Abstract
To reveal the underlying mechanisms responsible for the regional vulnerability to amyloid-β (Aβ) accumulation prior to the development of Alzheimer’s disease, we studied distribution of Aβ, apolipoprotein E (apoE), synaptic markers, and other molecules involved in Aβ metabolism in multiple brain areas of non-demented individuals. Twelve brain regions including neocortical, limbic, and subcortical areas were dissected from brains of non-demented individuals and extracted according to increasing insolubility by a sequential three-step method. The levels of Aβ40, Aβ42, apoE, APP, APP-CTFβ, BACE1, presenilin-1, neprilysin, insulysin, LRP1, LDLR, synaptophysin, PSD95, GFAP, and lactate were determined by ELISAs or enzymatic assays. The regional distribution of apoE showed moderate-to-strong inverse correlation with levels of Aβ, especially insoluble Aβ40. On the other hand, the regional distributions of synaptic markers, particularly PSD95, showed moderate-to-strong positive correlation with levels of Aβ, especially soluble Aβ40. The regional correlations between Aβ and LRP1, GFAP, or lactate were mild-to-moderate. Moderate-to-strong positive regional correlations were observed between apoE and GFAP or lactate and between PSD95 and LRP1. No significant regional correlations were detected between Aβ and APP, APP-CTFβ, BACE1, or presenilin-1, those involved in Aβ production. There were no significant negative regional correlations between Aβ and two major Aβ degrading enzymes, neprilysin and insulysin. These regional correlations remained consistent regardless of the degree of Aβ accumulation. The regional vulnerability to Aβ accumulation may be due to a net balance between two competing processes: (1) synapses involved in promoting the initial Aβ accumulation and (2) astrocyte-derived apoE involved in preventing Aβ accumulation.
Keywords: Alzheimer’s disease, Amyloid-β, Regional vulnerability, Apolipoprotein E, Synapses
Introduction
Accumulation and aggregation of amyloid-β (Aβ) peptides may initiate Alzheimer’s disease (AD) pathogenesis (so-called amyloid cascade hypothesis) [4, 26]. Accumulation of Aβ is determined by a balance between processes of production and clearance. Aβ is produced by proteolytic cleavages of amyloid precursor protein (APP) by β-site cleaving enzyme and subsequent cleavage of β-C-terminal fragments of APP (APP-CTFβ) by γ-secretase [65]. Neuronal and synaptic activity might be involved in this initial step of Aβ metabolism [7, 14]. Upon production, much of Aβ is efficiently cleared by Aβ degrading enzymes through cellular uptake to lysosomes or brain vasculature. Cellular clearance of Aβ by various cell types in brain parenchyma and vasculature is mediated by cell surface Aβ-binding receptors and regulated by apolipoprotein E (apoE). Aβ can also be cleared through perivascular drainage into the cerebrospinal fluid (CSF) [10, 29–31, 37]. Over production and/or inefficient clearance of Aβ can lead to its accumulation and aggregation and eventual synaptic and neuronal toxicity.
To understand the pathogenesis of AD, it is important to elucidate how Aβ accumulates in human brains. Aβ deposition appears to begin more than a decade before the onset of AD [4, 26]. Accumulating evidence has shown that risk factors of AD (aging and APOE ε4 allele) accelerate accumulation of Aβ prior to the development of the disease [47, 53]. Aβ deposition typically occurs first in neocortical areas, followed by limbic areas, brainstem regions, including the thalamus and striatum, and finally spreads to the cerebellum [64]. In AD brains, Aβ depositions are observed throughout the brain, and especially cortical deposition appears to plateau relatively early in the disease process or prior to the development of AD [8, 26,27].
Despite clinical and pathological evidence of regional specificity of Aβ depositions, it is not yet clear what factors determine selective vulnerability to Aβ accumulation. An emerging hypothesis is that a certain type of neuronal activity regulates region-specific Aβ levels, as evidenced by the fact that areas involving default neuronal activity overlap with areas associated with Aβ deposition [11, 70]. Regional associations presumably exist between levels of Aβ and molecules or markers involved in Aβ metabolism; however, the regional distribution of molecules involved in Aβ metabolism has not been fully investigated. Here, we provide a post-mortem analysis of regional neuroanatomical distribution of Aβ as well as molecules and markers related to Aβ metabolism, in brains of non-demented individuals within extracts based upon differential solubility in detergents and chaotropic agents.
Materials and methods
Sample preparation
Post-mortem tissues were obtained through the Mayo Clinic Brain Bank under procedures approved by the Mayo Clinic Institutional Review Board. All subjects had been enrolled in one of two NIH funded studies, Mayo Clinic Alzheimer Disease Research Center (P50 AG016574) or Mayo Clinic Study on Aging (U01 AG006786), and had standardized antemortem clinical and neuropsychological assessments. Brain samples from 21 non-demented individuals were analyzed. Demographic characteristics are shown in Table 1. Gray matter of 12 brain areas (Table 2) was dissected and kept frozen until extraction. Brain lysates were prepared according to the three-step extraction method described by Shankar et al. [55] with minor modifications. After removal of meninges and blood vessels, 100–200 mg of frozen brain tissue were homogenized in ice-cold TBS containing a protease inhibitor cocktail (PIC; Roche Diagnostics, Indianapolis, IN, USA) by Polytron homogenizer (KINEMATICA, Bohemia, NY,USA). After centrifugation at 100,000×g for 60 min at 4 °C, the supernatant was aliquoted and stored at −80 °C (referred to as TBS fraction). The residual pellet was rehomogenized in TBS plus 1 % Triton X-100 with PIC, incubated with mild agitation for 1 h at 4 °C and centrifuged as above. The resultant supernatant was aliquoted and stored at −80 °C (referred to as TBS-TX fraction). The residual pellet was rehomogenized in TBS plus 5 M guanidine hydrochloride, pH 7.6, and incubated with mild agitation for 12–16 h at 22 °C. After centrifugation as above, the resultant supernatant (referred to as GuHCl fraction) was diluted with 9 volume of TBS, aliquoted and stored at −80 °C.
Table 1.
Demographic characteristics of subjects
| Number | Age (year) | Male:Female | MMSE | CDR | Braak NFT Stage | PMI (h) |
|---|---|---|---|---|---|---|
| 21 | 91.3 ± 7.1 (76–101) | 4:17 | 27 (21–30) | 0 (0–0.5) | III (I–IV) | 11.4 ± 5.5 (3–23) |
Values are mean ± SD (range) (Age and PMI), median (range) (MMSE, CDR, and Braak NFT Stage), and number of subjects (Number and Male:Female)
MMSE score of mini-mental state examination, CDR clinical dementia rating scale, NFT neurofibrillary tangle, PMI post-mortem interval
Table 2.
Brain regions used for the analysis
| Cortical areas |
Subcortical areas | |
|---|---|---|
| Neocortical areas | Limbic areas | |
| Dorsolateral prefrontal (BA9, DF) | Posterior cingulate (BA31, PC) | Striatum (caudate, ST) |
| Orbitofrontal (BA12, OF) | Entorhinal (BA28, EC) | Thalamus (TL) |
| Inferior temporal (BA20, IT) | Amygdala (AM) | Hypothalamus (HT) |
| Inferior parietal (BA39/40, IP) | Cerebellum (CB) | |
| Primary visual (BA17, VC) | ||
BA brodmann area
Quantification of proteins and lactate
Total protein levels were determined by Bradford method (Bio-Rad, Hercules, CA, USA). The levels of Aβ1-40 and Aβ1-42 were determined by ELISA as previously described [13] using an end-specific monoclonal antibody (13.1.1 for Aβ x-40 and 2.1.3 for Aβ x-42) and a HRP-conjugated detector antibody (Ab5, human Aβ1-16 specific, all antibodies were in-house produced by Mayo Clinic). The levels of apoE were determined by ELISA using two different goat anti-apoE antibodies, as previously described [3]. The levels of APP were determined by ELISA on TBS-TX fraction using a rabbit anti-C-terminus of APP capture antibody (a gift from Dr. Pritam Das) and biotin-conjugated MAB890 detector antibody (R&D, Minneapolis, MN, USA). The recombinant human APP proteins (OriGene, Rockville, MD, USA) were used as standards. The levels of APP-CTFβ were determined by ELISA on TBS-TX fraction using an above-mentioned rabbit anti-C-terminus of APP capture antibody and biotin-conjugated 82E1 detector antibody (IBL-America, Minneapolis, MN, USA). Synthetic peptides, consisted of 15 a.a. of N-terminus of Aβ and 20 a.a. of C-terminus of APP, were used as standards. The levels of insulin degrading enzyme (IDE, or insulysin) were determined by ELISA on TBS fraction using 6H9 capture antibody and biotin-conjugated 6A1 detector antibody (gifts from Dr. Malcolm Leissring), as previously described [16]. The levels of β-site APP-cleaving enzyme 1 (BACE1), N-terminal fragments of presenilin-1, and Neprilysin (NEP) were determined on TBS-TX fraction by commercial ELISA kit (R&D). The levels of glial fibrillary acidic protein (GFAP) were determined by ELISA on TBS fraction using rabbit anti-GFAP capture antibody (US Biological, Swampscott, MA, USA) and biotin-conjugated GA-5 detector antibody (Abcam, Cambridge, MA, USA). GFAP proteins purified from human brain (Millipore, Billerica, MA, USA) were used as standards. The levels of low-density lipoprotein receptor (LDLR) were determined by ELISA on TBS-TX fraction using Irene capture antibody [52] and biotin-conjugated goat anti-LDLR detector antibody (R&D). The recombinant human LDLR proteins (R&D) were used as standards. The levels of LDLR-related protein 1 (LRP1) were determined by ELISA on TBS-TX fraction using 6F9 capture antibody and biotin-conjugated 5A6 detector antibody (Molecular Innovations, Novi, MI, USA). LRP1 proteins purified from human placenta were used as standards. The levels of postsynaptic density 95 (PSD95) were determined by ELISA on TBS fraction using rabbit anti-PSD95 capture antibody (Osenses, Keswick, SA, Australia) and biotin-conjugated mouse anti-PSD95 detector antibody (NeuroMab, Davis, CA, USA). The recombinant human PSD95 proteins (Novus Biologicals, Littleton, CO, USA) were used as standards. The levels of synaptophysin were determined by ELISA on TBS-TX fraction using rabbit anti-synaptophysin antibody (Osenses) and biotin-conjugated mouse anti-synaptophysin antibody (Acris Antibodies, San Diego, CA, USA). The recombinant human synaptophysin proteins (Novus Biologicals) were used as standards. Levels of specific proteins and lactate (with the exception of total protein, Aβ and apoE) were determined on one of the three fractions, based on their abundance among these fractions. Specifically, TBS fraction was used to measure levels of cytosolic/secreted proteins and molecules (i.e., IDE, GFAP, PSD95, and lactate); whereas TBS-TX fraction was used to measure levels of membrane proteins (i.e., APP, APP-CTFβ, BACE1, presenilin-1, NEP, LDLR, LRP1, and synaptophysin). Colorimetric quantification was performed on a Synergy HT plate reader (BioTek, Winooski, VT, USA) using horseradish peroxidase (HRP)-linked streptavidin (Vector, Burlingame, CA, USA) or Poly-HRP 40 streptavidin (Fitzgerald, Acton, MA, USA) and 3,3′,5,5′-tetramethyl-benzidine substrate (Sigma, St Louis, MO, USA). Brain lactate levels were determined on TBS fraction using enzymatic lactate assay kit according to the manufacture’s instruction (BioVision, Milpitas, CA, USA). Colorimetric quantification was performed on a Synergy HT plate reader.
Data analysis
All measured values were first normalized by total protein levels in the sample. Comparisons of cortical Aβ and apoE levels (averaged value of cortical areas) among three fractions were performed by ANOVA followed by paired t test with Holm correction for multiple comparisons (R, version 2.14.1; The R foundation, Vienna, Austria). When comparing regional differences, each measured value normalized by total protein levels was then normalized by the average value within an individual to adjust for the influence of difference between individuals. Comparisons of such normalized values among different brain areas were performed by ANOVA followed by paired t test with Holm correction for multiple comparisons (R). The non-parametric Spearman rank correlation coefficient was used to summarize the degree of correlation between median levels of each protein across 12 brain regions (JMP, version 7; SAS, Cary, NC). The p values of <0.05 were considered significant.
Results
Regional distribution of Aβ
We evaluated levels of Aβ extracted into three fractions: (1) TBS-soluble pool (TBS fraction), (2) TBS-insoluble but Triton-X soluble pool (TBS-TX fraction), and (3) TBS and Triton-X-insoluble but GuHCl soluble pool (GuHCl fraction). Average Aβ40 levels of cortical areas were highest in GuHCl fraction compared to other fractions (Supplementary Fig. 1). We observed a trend in differential Aβ40 distributions by anatomical region with neocortical areas being the highest, followed by limbic areas and then subcortical areas, though there was not a significant difference between neocortical and limbic areas (Fig. 1a–c). A mixed model statistical analysis showed significant differences in Aβ40 levels among cortical areas as well as between neocortical and limbic areas (see Supplementary Materials and Methods and Supplementary Fig. 2). Since we encountered difficulties in measuring Aβ42 in TBS and TBS-TX fraction in a pilot study, especially in individuals with low Aβ levels, we determined the regional distribution of Aβ42 only in GuHCl fraction (Fig. 1d). The regional distribution of Aβ42 in GuHCl fraction strongly correlated with that of Aβ40, suggesting the same regional specificity of Aβ40 and Aβ42 levels (Supplementary Fig. 3). To confirm that such regional distribution trends persist regardless of the degree of Aβ accumulation, we firstly, separated individuals into two groups: (1) low levels of cortical Aβ and (2) intermediate-to-high levels of cortical Aβ (see Supplementary Materials and Methods and Supplementary Table 1), and found similar trends of regional distribution of Aβ between the two groups (Supplementary Table 2). Moreover, we separated individuals on a histochemical basis by the degree of Aβ accumulation, namely “pathological aging” [17] (Supplementary Table 5). Indeed, regional distribution of Aβ was well preserved between individuals with/without pathological aging (Supplementary Table 6). In addition, we separated individuals on a genetic basis by the presence/absence of the APOE ε4 allele and found similar regional distribution of Aβ (Supplementary Table 9, 10). These results suggest that the regional distribution of Aβ is similar among non-demented individuals with different degrees of Aβ accumulation or with/without the strong genetic risk factor for AD.
Fig. 1.
Regional distribution of Aβ in fractions from the three-step extraction. After normalization within each individual, Aβ40 level in TBS fraction (a), TBS-TX fraction (b) and GuHCl fraction (c) and Aβ42 level in GuHCl fraction (d) are plotted by different brain areas with the average line of each area. In this and subsequent figures, blue areas are neocortical areas. Purple areas are limbic areas. Orange areas are subcortical areas. If two areas are not connected by a line on the upper portion of the graph, statistically significant difference was observed between these two areas. The p < 0.05 was considered significant; paired t test with Holm correction for multiple comparisons. TX TBS-TX
Regional distribution of molecules involved in Aβ metabolism
We next determined the regional distribution of molecules linked to Aβ metabolism. Cortical apoE levels in GuHCl fraction were around one-tenth of that in TBS or TBS-TX fraction, suggesting that TBS and TBS-TX fractions represent main functional fractions of apoE (data not shown). In these fractions, subcortical areas generally had higher apoE levels than cortical areas. Some limbic areas (AM and EC) had higher apoE levels compared to neocortical areas (Fig. 2a, b). Frontal-temporal neocortical areas (OF, DF, and IT) and the striatum had higher levels of APP compared with parietal-to-posterior cortical areas (VC, IP, and PC), amygdala, and cerebellum (Fig. 2c). APP-CTFβ levels were lowest in cerebellum among the 12 areas. Some neocortical areas (OF and IT) and the striatum had higher levels of APP-CTFβ compared with parietal-to-posterior cortical areas (VC, IP, and PC) and amygdala (Fig. 2d). Two of the limbic areas (AM and EC) had higher levels of BACE1 (β-secretase) than other areas (Fig. 2e). Thalamus and hypothalamus had relatively low levels of presenilin-1, the major enzymatic component of γ-secretase (Fig. 2f).NEP levels were much higher in striatum compared to other areas (Fig. 2g). Although IDE levels in thalamus and cerebellum were variable with some extreme outliers, some cortical areas (DF, IT, IP, and OF) had higher IDE levels (Fig. 2h). LDLR levels were lowest in the striatum (Fig. 2j), whereas LRP1 levels were lowest in the thalamus (Fig. 2i). Two cortical areas (OF and EC) had higher synaptophysin (a presynaptic marker) levels compared to amygdala (Fig. 2k). Neocortical areas generally had higher PSD95 (a postsynaptic marker) levels compared to subcortical areas. Frontal-to-temporal cortices (DF, OF, and IT) tended to have higher PSD95 levels compared to parietal-to-posterior cortical and limbic areas. Among subcortical areas, we observed higher PSD95 levels in the striatum than in the thalamus and cerebellum (Fig. 2l). Subcortical areas generally had higher lactate and GFAP (an astrocytic marker) levels compared to cortical areas. GFAP levels were also higher in some of limbic areas (AM and EC) compared to neocortical areas (Fig. 2m, n).
Fig. 2.
Regional distribution of molecules related with Aβ metabolism. After normalization within each individual, levels of apoE in TBS fraction (a) and in TBS-TX fraction (b), APP (c), APP-CTFβ (d), BACE1 (e), presenilin-1 (f), NEP (g), IDE (h), LRP1 (i), LDLR (j), synaptophysin (k), PSD95 (l), lactate (m), and GFAP (n) are plotted by different brain areas with the average line of each area. The p < 0.05 was considered significant; paired t test with Holm correction for multiple comparisons. PS1 presenilin-1, SYP synaptophysin
Regional associations among Aβ and molecules involved in Aβ metabolism
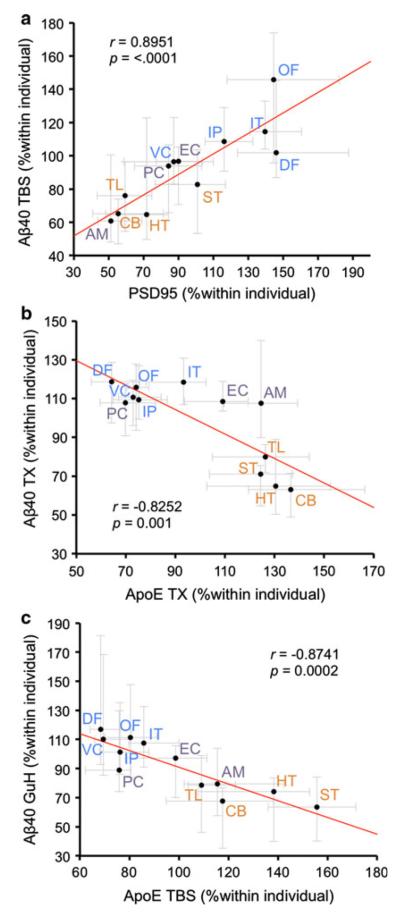
We then assessed the association between the regional distributions of Aβ and these related molecules. To reduce the influence of some particularly extreme outliers, a median value of 21 individuals was used to assign a representative value in each area. Since we did not have a priori assumptions of linear relationship between them, these regional distributions were compared by Spearman rank test. The results of this correlation analysis are summarized in Table 3. There were several significant regional associations between Aβ and apoE, IDE, LRP1, GFAP, lactate, synaptophysin, or PSD95. In particular, the regional distribution of Aβ40 in TBS fraction was most strongly associated with PSD95 levels (Fig. 3a; r = 0.8951, p < 0.0001). On the other hand, the regional distribution of Aβ40 in GuHCl fraction was most strongly associated with apoE levels in TBS fraction (Fig. 3c; r = −0.8741, p = 0.0002). Similarly, the regional distribution of Aβ40 in TBS-TX fraction was strongly associated with apoE levels (Fig. 3b). The regional distribution of Aβ42 in GuHCl fraction was also strongly associated with apoE and PSD95 (see Table 3). In addition, these strong regional correlations between Aβ and PSD95 or apoE persisted even when separating individuals by the degree of biochemically-determined Aβ accumulation (Supplementary Table 3 and 4), histochemically-determined Aβ accumulation (Supplementary Table 7 and 8), or the presence/absence of the APOE ε4 allele (Supplementary Table 11 and 12). On the contrary, no significant positive regional correlations were detected between Aβ and APP, APP-CTFβ, BACE1, or presenilin-1, those involved in Aβ production. Moreover, there were no significant negative regional correlations between Aβ and two major Aβ degrading enzymes, NEP and IDE, in total 21 non-demented individuals (see Table 3). These regional correlations remained consistent regardless of the degree of Aβ accumulation or the presence/absence of the APOE ε4 allele (see supplementary Table 3, 4, 7, 8, 11, and 12). Although we examined correlations between absolute levels of Aβ and related molecules within each area, consistent results (i.e., consistent correlations throughout areas that are or are not vulnerable to Aβ accumulation) could not be obtained (data not shown).
Table 3.
Regional associations between Aβ in each fraction and molecules related to Aβ metabolism
| Aβ40 TBS |
Aβ40 TX |
Aβ40 GuHCl |
Aβ42 GuHCl |
|||||
|---|---|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | r | p value | |
| ApoE TBS | −0.6364 | 0.0261 | −0.8182 | 0.0011 | −0.8741 | 0.0002 | −0.6783 | 0.0153 |
| ApoE TX | −0.6853 | 0.0139 | −0.8252 | 0.001 | −0.8112 | 0.0014 | −0.7972 | 0.0019 |
| APP | 0.3427 | 0.2756 | 0.2308 | 0.4705 | 0.1049 | 0.7456 | 0.4056 | 0.1908 |
| APP–CTFβ | 0.2098 | 0.5128 | 0.035 | 0.9141 | −0.035 | 0.9141 | 0.2378 | 0.4568 |
| BACE1 | −0.2517 | 0.4299 | −0.1748 | 0.5868 | −0.2028 | 0.5273 | −0.007 | 0.9828 |
| PS1 | 0.5524 | 0.0625 | 0.2587 | 0.4168 | 0.1608 | 0.6175 | 0.4056 | 0.1908 |
| NEP | 0.0839 | 0.7954 | −0.2098 | 0.5128 | −0.2867 | 0.3663 | −0.1818 | 0.5717 |
| IDE | 0.8182 | 0.0011 | 0.7133 | 0.0092 | 0.7413 | 0.0058 | 0.6364 | 0.0261 |
| LRP1 | 0.7343 | 0.0065 | 0.5245 | 0.08 | 0.4056 | 0.1908 | 0.6643 | 0.0185 |
| LDLR | −0.3357 | 0.2861 | −0.2937 | 0.3541 | −0.1399 | 0.6646 | −0.3077 | 0.3306 |
| GFAP | −0.7483 | 0.0051 | −0.6853 | 0.0139 | −0.6573 | 0.0202 | −0.5245 | 0.08 |
| Lactate | −0.6364 | 0.0261 | −0.6014 | 0.0386 | −0.7133 | 0.0092 | −0.6154 | 0.0332 |
| SYP | 0.5874 | 0.0446 | 0.5175 | 0.0849 | 0.5175 | 0.0849 | 0.4196 | 0.1745 |
| PSD95 | 0.8951 | <.0001 | 0.7902 | 0.0022 | 0.6993 | 0.0114 | 0.8112 | 0.0014 |
Correlation coefficient (r) and p value were acquired by the non-parametric Spearman rank test comparing median value of normalized levels of each protein from 21 individuals across 12 brain regions. Significant correlations are shown as bold text
PS1 presenilin-1, SYP synaptophysin
Fig. 3.
Strong regional associations between Aβ and PSD95 or apoE. a PSD95 levels in each brain area are plotted against Aβ40 levels in TBS fraction in each brain area. b ApoE levels in TBS-TX fraction in each brain area are plotted against Aβ40 levels in TBS-TX fraction in each brain area. c ApoE levels in TBS fraction in each brain area are plotted against Aβ40 levels in GuHCl fraction in each brain area. Values are median with 25 and 75 percentiles. Correlation coefficient (r) and p value were acquired by Spearman rank correlation test. GuH GuHCl
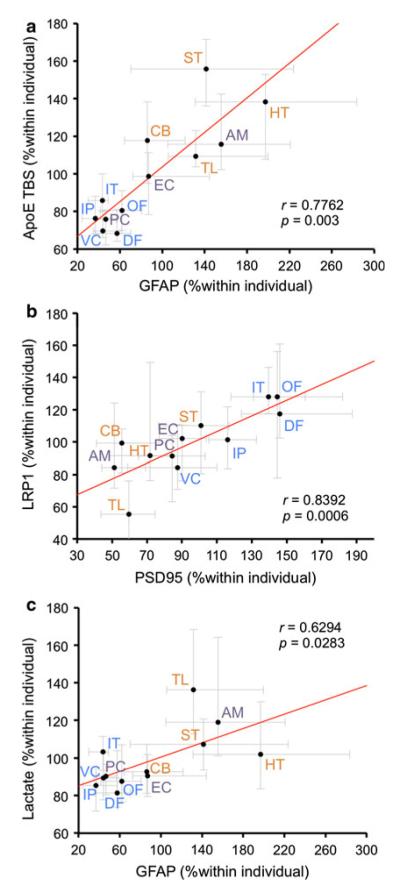
To investigate potential relationships among molecules related to Aβ metabolism, we next assessed whether correlations in regional distribution exist. The results of this correlation analysis are summarized in Table 4. Although no correlations were observed between the regional distribution of apoE and apoE receptors (LRP1 and LDLR), significant positive correlations were observed between the regional distribution of apoE and GFAP (Fig. 4a). This supports the notion that astrocytes are the primary cell type in the brain that produces apoE and dictate the regional distribution of apoE [10]. In addition, there was a strong positive correlation between LRP1 and PSD95 regional distribution (Fig. 4b). These results are consistent with the previous findings that LRP1 interacts with PSD95, and is mostly localized and functions in postsynaptic sites [41]. A negative correlation was observed between lactate and PSD95, whereas a positive correlation was observed between lactate and GFAP (Fig. 4c). Such data may support the notion that regional distribution of lactate is characterized by astrocytes, a main source of lactate [51].
Table 4.
Regional associations among molecules related to Aβ metabolism
| ApoE TX | APP | APP-CTFβ | BACE1 | PS1 | NEP | IDE | LRP1 | LDLR | GFAP | Lactate | SYP | PSD95 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ApoE TBS | 0.9021*** | 0.1888 | 0.3636 | 0.4406 | −0.0839 | 0.1189 | −0.6993* | −0.1329 | 0.1748 | 0.7762** | 0.7622** | −0.4476 | −0.5245 |
| ApoE TX | −0.0909 | 0.1469 | 0.3706 | −0.3077 | 0.021 | −0.6154* | −0.3217 | 0.4056 | 0.6783* | 0.7343** | −0.3706 | −0.6853* | |
| APP | 0.8741*** | 0.3636 | 0.3636 | 0.1818 | 0.042 | 0.5944* | 0 | 0.2378 | 0.1329 | 0.1888 | 0.5455 | ||
| APP-CTFβ | 0.5664 | 0.0559 | −0.0909 | −0.1469 | 0.4615 | 0.0839 | 0.4056 | 0.1538 | 0.1678 | 0.3916 | |||
| BACE1 | −0.4336 | −0.5035 | −0.6434* | −0.1888 | 0.0699 | 0.6014* | 0.5594 | 0.007 | −0.2797 | ||||
| PS1 | 0.5455 | 0.5315 | 0.7203** | −0.3916 | −0.4196 | −0.3007 | −0.042 | 0.5455 | |||||
| NEP | 0.2448 | 0.1818 | −0.007 | −0.0909 | 0.0769 | 0.1748 | 0.1189 | ||||||
| IDE | 0.6154* | 0.014 | −0.7762** | −0.7832** | 0.4266 | 0.7413 | |||||||
| LRP1 | −0.2448 | −0.3357 | −0.3916 | 0.1818 | 0.8392*** | ||||||||
| LDLR | 0.3497 | 0.0699 | −0.1259 | −0.3007 | |||||||||
| GFAP | 0.6294* | −0.2937 | −0.5455 | ||||||||||
| Lactate | −0.2587 | −0.6294** | |||||||||||
| SYP | 0.4685 |
Values are Correlation coefficient (r). Significant correlations are shown as bold text
p < 0.05,
p < 0.01,
p < 0.001; non-parametric Spearman rank test comparing median value of normalized levels of each protein from 21 individuals across 12 brain regions
Fig. 4.
Strong regional associations among molecules related to Aβ metabolism. a GFAP levels in each brain area are plotted against apoE levels in TBS fraction in each brain area. b PSD95 levels in each brain area are plotted against LRP1 levels in each brain area. c GFAP levels in each brain area are plotted against lactate levels in each brain area. Values are median with 25 and 75 percentiles. Correlation coefficient (r) and p value were acquired by Spearman rank correlation test
Discussion
To study the underlying mechanisms responsible for the regional vulnerability of Aβ accumulation, we evaluated the regional relationship between Aβ and several molecules related to Aβ metabolism in 21 non-demented individuals across 12 brain areas. We used ELISAs to determine the levels of these molecules, as it is difficult to measure them for comparison across multiple brain areas in the large data set by Western blotting, as well as detect Aβ (especially in individuals with low Aβ accumulation) and some of the molecules related to Aβ metabolism by immunohistochemical staining. Taking advantage of a comprehensive set of ELISAs, the regional distribution of Aβ and molecules related to Aβ metabolism were systematically compared across multiple areas in an unbiased manner. The regional distribution of Aβ40 in TBS fraction and in TBS-TX/GuHCl was most strongly associated with levels of PSD95 and apoE, respectively, even when considering interconnecting correlations (Fig. 5). The regional distribution of Aβ42 in GuHCl was also strongly associated with PSD95 and apoE. It is interesting to note that the regional associations of Aβ with PSD95 were positive and those of Aβ with apoE were negative. Moreover, these strong correlations persisted throughout individuals with low Aβ accumulation (a presumably normal phase before Aβ accumulation) and with pathological aging (a possible prodromal phase of AD [17, 46]) (see Supplementary Table 1–8). These results suggest that PSD95 and apoE are independently related to the mechanisms that govern region-specific Aβ accumulation prior to the development of AD. In light of accruing evidence, we propose that regional vulnerability to Aβ accumulation depends on a net balance between two competing processes: (1) synapses are involved in promoting the initial event of Aβ accumulation and (2) astrocyte-derived apoE is involved in preventing Aβ accumulation.
Fig. 5.

Correlation map based on the regional associations between Aβ and molecules related with Aβ metabolism. a Correlation map are depicted based on the regional associations among Aβ40 in TBS fraction, apoE in TBS fraction, GFAP, PSD95, and LRP1. b Correlation map are depicted based on the regional associations among Aβ40 in TBS-TX and GuHCl fraction, apoE in TBS fraction, GFAP,PSD95, and LRP1. Values indicate correlation coefficient (r). Red line indicates a positive association. Blue line indicates a negative association. Solid line indicates statistical significant associations observed between two molecules. Dashed line indicates no statistical significant associations between two molecules. Bold line indicates the strongest correlation between Aβ40 and PSD95, and between Aβ40 and apoE
When interpreting the results of our study, there are some caveats to consider. First, this study does not directly address a causal relationship between Aβ and related molecules. Therefore, it remains possible that strong regional associations observed in this study might be mediated by other confounding factors, though such strong associations would provide important implications in either case. Second, our rationale to use samples from non-demented elderly individuals in this study is based on: (1) longitudinal amyloid imaging studies indicate that amyloid accumulation in non-demented elderly individuals is a good predictor of AD development in the future [28, 49,69], (2) neuropathological and biochemical studies showed extensive similarities between accumulated Aβ in AD and pathological aging [17, 46], and (3) severe neurodegeneration and neuroinflammation observed in AD [45, 71] could make it difficult to study causal relationships between Aβ and molecules involved in Aβ metabolism. A third consideration is that protein levels determined by ELISAs do not always reflect activity levels as well as functional properties, as several proteins measured in this study, such as BACE1, presenilin-1, NEP, or IDE, undergo post-transcriptional modifications or possibly reside in cellular compartments different than those in the Aβ-related pathway [15, 48, 65]. Further experiments considering protein activity and function would complement key findings in this study.
We were careful to consider, and overcome, potential technical limitations associated with this study. First, we analyzed a particular population of non-demented individuals, though these samples were randomly selected based on the antemortem record. These individuals were mostly very elderly and female (see Table 1). Of note, after stratification by each one of the following variables: age, sex, PMI, and Braak stage, the resulting regional correlations were consistent with our previous findings (data not shown) suggesting that such potential confounding factors did not affect the regional associations observed in this study. Second, normalization of measured values by the average value within each individual might obscure the relationship among each molecule as well as differences among individuals in different stages of Aβ accumulation. overcome this, we also assessed the regional distribution by separating individuals according to Aβ levels, and observed a similar trend in regional associations regardless of the degree of Aβ accumulation (see Supplementary Table 1–8). Third, in our correlation analysis, the small number of brain regions studied (12 brain areas) might not be enough to detect regional associations. To improve the robustness of the analysis, we used the median value of 21 individuals and non-parametric tests for comparison. Indeed, several reasonable regional associations were obtained, which support this methodology (see Fig. 4). As such, strong regional associations observed in this study would have important implications for the regional specificity of Aβ levels.
Overall, Aβ levels tended to be higher in cortical areas when compared with subcortical areas, though there were no significant differences in Aβ levels among neocortical areas or between neocortical and limbic areas by paired t test with Holm correction. This result may partially contradict observations made with histological methods and with clinical amyloid imaging methods, which have reported regional heterogeneity of Aβ accumulation in cortical areas of aged individuals [8, 11, 64, 70]. However, our results actually imply a difference in Aβ levels among cortical areas, especially soluble Aβ (in TBS fraction). These statistically significant differences were achieved by a mixed model analysis shrinking extreme outliers (see Supplementary Fig. 1). Indeed, these outliers might be compatible with “considerable inter-individual differences” described by Braak et al. [8], which would make it difficult to define the order of Aβ accumulation. For example, eight individuals have an extremely outlying (defined as higher than the 75th percentile + 3 interquartile range for the particular area) Aβ42 level (as normalized value) in GuHCl fraction at variable areas, as some of them are apparent in Fig. 1d. The number of such extreme Aβ distribution outliers was generally greater than that of other proteins (data not shown). Moreover, the average of cortical Aβ40 or Aβ42 levels in these 8 individuals was lower than that of the other 13 individuals (data not shown). These results may suggest that individual differences in region-specific Aβ accumulation exist especially during the initial phase of Aβ accumulation. Although median values were chosen to examine regional correlations with other molecules to reduce the influence of such extreme outliers for the entire analysis, it may be worthwhile to further explore mechanisms underlying the heterogenic nature of region-specific Aβ accumulation.
Several previous attempts to implicate the involvement of the Aβ production factors in Aβ regional specificity have not been successful. Such studies examined the regional distribution of APP, presenilin-1, presenilin-2, and BACE1 in human or rodent brains by histochemical or biochemical techniques [5, 20, 50, 62, 66]. As the level of APP-CTFβ may better reflect the rate of Aβ production more than the level of APP, we determined the regional distribution of APP-CTFβ in addition to APP, BACE1, and presenilin-1. Our results support the conclusion of previous studies. However, if regional alteration of γ-secretase activity (probably reflected by presenilin-1 level) affects APP-CTFβ levels, it is difficult to exclude the involvement of Aβ production in the regional specificity of Aβ levels by our method of analysis. Further investigations are needed to test this possibility. It is noteworthy that there was a strong positive regional correlation between APP-CTFβ and APP (see Table 4). This suggests that the regional distribution of APP-CTFβ is actually determined by the regional distribution of APP. Also, our results indicate that Aβ production rate in striatum is comparable to or slightly higher than that of cortical areas. Such results may have implications for understanding the striatal vulnerability of Aβ deposition in familial AD [33, 68], and further investigation of this topic would require an examination of samples from familial AD cases.
IDE and NEP are major Aβ degrading enzymes, the effects of which have been well characterized in vitro and in vivo by several groups. Importantly, manipulation of levels of these enzymes negatively correlated with Aβ levels [18, 25, 39, 42, 43]. In our study, the regional distribution of these two major Aβ degrading enzymes did not show significant negative correlations with Aβ levels. On the contrary, those of IDE were positively correlated with Aβ levels. This appeared to be mediated by regional associations between neuronal markers and IDE, which coincide with the notion that IDE mainly resides in neurons [6]. High levels of NEP in striatum are consistent with previous immunohistochemical studies [1]. Therefore, NEP might have region-specific effects for Aβ degradation, especially in striatum. However, deletion of NEP raised Aβ levels in cortical and striatum at similar rates in rodent brains [25], which refutes such region-specific regulation of Aβ levels by NEP.
Our results imply that apoE affects the region-specific Aβ levels by preventing Aβ accumulation. Although apoE is intimately involved in Aβ metabolism, its effects are still not clear, especially in vivo or in humans. Previous studies using transgenic mouse models have shown that apoE promotes Aβ accumulation [24, 32], while other studies suggest apoE prevents Aβ accumulation in brain [29, 37,56]. Fundamental differences in lipoprotein metabolism between humans and rodents [12, 21] might generate controversies in apoE effects on Aβ metabolism. Of note, several studies showed that levels of apoE are increased by a protective APOE allele [22, 23, 44, 54, 60]. Our results may support such dose-dependent, beneficial effects of apoE on Aβ accumulation. While it cannot be ruled out that confounding factors mediate strong correlations between apoE and Aβ, the opposite notion that Aβ accumulation affects apoE distribution is at least unlikely because the strong associations are observed even in individuals with low Aβ levels (see Supplemental Table 3). Because the regional distribution of GFAP was also strongly associated with Aβ as well as apoE (though sometimes apparently weaker compared to the association between Aβ and apoE (e.g., correlation with Aβ42 in GuHCl, see Table 3)), astrocytes may be involved in apoE-mediated or non-apoE-mediated Aβ disaggregation [37]. Therefore, another marker protein that can distinguish astrocyte subtypes [9,63] might show stronger and consistent association with Aβ. In addition, it may be worth comparing regional vulnerability to Aβ accumulation among carriers of different APOE allele to elucidate how the risk allele or protective allele is involved in AD pathogenesis. In this study, we failed to detect a difference in the regional distribution of Aβ or regional relationship between Aβ and molecules related to Aβ metabolism in the presence/absence of the APOE ε4 allele (see supplementary Table 9–12). However, this result might be affected by the relatively small number of samples examined as well as our analysis of non-demented individuals. Further studies analyzing a larger number of samples, including AD patients, would be helpful to test this hypothesis.
Synapses are an important site for Aβ production and accumulation [34, 36, 38, 40, 57, 61]. The strong regional association between PSD95 and Aβ levels, especially in TBS fraction, even in individuals with low Aβ accumulation, may suggest the involvement of neuronal or synaptic activity in the regional specificity of Aβ levels [7, 14]. However, it cannot be ruled out that Aβ is preferably accumulating in postsynaptic structures, as previously observed, in which LRP1 might play an important role [19, 34, 36, 41, 61]. There might also exist a cross-talk between apoE-mediated Aβ clearance and synaptic accumulation of Aβ, as previously reported by Arold et al. and other groups [2, 35]. Recent studies showed that glutamatergic neurons are involved in Aβ accumulation or Aβ secretion [58, 59, 67]. Along these lines, specific presynaptic markers of this neuronal type, such as vGluT1 or vGluT2, might be useful to further define the mechanisms underlying strong associations between Aβ and PSD95 [58]. Therefore, even if neural activity is involved, a deeper understanding of the underlying mechanism is warranted since our study, as well as previous studies [7, 14], failed to show the contribution of the Aβ production pathway. In addition, we observed regional differences in the distribution of lactate in TBS fraction that correlated with GFAP, reflecting the distribution of astrocytes. Other makers are needed to further prove the involvement of neural activity in regional specificity of Aβ accumulation in post-mortem tissue [7, 51].
One potential way of interpreting results in this study is to consider the effect of Aβ accumulation. It is well-known that Aβ accumulation causes cellular toxicity and eventual cellular degeneration; however, these effects require a long period to become apparent in humans (namely, apparent in AD patients) [26]. Therefore, it seems unreasonable to conclude that Aβ accumulation is irrelevant to the fall of synaptic proteins and/or to the increase in glial cells from results of this study using non-demented individuals. Analysis of AD samples would be necessary to test such a possibility.
In summary, we show that two independent (i.e., synapse-mediated and apoE-mediated) processes might contribute to region-specific Aβ accumulation in non-demented individuals. Further development of clinical imaging techniques that can detect individual molecular distributions or a post-mortem approach that can link antemortem state will validate the underlying mechanisms of the regional vulnerability to Aβ accumulation.
Supplementary Material
Acknowledgments
We thank Dr. Pritam Das for ELISA reagents detecting Aβ and an antibody against C-terminus region of APP, Drs. Malcolm Leissring and Samir Abdul-Hay for ELISA reagents detecting IDE, Mr. John Gonzalez for assisting with dissection of brain tissues, Ms. Caroline Stetler for careful reading of this manuscript, and Dr. Takahisa Kanekiyo for helpful discussion. This research was supported by grants from the National Institutes of Health (NIH) (P01 AG030128-Project 3 & P01 NS074969-Project 3 to G.B.); Alzheimer’s Drug Discovery Foundation (ADDF) (to G.B.); American Health Assistance Foundation (AHAF) (to G.B.); Mayo Clinic Alzheimer’s Disease Research Center (ADRC) (P50 AG016574) (to D.W.D and M.S.); Japan Heart Foundation and Naito Foundation (to M.S.). The authors also acknowledge the many individuals who contribute to the Mayo Clinic Alzheimer Disease Research Center (PI: R.C.P., P50 AG016574) and Mayo Clinic Study on Aging (PI: R.C.P., U01 AG006786), as well as the neuropathology core in Rochester, MN (Dr. Joseph Parisi), without whose contributions this study would not have been possible.
Footnotes
Conflict of interest R.C.P. has been chair of a safety monitoring committee for Pfizer (Wyeth) and Janssen Alzheimer Immunotherapy (Elan); and a consultant for Elan Pharmaceuticals and GE Healthcare. All other authors declare that they have no conflicts of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s00401-013-1086-9) contains supplementary material, which is available to authorized users.
Contributor Information
Mitsuru Shinohara, Department Neuroscience, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224, USA.
Ronald C. Petersen, Department of Neurology, Mayo Clinic, Rochester, MN, USA
Dennis W. Dickson, Department Neuroscience, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224, USA
Guojun Bu, Department Neuroscience, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224, USA.
References
- 1.Akiyama H, Kondo H, Ikeda K, Kato M, McGeer PL. Immunohistochemical localization of neprilysin in the human cerebral cortex: inverse association with vulnerability to amyloid beta-protein (Abeta) deposition. Brain Res. 2001;902:277–281. doi: 10.1016/s0006-8993(01)02390-3. [DOI] [PubMed] [Google Scholar]
- 2.Arold S, Sullivan P, Bilousova T, et al. Apolipoprotein E level and cholesterol are associated with reduced synaptic amyloid beta in Alzheimer’s disease and apoE TR mouse cortex. Acta Neuropathol. 2012;123:39–52. doi: 10.1007/s00401-011-0892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bales KR, Liu F, Wu S, et al. Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci. 2009;29:6771–6779. doi: 10.1523/JNEUROSCI.0887-09.2009. doi:10.1523/jneurosci.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. doi:10.1016/s0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 5.Benkovic SA, McGowan EM, Rothwell NJ, Hutton M, Morgan DG, Gordon MN. Regional and cellular localization of presenilin-2 RNA in rat and human brain. Exp Neurol. 1997;145:555–564. doi: 10.1006/exnr.1997.6487. doi:10.1006/exnr.1997.6487. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein HG, Ansorge S, Riederer P, Reiser M, Frolich L, Bogerts B. Insulin-degrading enzyme in the Alzheimer’s disease brain: prominent localization in neurons and senile plaques. Neurosci Lett. 1999;263:161–164. doi: 10.1016/s0304-3940(99)00135-4. [DOI] [PubMed] [Google Scholar]
- 7.Bero AW, Yan P, Roh JH, et al. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. doi:10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 9.Bramanti V, Tomassoni D, Avitabile M, Amenta F, Avola R. Biomarkers of glial cell proliferation and differentiation in culture. Front Biosci. 2010;2:558–570. doi: 10.2741/s85. [DOI] [PubMed] [Google Scholar]
- 10.Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. doi:10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. doi:10.1523/jneurosci.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camus MC, Chapman MJ, Forgez P, Laplaud PM. Distribution and characterization of the serum lipoproteins and apoproteins in the mouse, Mus musculus. J Lipid Res. 1983;24:1210–1228. [PubMed] [Google Scholar]
- 13.Chakrabarty P, Jansen-West K, Beccard A, et al. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010;24:548–559. doi: 10.1096/fj.09-141754. doi:10.1096/fj.09141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cirrito JR, Yamada KA, Finn MB, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. doi:10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 15.De Strooper B, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol. 2010;6:99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delledonne A, Kouri N, Reinstatler L, et al. Development of monoclonal antibodies and quantitative ELISAs targeting insulin-degrading enzyme. Mol Neurodegener. 2009;4:39. doi: 10.1186/1750-1326-4-39. doi:10.1186/1750-1326-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickson DW, Crystal HA, Mattiace LA, et al. Identification of normal and pathological aging in prospectively studied non-demented elderly humans. Neurobiol Aging. 1992;13:179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- 18.Eckman EA, Adams SK, Troendle FJ, et al. Regulation of steady-state beta-amyloid levels in the brain by neprilysin and endothelin-converting enzyme but not angiotensin-converting enzyme. J Biol Chem. 2006;281:30471–30478. doi: 10.1074/jbc.M605827200. doi:10.1074/jbc.M605827200. [DOI] [PubMed] [Google Scholar]
- 19.Fuentealba RA, Liu Q, Zhang J, et al. Low-density lipoprotein receptor-related protein 1 (LRP1) mediates neuronal Abeta42 uptake and lysosomal trafficking. PLoS One. 2010;5:e11884. doi: 10.1371/journal.pone.0011884. doi:10.1371/journal.pone.0011884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goedert M. Neuronal localization of amyloid beta protein precursor mRNA in normal human brain and in Alzheimer’s disease. EMBO J. 1987;6:3627–3632. doi: 10.1002/j.1460-2075.1987.tb02694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goulinet S, Chapman MJ. Plasma lipoproteins in the golden Syrian hamster (Mesocricetus auratus): heterogeneity of apoB- and apoA-I-containing particles. J Lipid Res. 1993;34:943–959. [PubMed] [Google Scholar]
- 22.Gupta VB, Laws SM, Villemagne VL, et al. Plasma apolipoprotein E and Alzheimer disease risk: the AIBL study of aging. Neurology. 2011;76:1091–1098. doi: 10.1212/WNL.0b013e318211c352. [DOI] [PubMed] [Google Scholar]
- 23.Haddy N, De Bacquer D, Chemaly MM, et al. The importance of plasma apolipoprotein E concentration in addition to its common polymorphism on inter-individual variation in lipid levels: results from Apo Europe. Eur J Hum Genet. 2002;10:841–850. doi: 10.1038/sj.ejhg.5200864. [DOI] [PubMed] [Google Scholar]
- 24.Holtzman DM, Fagan AM, Mackey B, et al. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann Neurol. 2000;47:739–747. [PubMed] [Google Scholar]
- 25.Iwata N, Tsubuki S, Takaki Y, et al. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. doi:10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- 26.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. doi:10.1016/s1474-4422(09) 70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. doi:10.1093/brain/ awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack CR, Jr, Wiste HJ, Vemuri P, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010;133:3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Q, Lee CY, Mandrekar S, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanekiyo T, Liu CC, Shinohara M, Li J, Bu G. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-beta. J Neurosci. 2012;32:16458–16465. doi: 10.1523/JNEUROSCI.3987-12.2012. doi: 10.1523/jneurosci.3987-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanekiyo T, Zhang J, Liu Q, Liu CC, Zhang L, Bu G. Heparan sulphate proteoglycan and the low-density lipoprotein receptor-related protein 1 constitute major pathways for neuronal amyloid-beta uptake. J Neurosci. 2011;31:1644–1651. doi: 10.1523/JNEUROSCI.5491-10.2011. doi:10.1523/jneurosci.5491-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J, Jiang H, Park S, et al. Haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of amyloid-beta amyloidosis. J Neurosci. 2011;31:18007–18012. doi: 10.1523/JNEUROSCI.3773-11.2011. doi:10.1523/jneurosci.3773-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klunk WE, Price JC, Mathis CA, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–6184. doi: 10.1523/JNEUROSCI.0730-07.2007. doi:10.1523/ jneurosci.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koffie R, Hyman B, Spires-Jones T. Alzheimer’s disease: synapses gone cold. Molecular Neurodegeneration. 2011;6:63. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koffie RM, Hashimoto T, Tai HC, et al. Apolipoprotein E4 effects in Alzheimer’s disease are mediated by synaptotoxic oligomeric amyloid-beta. Brain. 2012;135:2155–2168. doi: 10.1093/brain/aws127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koffie RM, Meyer-Luehmann M, Hashimoto T, et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci USA. 2009;106:4012–4017. doi: 10.1073/pnas.0811698106. doi:10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koistinaho M, Lin S, Wu X, et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. doi:10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- 38.Lazarov O, Lee M, Peterson DA, Sisodia SS. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J Neurosci. 2002;22:9785–9793. doi: 10.1523/JNEUROSCI.22-22-09785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leissring MA, Farris W, Chang AY, et al. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- 40.Marquez-Sterling NR, Lo AC, Sisodia SS, Koo EH. Trafficking of cell-surface beta-amyloid precursor protein: evidence that a sorting intermediate participates in synaptic vesicle recycling. J Neurosci. 1997;17:140–151. doi: 10.1523/JNEUROSCI.17-01-00140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.May P, Rohlmann A, Bock HH, et al. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol Cell Biol. 2004;24:8872–8883. doi: 10.1128/MCB.24.20.8872-8883.2004. doi:10.1128/mcb.24.20.8872-8883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meilandt WJ, Cisse M, Ho K, et al. Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. J Neurosci. 2009;29:1977–1986. doi: 10.1523/JNEUROSCI.2984-08.2009. doi:10.1523/jneurosci.2984-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller BC, Eckman EA, Sambamurti K, et al. Amyloid-beta peptide levels in brain are inversely correlated with insulysin activity levels in vivo. Proc Natl Acad Sci USA. 2003;100:6221–6226. doi: 10.1073/pnas.1031520100. doi:10.1073/pnas.1031520100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mooijaart SP, Berbee JF, van Heemst D, et al. ApoE plasma levels and risk of cardiovascular mortality in old age. PLoS Med. 2006;3:9. doi: 10.1371/journal.pmed.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore AH, O’Banion MK. Neuroinflammation and anti-inflammatory therapy for Alzheimer’s disease. Adv Drug Deliv Rev. 2002;54:1627–1656. doi: 10.1016/s0169-409x(02)00162-x. [DOI] [PubMed] [Google Scholar]
- 46.Moore BD, Chakrabarty P, Levites Y, et al. Overlapping profiles of Abeta peptides in the Alzheimer’s disease and pathological aging brains. Alzheimers Res Ther. 2012;4(3):18. doi: 10.1186/alzrt121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. doi:10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nalivaeva NN, Beckett C, Belyaev ND, Turner AJ. Are amyloid-degrading enzymes viable therapeutic targets in Alzheimer’s disease? J Neurochem. 2012;1:167–185. doi: 10.1111/j.1471-4159.2011.07510.x. [DOI] [PubMed] [Google Scholar]
- 49.Okello A, Koivunen J, Edison P, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73:754–760. doi: 10.1212/WNL.0b013e3181b23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Page K, Hollister R, Tanzi RE, Hyman BT. In situ hybridization analysis of presenilin 1 mRNA in Alzheimer disease and in lesioned rat brain. Proc Natl Acad Sci USA. 1996;93:14020–14024. doi: 10.1073/pnas.93.24.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pocivavsek A, Burns MP, Rebeck GW. Low-density lipoprotein receptors regulate microglial inflammation through c-Jun N-terminal kinase. Glia. 2009;57:444–453. doi: 10.1002/glia.20772. doi:10.1002/glia.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. doi:10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riddell DR, Zhou H, Atchison K, et al. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci. 2008;28:11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shankar GM, Li S, Mehta TH, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. doi:10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shibata M, Yamada S, Kumar SR, et al. Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. doi:10.1172/jci10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shigematsu K, McGeer PL, McGeer EG. Localization of amyloid precursor protein in selective postsynaptic densities of rat cortical neurons. Brain Res. 1992;592:353–357. doi: 10.1016/0006-8993(92)91697-d. [DOI] [PubMed] [Google Scholar]
- 58.Sokolow S, Luu SH, Nandy K, et al. Preferential accumulation of amyloid-beta in presynaptic glutamatergic terminals (VGluT1 and VGluT2) in Alzheimer’s disease cortex. Neurobiol Dis. 2012;45:381–387. doi: 10.1016/j.nbd.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suh J, Lyckman A, Wang L, Eckman EA, Guenette SY. FE65 proteins regulate NMDA receptor activation-induced amyloid precursor protein processing. J Neurochem. 2011;119:377–388. doi: 10.1111/j.1471-4159.2011.07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sullivan PM, Han B, Liu F, et al. Reduced levels of human apoE4 protein in an animal model of cognitive impairment. Neurobiol Aging. 2011;32:791–801. doi: 10.1016/j.neurobiolaging.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi RH, Milner TA, Li F, et al. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takami K, Terai K, Matsuo A, Walker DG, McGeer PL. Expression of presenilin-1 and -2 mRNAs in rat and Alzheimer’s disease brains. Brain Res. 1997;748:122–130. doi: 10.1016/s0006-8993(96)01274-7. [DOI] [PubMed] [Google Scholar]
- 63.Thal DR. The role of astrocytes in amyloid beta-protein toxicity and clearance. Exp Neurol. 2012;236:1–5. doi: 10.1016/j.expneurol.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 64.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 65.Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. doi:10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vassar R, Bennett BD, Babu-Khan S, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 67.Verges DK, Restivo JL, Goebel WD, Holtzman DM, Cirrito JR. Opposing synaptic regulation of amyloid-beta metabolism by NMDA receptors in vivo. J Neurosci. 2011;31:11328–11337. doi: 10.1523/JNEUROSCI.0607-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Villemagne VL, Ataka S, Mizuno T, et al. High striatal amyloid beta-peptide deposition across different autosomal Alzheimer disease mutation types. Arch Neurol. 2009;66:1537–1544. doi: 10.1001/archneurol.2009.285. doi: 10.1001/archneurol.2009.285. [DOI] [PubMed] [Google Scholar]
- 69.Villemagne VL, Pike KE, Chetelat G, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vlassenko AG, Vaishnavi SN, Couture L, et al. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proc Natl Acad Sci USA. 2010;107:17763–17767. doi: 10.1073/pnas.1010461107. doi:10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wenk GL. Neuropathologic changes in Alzheimer’s disease. J Clin Psychiatry. 2003;9:7–10. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.