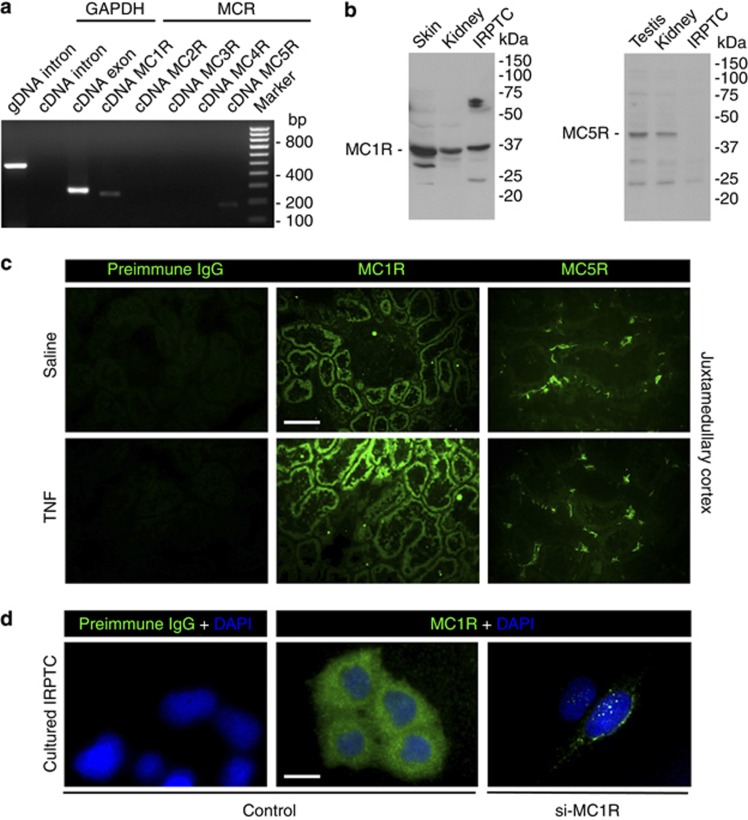
To explore the steroid-independent kidney-protective mechanisms, the expression of all MCRs was profiled in rat kidneys by reverse transcriptase–PCR (RT-PCR). The MCRs are the product of small, intronless genes; hence, genomic contamination complicates mRNA-based expression analyses, including RT-PCR.9, 27 To avoid this issue, samples of mRNA extracted from kidney tissues were subjected to DNase treatment before further processes. Shown in Figure 6a, rat kidney tissues evidently expressed melanocortin 1 receptor (MC1R) mRNA and to a much lesser extent MC5R mRNA. A parallel PCR amplification of an intron of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was carried out simultaneously using the same cDNA samples (Table 1) to confirm the quality of mRNA: no band of the GAPDH intron was detected (Figure 6a), indicating no genomic contamination of the mRNA samples. To validate the RT-PCR results, immunoblot analysis of kidney homogenates was performed and showed that both MC1R and MC5R were expressed in the kidney; however, only MC1R is expressed in both rat kidney and cultured proximal tubular epithelial (IRPTC) cells (Figure 6b). To localize the MCR expression in the kidney, fluorescent immunohistochemistry staining was conducted and showed that MC1R was expressed intensely and predominantly by renal tubules and very weakly by glomeruli, whereas MC5R was weakly and sporadically expressed by the interstitial cells (Figure 6c). TNF injury enhanced the intensity of MC1R expression in the tubules, but had minor effect on MC5R expression (Figure 6c). In cultured IRPTC cells, fluorescent immunocytochemistry staining consistently revealed punctate staining of MC1R (Figure 6d), in agreement with a typical staining pattern of cytoplasmic membrane receptors. No fluorescent staining was seen when preimmune IgG was used as primary antibodies for either immunohistochemistry or immunocytochemistry staining (Figure 6c and d). Moreover, in cells transfected with MC1R-specific siRNA, the immunofluorescent staining signal was drastically diminished (Figure 6d), denoting a high specificity of the MC1R immunostaining. Collectively, the above findings suggest that MC1R is the major MCR for ACTH in the kidney that is located to renal tubular cells.
Figure 6.
Melanocortin 1 receptor (MC1R) is a major intrarenal melanocortin receptor predominantly expressed by renal tubular epithelial cells. (a) Genomic DNA (gDNA) and mRNA were extracted from rat kidney, and cDNA was prepared from mRNA following DNase treatment of mRNA samples. Both gDNA and cDNA samples were subjected to PCR amplification for the indicated intron or exon of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), as well as indicated melanocortin receptor (MCR) subtypes. PCR results were resolved in agarose gels and visualized under ultraviolet light and indicated that rat kidney expresses mainly MC1R and, to a much lesser extent, MC5R. (b) To validate the RT-PCR data, protein was prepared from rat kidneys as well as cultured rat proximal tubular epithelial cells (IRPTC), and subjected to immunoblot analysis for MC1R and MC5R. Samples prepared from skin and testis served as positive controls. Rat kidney expresses both MC1R and MC5R; however, only MC1R is expressed in both rat kidney and cultured IRPTC cells. (c) Fluorescent immunohistochemistry staining in control (saline) or tumor necrosis factor (TNF)–treated kidney indicates that MC1R (green) is abundantly expressed by renal tubules and induced after TNF injury, whereas MC5R (green) is expressed not by the tubules but weakly and sporadically by interstitial cells in rat kidneys; bar=100 μm. (d) Cultured IRPTC cells (control) or those transfected with MC1R-specific (si-MC1R) siRNA for RNA interference were subjected to fluorescent immunocytochemistry of MC1R, which reveals punctate staining (green) consistent with a typical staining pattern of cytoplasmic membrane receptors; counterstained with DAPI (blue); bar=20 μm.
Table 1. Nucleotide sequences of the primers used for PCR.
| Molecule | Forward primer (5′-3′) | Reverse primer (5′-3′) | Amplimer size (bp) |
|---|---|---|---|
| MC1R | TGTGGTAGCCATCACCAAAA | CCAGGAAGCAGAGACTGGAC | 230 |
| MC2R | ATCTGCAGTTTGGCCATTTC | GCAATCACAGACAGGCTGAA | 188 |
| MC3R | AGGACCAATTCATCCAGCAC | AGGGCTTTCCTAACCGTCAT | 160 |
| MC4R | CTTTTACGCGCTCCAGTACC | GTAATTGCGCCCTTCATGTT | 285 |
| MC5R | CCTCGGAGGACAACATCCTA | CTAGGCTGCCCACAAAGAAG | 189 |
| GAPDH exon | GGTGATGCTGGTGCTGAGTA | ACTGTGGTCATGAGCCCTTC | 272 |
| GAPDH intron | AGCTGTTGCTCCCCTTGTAA | ATCCATCACCTGGCCTACAG | 417 |
MC1R mediates the anti-apoptotic action of ACTH on renal tubular cells
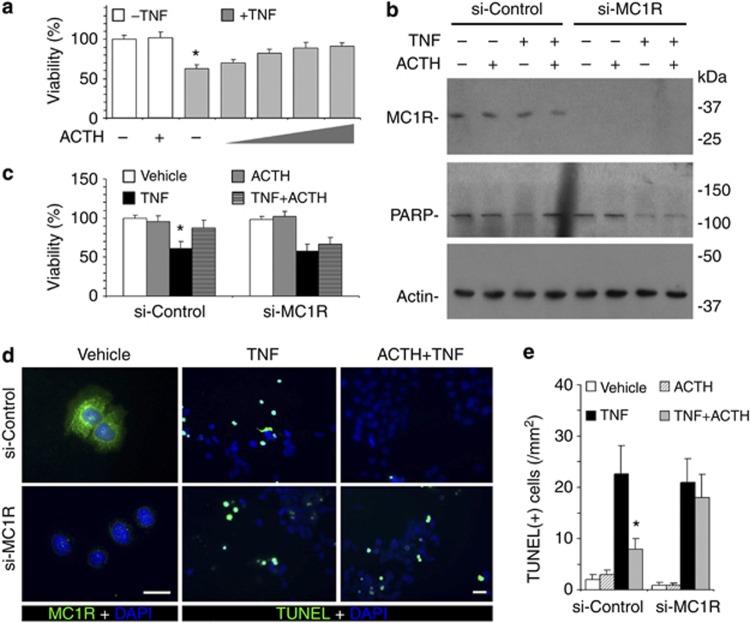
In IRPTCs stimulated with TNF in a culture model in which the steroidogenic circuit is absent, ACTH treatment markedly reinstated cellular viability in a dose-dependent manner with a maximal effect observed above 10−8M (Figure 7a), again suggesting that ACTH has a direct kidney-protective activity that is independent of steroidogenesis. To examine whether this steroid-independent anti-apoptotic/prosurvival effect of ACTH is mediated via its cognate MC1R on tubular epithelial cells, RNA interference (RNAi) was applied to specifically silence the MC1R expression in cultured IRPTC cells. Shown in Figure 7b, RNAi with MC1R-specific siRNA (si-MC1R) drastically knocked down MC1R expression. By contrast, RNAi with scramble siRNA (si-Control) barely affected its expression, as validated by immunoblot analysis (Figure 7b) and immunocytochemistry staining (Figure 7d). TNF stimulation impaired cellular viability, as estimated by tetrazolium (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) assay (Figure 7c), and induced apoptosis, as determined by PARP activation (Figure 7b) and TUNEL staining (Figure 7d and e). In cells transfected with scrambled siRNA, ACTH significantly counteracts TNF-induced apoptotic responses (Figure 7b–e). The beneficial effects of ACTH were largely abolished in MC1R-silenced cells, suggesting that MC1R is indispensable for ACTH's anti-apoptotic action in renal tubular cells.
Figure 7.
Melanocortin 1 receptor (MC1R) mediates the anti-apoptotic effect of adrenocorticotropic hormone (ACTH) in renal tubular epithelial cells. (a) ACTH reinstates the viability of tumor necrosis factor (TNF)–injured IRPTC cells in a dose-dependent manner. IRPTC cells were treated with vehicle, TNF (10 ng/ml), ACTH (10−8M), or TNF in combination with ACTH (10−10M, 10−9M, 10−8M, 10−7M) for 6 h before cellular viability was estimated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay; *P<0.05 versus other groups. (b) After MC1R silencing, ACTH fails to prevent TNF-induced PARP cleavage. IRPTC cells were transfected with scrambled (si-Control) or MC1R-specific (si-MC1R) siRNA for RNA interference. Cells were then treated with vehicle, TNF (10 ng/ml), and/or ACTH (10−8M) for 6 h. (b) Cell lysates were subjected to immunoblot analysis for MC1R, PARP, and actin; cellular viability assessed by MTT assay (c); *P<0.05 versus other groups in si-Control. (d) Fixed cells were prepared for TdT-mediated dUTP nick-end labeling (TUNEL) staining (green), fluorescent immunocytochemistry staining for MC1R (green), and counterstaining of DAPI (blue); bar=30 μm. (e) Absolute counts of TUNEL-positive cells in cell cultures; *P<0.05 versus other groups in si-Control.
ACTH gel protects from septic AKI via an additive action
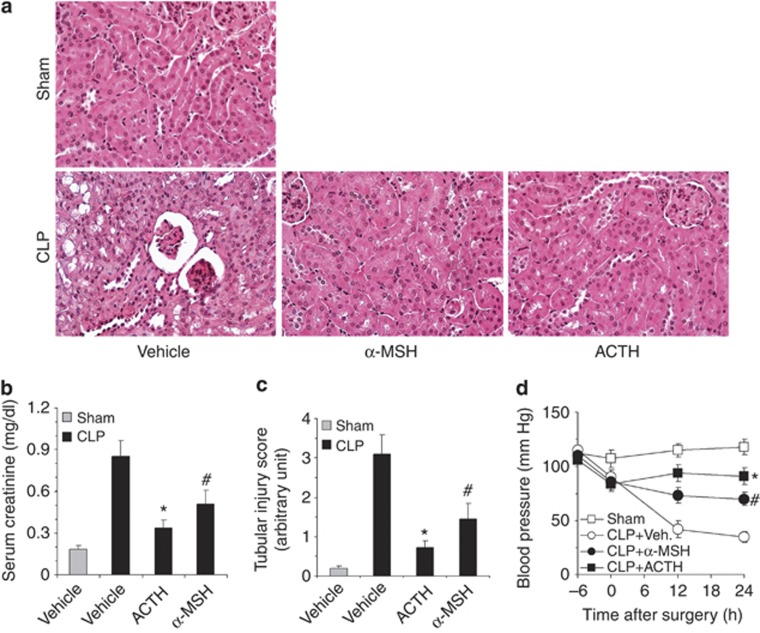
To explore whether ACTH has a kidney-protective efficacy comparable to α-MSH and to ascertain whether the kidney-protective activity of ACTH is also applicable to other forms of AKI, the effect of ACTH gel was compared with that of α-MSH and reexamined in a murine model of septic AKI induced by CLP. Mice were treated with ACTH gel (10 IU/kg) or an equal molar amount of α-MSH (71 μg/kg), subjected to CLP and followed up for 24 h. CLP-induced polymicrobial sepsis disturbed systemic hemodynamics, caused hypotensive shock, and resulted in a histology of acute tubular injury, as well as kidney dysfunction, reflected by an increase in the serum creatinine level. Consistent with previous reports,16 α-MSH treatment markedly ameliorated histological AKI (Figure 8a and c) and kidney dysfunction (Figure 8b) and corrected hypotension (Figure 8d) in septic mice. Compared with α-MSH, which is equal in biological activity to ACTH, except for steroidogenesis, ACTH gel exerted a much more potent protective effect, evidenced by significantly better kidney histology and more improvements in tubular injury scores, serum creatinine levels, and blood pressure (Figure 8). These data suggest that the kidney-protective effect exerted by ACTH might be ascribed to an additive beneficial action achieved by both steroid-dependent mechanisms and MC1R-mediated α-MSH-like activities.
Figure 8.
Adrenocorticotropic hormone (ACTH) gel protects from cecal ligation puncture (CLP)–induced septic acute kidney injury better than α-melanocyte-stimulating hormone (MSH). Mice were pretreated with ACTH gel (10 IU/kg) or an equal molar amount of α-MSH (71 μg/kg) or an equal volume of vehicle (normal saline) and subjected to CLP or sham surgery. Mice were followed up for 24 h and killed. (a) Kidney sections prepared for hematoxylin and eosin staining to evaluate histological injury; original magnification, × 400. (b) Blood was collected and serum creatinine levels were determined; *, #P<0.01 versus vehicle-treated CLP group (n=8); *P<0.05 versus α-MSH-treated CLP group (n=8). (c) Tubular injury score was estimated; *, #P<0.01 versus vehicle (Veh.)-treated CLP group (n=8); *P<0.05 versus α-MSH-treated CLP group (n=8). (d) Systolic blood pressure was measured at indicated time points; *, #P<0.01 versus vehicle (Veh.)-treated CLP group (n=8); *P<0.05 versus α-MSH-treated CLP group (n=8).
DISCUSSION
AKI remains a therapeutic challenge despite advances in supportive care. In the present study, supplementation of exogenous ACTH attenuated acute renal histological injury, improved kidney dysfunction, and promoted general survival in animal models of AKI. To the best of our knowledge, this study is the first to demonstrate a kidney-protective action of ACTH on AKI.
ACTH carries vital biologic information from the brain to the adrenal cortex and to other parts of the mammalian body.8, 9 As a crucial component of the hypothalamic–pituitary–adrenal axis, ACTH stimulates the adrenal production of corticosteroids.8, 9 The efficacy of corticosteroids on AKI remains highly controversial;28, 29 however, its potent anti-inflammatory activity has been widely acknowledged.30 Thus, the beneficial effect of ACTH on AKI observed in this study might be explained partially by the steroidogenic action through which ACTH upregulates corticosterone production and subsequently suppresses TNF-induced acute inflammatory injury in the kidney. In support of this, severity of the TNF-induced tubulointerstitial injury inversely correlated with steroidogenic response to ACTH, as determined by the plasma levels of corticosterone at low doses. However, the steroidogenic response to ACTH above a dose of 5 IU/kg reached a plateau and the renoprotective effect continued to increase with even higher doses, suggesting that steroid-independent mechanisms also contribute.
Besides governing steroidogenesis, ACTH is also an important physiological agonist of the melanocortin system, which has an integral role in the homeostatic control of a diverse array of physiological functions, including cellular survival, proliferation, anti-inflammation, and immunomodulation.9, 13 The anti-apoptotic and kidney-protective action on AKI by α-MSH, another agonist of the melanocortin system, has been extensively examined and reproducibly demonstrated in multiple animal models of AKI, including ischemia–reperfusion injury,31, 32, 33 renal toxicity,34, 35 and ureteral obstruction.36 α-MSH shares the same amino-acid sequence as the N-terminus of ACTH, and possesses all of ACTH's biological activities with similar potency, except steroidogenesis.9, 13 Therefore, the steroid-independent mechanisms accounting for the kidney-protective effect of ACTH might be attributable to a non-steroidogenic melanocortin activity or an α-MSH-like activity. Thus, ACTH might have an additive action attributable to both corticosteroid–mediated mechanisms and the non-steroidogenic α-MSH-like activities. Accordingly, owing to its unique steroidogenic effect, ACTH might be superior to α-MSH in protecting kidney from acute injuries, as revealed in our study of CLP-induced sepetic AKI.
The detailed molecular mechanism underlying the anti-apoptotic and kidney-protective effect of ACTH and α-MSH remains elusive, and whether ACTH and α-MSH directly targets kidney parenchymal cells has been barely investigated. Our study found for the first time that MC1R, a cognate receptor with high affinity for both α-MSH and ACTH, is the major MCR that is abundantly expressed in the kidney and predominantly distributed on tubules, denoting renal tubules as a direct therapeutic target for both ACTH and α-MSH. Indeed, ACTH treatment of tubular epithelial cells in a culture model in which the steroidogenic circuit is absent drastically obliterated TNF-induced apoptosis and reinstated cellular viability. Furthermore, MC1R silencing largely abrogated ACTH's beneficial effect, suggesting that MC1R mediates this cytoprotective effect. In agreement with our observations, Masser et al.37 demonstrated the dramatic worsening of experimental intestinal injury in mice with a nonfunctional MC1R. Moreover, the anti-apoptotic/prosurvival effect of MC1R signaling has been demonstrated previously in human melanocytes.38, 39 Treatment with α-MSH results in the reduction of ultraviolet radiation–elicited apoptosis, and this effect was independent of melanin synthesis, in agreement with an MC1R-mediated non-pigmentary anti-apoptotic mechanism.38, 39 Consistently, our data suggest that ACTH intercepts the caspase-directed proapoptotic signaling pathway and obliterates GSK3β activation, which promotes apoptosis and plays dirty in AKI.26
Systemic inflammatory response, as well as immune dysfunction, also plays an important role in the pathogenesis of AKI.40, 41 Evidence suggests that melanocortins such as α-MSH and ACTH display a potent systemic protective activity directly via MCRs expressed on most immune and non-immune cells.27 Therefore, in addition to a direct kidney effect, ACTH might prevent AKI via systemic protection, immunomodulation, and anti-inflammation. Indeed, our data indicate that ACTH gel treatment has a systemic beneficial action that corrected hypotension and improved histological injuries and inflammation in multiple organs including the liver and lung.
In summary, ACTH gel, an FDA-approved formulation of natural ACTH for the treatment of nephrotic syndrome, can also ameliorate acute renal histological injury, correct acute kidney dysfunction, and improve general survival in animal models of AKI. ACTH ameliorates AKI by an additive action exerted possibly via both steroid-dependent mechanisms and MC1R-directed anti-apoptotic signaling. Our findings suggest that ACTH may represent a novel and pragmatic kidney-protective strategy to prevent or treat AKI.
MATERIALS AND METHODS
Cell culture
IRPTC cells, provided by Dr Julie Ingelfinger, were grown in Dulbecco's modified Eagle's medium/F12 that contained 5% fetal bovine serum. Recombinant rat TNF (R&D Systems, Minneapolis, MN) and porcine ACTH (Sigma, St Louis, MO) were used to treat the culture at indicated concentrations. RAM cells were purchased from American Type Culture Collection (ATCC, Manassas, VA) and were cultured in Ham's F12K containing 15% fetal bovine serum. For fluorescent viable labeling, RAM cells (1 × 107) were incubated in medium containing 5 mg/ml Calcein-AM (Molecular Probes, Eugene, OR) at 37 °C for 30 min.
Cellular viability assay
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide assay was used to assess cell viability as described previously.
Fluorescent immunocytochemistry
Cells were fixed with 4% paraformaldehyde. After serum blocking for 30 min, cells were incubated with the primary antibody to indicated MCR (Alomone Labs, Jerusalem, Israel) or preimmune IgG and then with the Alexa fluorophore 488–conjugated secondary antibody (Invitrogen, Carlsbad, CA). Finally, cells were counterstained with 4′,6-diamidino-2-phenylindole and mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and visualized using a fluorescence microscope.22
RNA interference
Predesigned siRNA duplex was chemically synthesized by Ambion (Austin, TX). MC1R-specific siRNA sequence (5′-GAGGUUGAAGUUCUUGAAGtt-3′) was designed according to the complete coding sequence of rat MC1R gene (GenBank accession no. AB576619.2). In addition, a scrambled siRNA sequence (5′-GCGAGUAGCGCUAGGAAGUtt-3′) without sequence similarity to any known gene sequences from mouse, rat, or human was designed as control for RNAi.42 Efficiency of lipofectamine (Invitrogen)-mediated gene-silencing efficiency was assessed by immunocytochemistry staining or immunoblot analysis for MC1R. Near-complete (∼80%) suppression of MC1R protein expression was observed in cells transfected with MC1R-specific siRNA.
RNA extraction and RT-PCR
Genomic DNA (gDNA) and mRNA were extracted from kidney cortex tissues or cultured cells, and the first-strand cDNA was prepared after mRNA was treated with DNase to eliminate potential gDNA contamination. PCR amplification of the intron or exon of GAPDH, as well as indicated MCRs, was carried out as previously described using primers listed in Table 1. To confirm no contamination of mRNA by gDNA, cDNA samples were subjected to PCR amplification of an intron and an exon of GAPDH gene; a sample of gDNA served as the positive control. PCR products resolved in agarose gels were photographed under ultraviolet light.
Animal experimental design
Animal experimental studies were approved by the institution's Animal Care and Use Committee, and they conform to the USDA regulation and NIH guidelines for human care and use of laboratory animals.
Rat model of TNF induced AKI
Male Sprague–Dawley rats with initial weights of 200–250 g were pretreated subcutaneously with saline or ACTH gel at indicated doses 60 min before injection of recombinant rat TNF (2 mg/kg wt) (R&D system) via tail vein. Rats were evaluated hourly and survival was recorded. To determine an optimal dose of ACTH gel for kidney protection, RAM infusion assay was performed in some rats as reported before.22 In brief, rats were anesthetized, at indicated time points after TNF injection, placed on a heated table to keep constant body temperature, and maintained at an euvolemic state. Fluorescent-labeled RAM cells (1 × 104) resuspended in normal saline (NS) were infused via the carotid artery. After a 15-min equilibration period, MAP was measured and glomerular filtration rate was determined by inulin clearance assay as described before.22 Rats were killed 60 min after RAM cell infusion, and ureteral urine and blood were collected. Organs were collected for further investigation. To quantify the fluorescent RAM cells sequestrated in the organs, tissue homogenates with an equal amount of protein (100 mg) were subjected to fluorometric analysis.
Mouse model of septic AKI induced by CLP
Male CD-1 mice at the age of 8 weeks were subjected to CLP surgery to induce sepsis as previously described.16, 23, 24 In brief, under isoflurane anesthesia, 1.5 cm of the cecal tip was ligated using a 4-0 silk suture and punctured twice with a 21-gauge needle. An approximately 1-mm column of fecal material was expressed. In sham-operated mice, the cecum was isolated but neither ligated nor punctured. After surgery, all mice received 1 ml of prewarmed NS intraperitoneally. Treatment with fluid and antibiotic was started at 12 h after surgery with subcutaneous injection of imipenem/cilastatin (14 mg/kg) in 1 ml of NS. Acthar gel (10 IU/kg) or equal molar amounts of α-MSH (71 μg/kg, Sigma; prepared in saline containing 16% gelatin) was administered subcutaneously 60 min before surgery and at 12 h post surgery. Vehicle-treated animals received an equal volume of NS subcutaneously at the indicated times. Mouse blood pressure was measured using the tail cuff method as described previously.43 Mice were killed 24 h after surgery and urine and blood were collected. Organs were collected for further investigation.
Western immunoblot analyses
Cultured tubular epithelial cells were lysed and rat kidneys homogenized in RIPA buffer supplemented with protease inhibitors. Samples with equal amounts of total protein (50 mg/ml) were processed for immunoblot as described previously.22 The antibodies against caspase-3, PARP, p-GSK3β, GSK3β, and actin were purchased from Cell Signaling Technology (Beverly, MA), and those against MCR were purchased from Alomone Labs.
Assessment of pulmonary edema
Lungs were removed from animals, placed in a tared microcentrifuge tube, and weighed. Lungs were then desiccated under vacuum at 25 °C and weighed again. The wet lung mass was divided by the dry lung mass to give the wet–dry ratio, which is the fraction of the wet lung weight that was water.44
Measurement of blood and urine chemistry
Albumin levels in the ureteral urine were measured using a rat Albumin ELISA Quantification kit (Bethyl Laboratories, Montgomery, TX). Plasma corticosterone levels were measured by Corticosterone EIA Kits (Cayman Chemical, Ann Arbor, MI). Serum creatinine levels were determined by a routine procedure as described previously.25, 45 To assess hepatotoxicity, serum activity of aspartate aminotransferase and alanine aminotransferase were measured using spectrophotometric ELISA kits (Uscn Life Science, Houston, TX).
Morphological studies
Formalin-fixed kidneys were embedded in paraffin and prepared in 3-μm-thick sections. Sections were stained with hematoxylin–eosin to estimate gross histological kidney injury. One observer performed computerized, as well as semiquantitative morphometric, analysis in a blinded manner. Severity of tubulointerstitial injury and tubular dilation/sloughing was semiquantitatively scored on a scale from 0 to 3 and reported as the mean of 20 random high-power ( × 400) fields per hematoxylin–eosin-stained section: 0, absent; 1, slight; 2, moderate; 3, marked; 4, severe.22 Necroapoptotic nuclei were defined as nuclei dropped off into the lumen or interstitium or those with chromatin condensation, pyknosis, or fragmentation. Indirect immunofluorescent staining of MCR was carried out on methanol/acetone-fixed (1:1) frozen cryostat sections using anti-MCR antibodies (Alomone Labs). Next, the Alexa Fluor 488–conjugated secondary antibodies (Invitrogen) were applied. As a negative control, the primary antibodies were replaced by preimmune IgG from the same species; no staining occurred. Finally, all sections were mounted with Vectashield mounting medium (Vector Laboratories). For visualization of fluorescence RAM cells that were sequestrated in the organs, cryosections were fixed with methanol/acetone and then subjected to Evans blue counterstaining.
TUNEL staining
Apoptotic cell death in kidney sections was detected by using the TUNEL kit (Roche Molecular Biochemicals, Mannheim, Germany).25 Ten randomly selected × 400 high-power fields of juxtamedullary cortex were counted to determine the number of apoptotic nuclei.
Statistical analyses
For immunoblot analysis, bands were scanned and the integrated pixel density was determined using a densitometer and the NIH image analysis program. All data are expressed as means±s.d. Statistical analysis of the data from multiple groups was performed by ANOVA followed by Student–Newman–Keuls tests. Data from two groups were compared by t-test. Log-rank test was used for Kaplan–Meier survival analysis. P<0.05 was considered significant.
Acknowledgments
Research work by RG has been supported by research grants from Questcor and National Institutes of Health grant R01DK092485. SC was funded by the National Natural Science Foundation of China (no. 30900766). We thank Dr. Dworkin for helpful discussions and Ms. Tolbert for technical assistance with the rat study.
All the authors declared no competing interests.
Footnotes
SUPPLEMENTARY MATERIAL
Figure S1. ACTH gel prevents acute liver and lung injuries and reduces macrophage sequestration in the liver and lung.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
Supplementary Material
References
- Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr. 2011;23:194–200. doi: 10.1097/MOP.0b013e328343f4dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J Am Soc Nephrol. 2011;22:810–820. doi: 10.1681/ASN.2010080796. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Ford GM, Waikar SS, et al. A rapid urine test for early detection of kidney injury. Kidney Int. 2009;76:108–114. doi: 10.1038/ki.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P, Udani S, Koyner JL. Does renal replacement therapy improve outcome? Controversies in acute kidney injury. Contrib Nephrol. 2011;174:212–221. doi: 10.1159/000329399. [DOI] [PubMed] [Google Scholar]
- Palevsky PM. Renal support in acute kidney injury--how much is enough. N Engl J Med. 2009;361:1699–1701. doi: 10.1056/NEJMe0907831. [DOI] [PubMed] [Google Scholar]
- Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–365. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- Dores RM. Adrenocorticotropic hormone, melanocyte-stimulating hormone, and the melanocortin receptors: revisiting the work of Robert Schwyzer: a thirty-year retrospective. Ann N Y Acad Sci. 2009;1163:93–100. doi: 10.1111/j.1749-6632.2009.04434.x. [DOI] [PubMed] [Google Scholar]
- Gong R. The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol. 2012;8:122–128. doi: 10.1038/nrneph.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arneil GC, Wilson HE. A.C.T.H. in nephrosis. Arch Dis Child. 1953;28:372–380. doi: 10.1136/adc.28.141.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AL, Arnadottir M. ACTH revisited--potential implications for patients with renal disease. Nephrol Dial Transplant. 2000;15:940–942. doi: 10.1093/ndt/15.7.940. [DOI] [PubMed] [Google Scholar]
- Bomback AS, Radhakrishnan J. Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) Discov Med. 2011;12:91–96. [PubMed] [Google Scholar]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- Blagaic V, Houra K, Turcic P, et al. The influence of alpha-, beta-, and gamma-melanocyte stimulating hormone on acetaminophen induced liver lesions in male CBA mice. Molecules. 2010;15:1232–1241. doi: 10.3390/molecules15031232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda Y, Chiao H, Star RA. Alpha-melanocyte-stimulating hormone and acute renal failure. Curr Opin Nephrol Hypertens. 1998;7:413–417. doi: 10.1097/00041552-199807000-00011. [DOI] [PubMed] [Google Scholar]
- Doi K, Hu X, Yuen PS, et al. AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int. 2008;73:1266–1274. doi: 10.1038/ki.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsernyes M, Juhasz B, Der P, et al. The administration of alpha-melanocyte-stimulating hormone protects the ischemic/reperfused myocardium. Eur J Pharmacol. 2003;470:177–183. doi: 10.1016/s0014-2999(03)01780-1. [DOI] [PubMed] [Google Scholar]
- Huang Q, Tatro JB. Alpha-melanocyte stimulating hormone suppresses intracerebral tumor necrosis factor-alpha and interleukin-1beta gene expression following transient cerebral ischemia in mice. Neurosci Lett. 2002;334:186–190. doi: 10.1016/s0304-3940(02)01088-1. [DOI] [PubMed] [Google Scholar]
- Hassoun HT, Zou L, Moore FA, et al. Alpha-melanocyte-stimulating hormone protects against mesenteric ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1059–G1068. doi: 10.1152/ajpgi.00073.2001. [DOI] [PubMed] [Google Scholar]
- Kettelhut IC, Fiers W, Goldberg AL. The toxic effects of tumor necrosis factor in vivo and their prevention by cyclooxygenase inhibitors. Proc Natl Acad Sci USA. 1987;84:4273–4277. doi: 10.1073/pnas.84.12.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauwels A, Janssen B, Waeytens A, et al. Caspase inhibition causes hyperacute tumor necrosis factor-induced shock via oxidative stress and phospholipase A2. Nat Immunol. 2003;4:387–393. doi: 10.1038/ni914. [DOI] [PubMed] [Google Scholar]
- Gong R, Rifai A, Dworkin LD. Hepatocyte growth factor suppresses acute renal inflammation by inhibition of endothelial E-selectin. Kidney Int. 2006;69:1166–1174. doi: 10.1038/sj.ki.5000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi K, Leelahavanichkul A, Yuen PS, et al. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119:2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22:999–1006. doi: 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- Bao H, Ge Y, Zhuang S, et al. Inhibition of glycogen synthase kinase-3beta prevents NSAID-induced acute kidney injury. Kidney Int. 2012;81:662–673. doi: 10.1038/ki.2011.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PJ, Cantley L. GSK3beta plays dirty in acute kidney injury. J Am Soc Nephrol. 2010;21:199–200. doi: 10.1681/ASN.2009121214. [DOI] [PubMed] [Google Scholar]
- Catania A. The melanocortin system in leukocyte biology. J Leukoc Biol. 2007;81:383–392. doi: 10.1189/jlb.0706426. [DOI] [PubMed] [Google Scholar]
- Kumar S, Allen DA, Kieswich JE, et al. Dexamethasone ameliorates renal ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20:2412–2425. doi: 10.1681/ASN.2008080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S, Derham C, Orsi NM, et al. Randomized clinical trial of the effects of methylprednisolone on renal function after major vascular surgery. Br J Surg. 2008;95:50–56. doi: 10.1002/bjs.5978. [DOI] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Chiao H, Kohda Y, McLeroy P, et al. Alpha-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats. J Clin Invest. 1997;99:1165–1172. doi: 10.1172/JCI119272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao H, Kohda Y, McLeroy P, et al. Alpha-melanocyte-stimulating hormone inhibits renal injury in the absence of neutrophils. Kidney Int. 1998;54:765–774. doi: 10.1046/j.1523-1755.1998.00075.x. [DOI] [PubMed] [Google Scholar]
- Gong H, Wang W, Kwon TH, et al. EPO and alpha-MSH prevent ischemia/reperfusion-induced down-regulation of AQPs and sodium transporters in rat kidney. Kidney Int. 2004;66:683–695. doi: 10.1111/j.1523-1755.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- Kolgazi M, Arbak S, Alican I. The effect of alpha-melanocyte stimulating hormone on gentamicin-induced acute nephrotoxicity in rats. J Appl Toxicol. 2007;27:183–188. doi: 10.1002/jat.1191. [DOI] [PubMed] [Google Scholar]
- Lee SY, Jo SK, Cho WY, et al. The effect of alpha-melanocyte-stimulating hormone on renal tubular cell apoptosis and tubulointerstitial fibrosis in cyclosporine A nephrotoxicity. Transplantation. 2004;78:1756–1764. doi: 10.1097/01.tp.0000144332.44435.ab. [DOI] [PubMed] [Google Scholar]
- Li C, Shi Y, Wang W, et al. Alpha-MSH prevents impairment in renal function and dysregulation of AQPs and Na-K-ATPase in rats with bilateral ureteral obstruction. Am J Physiol Renal Physiol. 2006;290:F384–F396. doi: 10.1152/ajprenal.00282.2004. [DOI] [PubMed] [Google Scholar]
- Maaser C, Kannengiesser K, Specht C, et al. Crucial role of the melanocortin receptor MC1R in experimental colitis. Gut. 2006;55:1415–1422. doi: 10.1136/gut.2005.083634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser JE, Kadekaro AL, Kavanagh RJ, et al. Melanin content and MC1R function independently affect UVR-induced DNA damage in cultured human melanocytes. Pigment Cell Res. 2006;19:303–314. doi: 10.1111/j.1600-0749.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- Abdel-Malek ZA, Ruwe A, Kavanagh-Starner R, et al. Alpha-MSH tripeptide analogs activate the melanocortin 1 receptor and reduce UV-induced DNA damage in human melanocytes. Pigment Cell Melanoma Res. 2009;22:635–644. doi: 10.1111/j.1755-148X.2009.00598.x. [DOI] [PubMed] [Google Scholar]
- Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109:e102–e107. doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves GM, Zamboni DS, Camara NO. The role of innate immunity in septic acute kidney injuries. Shock. 2010;34 (Suppl 1:22–26. doi: 10.1097/SHK.0b013e3181e7e69e. [DOI] [PubMed] [Google Scholar]
- Chen S, Ge Y, Si J, et al. Candesartan suppresses chronic renal inflammation by a novel antioxidant action independent of AT1R blockade. Kidney Int. 2008;74:1128–1138. doi: 10.1038/ki.2008.380. [DOI] [PubMed] [Google Scholar]
- Whitesall SE, Hoff JB, Vollmer AP, et al. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol. 2004;286:H2408–H2415. doi: 10.1152/ajpheart.01089.2003. [DOI] [PubMed] [Google Scholar]
- Rojas M, Woods CR, Mora AL, et al. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005;288:L333–L341. doi: 10.1152/ajplung.00334.2004. [DOI] [PubMed] [Google Scholar]
- Keppler A, Gretz N, Schmidt R, et al. Plasma creatinine determination in mice and rats: an enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int. 2007;71:74–78. doi: 10.1038/sj.ki.5001988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.