Abstract
Background & Aims
p53 and its transcriptional target miRNA34a have been implicated in the pathogenesis of fatty liver. We tested the efficacy of a p53 inhibitor, pifithrin-α p-nitro (PFT) in attenuating steatosis, associated oxidative stress and apoptosis in a murine model of non-alcoholic fatty liver disease (NAFLD).
Methods
C75Bl/6J mice were fed a high-fat (HFD) or control diet for 8 weeks, PFT or DMSO (vehicle) were administered three times per week. Markers of oxidative stress and apoptosis as well as mediators of hepatic fatty acid metabolism were assessed by immunohistochemistry, Western-blot, real-time PCR and biochemical assays.
Results
PFT administration suppressed HFD-induced weight gain, ALT elevation, steatosis, oxidative stress and apoptosis. PFT treatment blunted the HFD-induced upregulation of miRNA34a and increased SIRT1 expression. In the livers of HFD-fed, PFT-treated mice activation of the SIRT1/PGC1α/PPARα axis increased the expression of malonyl-CoA decarboxylase (MLYCD), an enzyme responsible for malonyl-CoA (mCoA) degradation. Additionally, the SIRT1/LKB1/AMPK pathway (upstream activator of MLYCD) was promoted by PFT. Thus, induction of these two pathways by PFT diminished the hepatic mCoA content by enhancing MLYCD expression and function. Since mCoA inhibits carnitine palmitoyltransferase 1 (CPT1), the decrease of hepatic mCoA in the PFT-treated, HFD-fed mice increased CPT1 activity, favored fatty acid oxidation and decreased steatosis. Additionally, we also demonstrated that PFT abrogated steatosis and promoted MLYCD expression in palmitoleic acid- treated human HepaRG cells.
Conclusions
The p53 inhibitor PFT diminished hepatic triglyceride accumulation and lipotoxicity in mice fed a HFD by depleting mCoA and favoring the β-oxidation of fatty acids.
Keywords: SIRT1, miRNA34a, malonyl-CoA
Introduction
There is accumulating evidence that p53 plays a central role in the pathogenesis of alcoholic [1–4] and non-alcoholic fatty liver disease (NAFLD) [5–7]. For example, chronic ethanol feeding in rats has been shown to increase the hepatic mRNA abundance, acetylation and transcriptional activity of p53 [1, 3, 4, 8]. The importance of p53 and its pro-apoptotic and pro-oxidant downstream target p66shc in alcoholic liver disease (ALD) is highlighted by the observations that genetic ablation of p53 or p66shc significantly reduced ethanol-induced hepatocellular injury and oxidative stress [2, 9]. The pivotal role of p53 in the pathogenesis of ALD has been further elucidated by a recent study, where rat strain- specific susceptibility to ethanol-induced liver injury was associated with differences in hepatic p53 activation and corresponding downstream changes in signaling cascades regulating apoptosis, oxidative stress, insulin signaling and fatty acid metabolism [4].
Similar to ALD, p53 was found to be upregulated in the livers of various murine models of NAFLD [7]. Hepatocyte apoptosis was linked to p53 activation in mice fed a HFD [5]. In addition, there was a positive correlation between the level of steatosis and p53 expression in human liver samples [6]. Based on these findings, p53 activation may be a broader metabolic event important in the pathogenesis of fatty liver, irrespective of etiology, that facilitates not only apoptosis and oxidative stress, but also generates hepatic abnormalities such as steatosis and insulin resistance, which contribute to injury. Regarding the role of p53 in steatosis, it has recently been observed that the regulatory control of p53 appears to fail in fatty liver disease since activated p53 upregulates the transcription of miRNA34a [10] as a direct downstream target of p53 [11]. In this regard, miRNA34a has been shown to be upregulated in the livers of mice fed a HFD as well as in patients with the metabolic syndrome and non-alcoholic steatohepatitis (NASH) [12, 13]. Its expression level also correlated with the susceptibility to NASH development in various murine strains [13]. It has been demonstrated that silent mating type information regulation 2 homolog 1 (SIRT1) expression in the liver is suppressed by miRNA34a [10]. SIRT1 has a central role in regulating hepatic fatty acid metabolism; it abrogates ectopic fat accumulation by facilitating fatty acid oxidation and curbing de novo fatty acid synthesis. Accordingly, hepatocyte-specific ablation of SIRT1 results in hepatic steatosis and inflammation upon HFD feeding in mice [14].
The mechanisms, by which SIRT1 activation may attenuate steatosis include the deacetylation and inactivation [15] of sterol regulatory element-binding protein 1c (SREBP1c, as a master transcriptional regulator of de novo fatty acid synthesis) as well as promoting the deacetylation and activation [16] of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α). This latter event may increase the PPARα-mediated gene expression (e.g., fatty acid oxidizing enzymes and malonyl-CoA decarboxylase (MLYCD)) [14, 17]. Additionally, the interaction between SIRT1 and PPARα was shown to be necessary for efficient PGC1α activation [14]. Finally, SIRT1 can deacetylate and promote the cytosolic translocation [18] of liver kinase B1 (LKB1) that initiates the LKB1/5′ adenosine monophosphate-activated protein kinase (AMPK)/acetyl-CoA carboxylase (ACC) signaling cascade, ultimately leading to decreased intrahepatic mCoA [19]. The mechanisms of AMPK-mediated malonyl-CoA decrease have been described and include the phosphorylation and subsequent inactivation of ACC [20] (involved in mCoA synthesis) as well as the phosphorylation and activation of MLYCD [21] (responsible for mCoA degradation). Malonyl-CoA is a key physiological regulator of cellular fatty acid oxidation and lipid partitioning by allosterically inhibiting carnitine palmitoyltransferase 1 (CPT1), which regulates the mitochondrial uptake of long-chain fatty-acyl CoA molecules for oxidation. The transcriptional and posttranslational activation of MLYCD via the SIRT1/PGC1α/PPARα and SIRT1/LKB1/AMPK signaling cascades, respectively, in concert with the inactivation of ACC may decrease the hepatic mCoA content. This biochemical event associated with SIRT1 activation favors fatty acid oxidation and decreases the likelihood of excess lipid accumulation.
Given these observations, we hypothesized that dysregulation of p53 in the liver during diet-induced obesity may favor excess accumulation of lipids by activating miRNA34a and altering SIRT1 expression. Steatosis promotes oxidative stress and increases the vulnerability of hepatocytes to acute injury [22]. Additionally, increased oxidative stress may further facilitate p53 stabilization in a feed-forward regulatory mechanism, activating downstream genes that are involved in apoptosis, oxidative stress and insulin resistance [4, 23]. Here we demonstrate that pharmacologic inhibition of p53 with a specific transcriptional inhibitor: pifithrin-α p-nitro [24] attenuates hepatic steatosis and liver injury in mice fed a HFD.
Materials and methods
Additional information regarding biochemical measurements, determination of CPT1 activity, miRNA34a quantification, Western-blot analysis, assessment of protein carbonylation, cell culture experiments and statistical analysis can be found in the Supplementary Material.
Animals and treatment
3-week-old male, C57Bl/6J mice (12 per group, Jackson Laboratory, Bar Harbor, ME) were fed ad libitum with a modified high-fat or control diet (Bioserv, Frenchtown, NJ) for 8 weeks. The calorie profile of the modified HFD resembled the composition of a previously published diet that effectively induced obesity, steatosis and insulin resistance in this mouse strain [25]. The exact formulations of the diets are available upon request. Body weights and food intake were recorded during the study. The animals were injected with the p53 inhibitor, pifithrin-α p-nitro (2.2 mg/kg [26]) or control vehicle, DMSO, dissolved in physiological saline three times per week. This inhibitor of p53 (EMD Chemicals, Inc. Gibbstown, NJ) is a modified version of the parent compound with enhanced in vivo efficacy. During the last week of the study venous blood was collected from the tail vein after 4 hours of fasting and blood glucose concentration was measured by a glucometer (TRUEresult™, Fort Lauderdale, FL). After completing the feeding regimen, the animals were fasted for 4 hours before euthanasia; serum, liver, epididymal fat, soleus muscle and colon samples were collected for further analyses. The Lifespan Animal Welfare Committee of Rhode Island Hospital, Providence, RI approved all animal experiments.
Histological studies and image analysis
Formalin-fixed liver specimens were stained with hematoxylin-eosin, Oil Red O and analyzed, as previously described [4]. Additionally, a quantification method was employed to assess the degree of hepatocellular apoptosis in the liver using the ISOL kit (Millipore, Billerica, MA) according to the manufacturer’s instructions [4].
Results
Diminished weight gain and liver steatosis following inhibition of p53
A single planned comparison of the final week (ninth week) revealed a significant interaction between diet and drug: an 8.75% (95% CI 16.7–0.01%) percent reduction in weight gain was observed in the HFD-fed mice injected with PFT relative to those injected with DMSO only (Supplementary Figure 1A, right panel, p=0.0478). A trend for an inhibitory effect of PFT on HFD-induced weight gain was detected as early as the sixth week of PFT administration that became significant by the ninth week. Body weight measurements at the time of sacrifice (end of the ninth week) also confirmed this finding (Supplementary Figure 1B). Representative images of mice from the various treatment groups are shown in Supplementary Figure 1C, demonstrating diminished subcutaneous fat accumulation in the PFT-treated HFD-fed animals compared to the DMSO-injected mice. Importantly, we did not find significant difference in food intake between the various experimental groups (data not shown). Additionally, PFT treatment did not induce abnormal cellular proliferation in the colon (Supplementary Figure 2A), in agreement with previous studies using this agent [27]. The reason for this finding may be that this form of PFT partially inhibits the transcriptional activity of p53 and leaves its non-transcriptional function (i.e, mitochondrial translocation of p53 in response to genotoxic stress [28]) intact, as we demonstrated it in PFT-treated HCT116 cells that had been challenged by a severe genotoxic stimulus (Supplementary Figure 2B). In contrast to p53 knock-out HCT116 cells, increasing doses of PFT did not enhance the phosphorylation of histone H2AX – an accurate measure of DNA double-strand breaks – in CPT-treated, p53 wild-type HCT116 cells, underlining the fundamental difference between the partial inhibition of the transcriptional function of p53 and the genetic ablation of p53 (Supplementary Figure 2C). The histopathological evaluation of formalin-fixed, hematoxylin-eosin-stained liver slides revealed less micro-and macrovesicular steatosis in the livers of PFT-treated HFD-fed animals compared to the DMSO-injected mice on the same diet (Figure 1A, left side). Oil Red O staining (Figure 1A, right side), intrahepatic triglyceride and cholesterol (Figure 1B and Supplementary Figure 3C) quantification were performed on the flash-frozen liver tissues and further demonstrated that PFT treatment abrogated steatosis in the livers of HFD-fed animals, and revealed that the intrahepatic ectopic lipid accumulation was principally comprised of triglycerides and not cholesterol. The diminished hepatic triglyceride content induced by PFT administration was coupled with reduced serum ALT levels, suggesting blunted hepatocellular injury (Figure 1C). Eight weeks of HFD feeding promoted hyperglycemia and hyperinsulinemia (Supplementary Figure 3D&E) regardless of the presence or absence of PFT treatment. Furthermore, there was no significant difference in the levels of circulating free fatty acids between the various treatment groups (Supplementary Figure 3F). These findings suggested that insulin could still effectively suppress lipolysis in peripheral adipose tissue and increased fatty acid influx was an unlikely source of the ectopic fat accumulation in the livers of HFD-fed mice. To further support this hypothesis, the phosphorylation of the hormone sensitive lipase (HSL) at Ser660 was assessed. The phosphorylation of HSL is inhibited by insulin. The phospho-HSL expression was no different in the two HFD-fed groups in agreement with similar levels of circulating free fatty acids. (Supplementary Figure 3A&B).
Figure 1. PFT diminishes hepatic steatosis, cell injury, oxidative stress and apoptosis.
Hematoxylin-eosin (Fig. 1A, left panel) and Oil Red O staining (Fig. 1A, right panel) revealed markedly diminished micro-and macrovesicular steatosis in the livers of PFT-injected mice on HFD. Representative images are shown (200x magnification). Hepatic triglyceride (Fig. 1B) was measured. Attenuated steatosis was associated with diminished serum ALT elevation in the PFT-injected mice on HFD (Fig. 1C). Oxidative modification of lipids was assessed by measuring the hepatic MDA content (Fig. 1D). Results are expressed as ratio to controls. Apoptosis was assessed by ISOL staining (see Suppl. Fig. 4B). The total number of apoptotic cells was normalized to the area of the tissue slice (Fig. 1E). (*): high-fat vs control (#): pifithrin vs DMSO, p<0.05.
Pifithrin treatment suppresses oxidative stress and apoptosis
Diminished HFD-induced steatosis in the liver by PFT was also associated with attenuated oxidative stress and apoptosis. The oxidative stress-mediated malondialdehyde (MDA) formation was blunted by PFT in the HFD-fed mice (Figure 1D). Similarly, protein carbonylation (i.e., oxidative stress-induced protein modification [29]) was diminished in these mice, as well (Supplementary Figure 4A). In situ oligo labeling of apoptotic cells [30] (Figure 1E and Supplementary Figure 4B) revealed that PFT substantially decreased apoptosis in the livers of HFD-fed mice.
Pifithrin blunts p53-induced miRNA34a expression and increases SIRT1 expression
There is evidence to suggest that p53 may have a direct role in regulating fatty acid metabolism by inducing miRNA34a, which in turn suppresses SIRT1 expression in the fatty liver [10]. SIRT1 is a key suppressor of fatty acid synthesis and activator of fatty acid oxidation [14–16, 19]. Therefore, we hypothesized that PFT exerted its ‘anti-steatosis’ effect by blunting the p53-driven miRNA34a upregulation in the fatty liver to induce downstream activation of SIRT1. PFT treatment decreased the transcriptional activity of p53 in HFD-fed mice, as the HFD-induced upregulation of miRNA34a was markedly blunted in the livers of PFT-injected mice compared to their DMSO-injected counterparts (Figure 2A). Importantly, the expression of SIRT1 was significantly increased in the livers of HFD-fed, PFT-injected mice, which was not observed in the DMSO-injected mice on the same diet (Figure 2B). The two-fold induction of SIRT1 by PFT treatment was also associated with decreased acetylation of p53 (Figure 2C), and was limited to the liver and not observed in the white adipose tissue or skeletal muscle (data not shown). The effect of SIRT1 on fatty acid metabolism is mediated by the deacetylation of key target proteins such as LKB1 [18], PGC1α [16] and SREBP1c [15]. Therefore, we investigated changes in these pathways to demonstrate that PFT treatment improved the dysregulation of the AMPK/mCoA signaling network in the HFD-fed mice, as shown by the diagram depicted in Figure 4C.
Figure 2. PFT blunts HFD-induced miRNA34a upregulation and activates the SIRT1/AMPK/ACC signaling pathway.
miRNA34a expression was determined by real-time quantitative RT-PCR and normalized to the RNU6B content (Fig. 2A). Hepatic SIRT1 levels were determined by Western- blotting and normalized to β-actin. Representative blot and the result of densitometry analysis are shown (Fig. 2B). The abundance of acetylated p53 was assessed by Western-blotting and normalized to β-actin (Fig. 2C). Western-blots were performed to assess the nuclear and cytosolic levels of LKB1, the phosphorylation of AMPK and ACC, as well as the abundance of total AMPK and ACC. Representative images are shown (Fig. 2D). Densitometry was performed and ratios between the cytosolic and nuclear LKB1, the phosphorylated and total AMPK/ACC were calculated and are shown in bar graphs. (*): high-fat vs control (#): pifithrin vs DMSO, p<0.05.
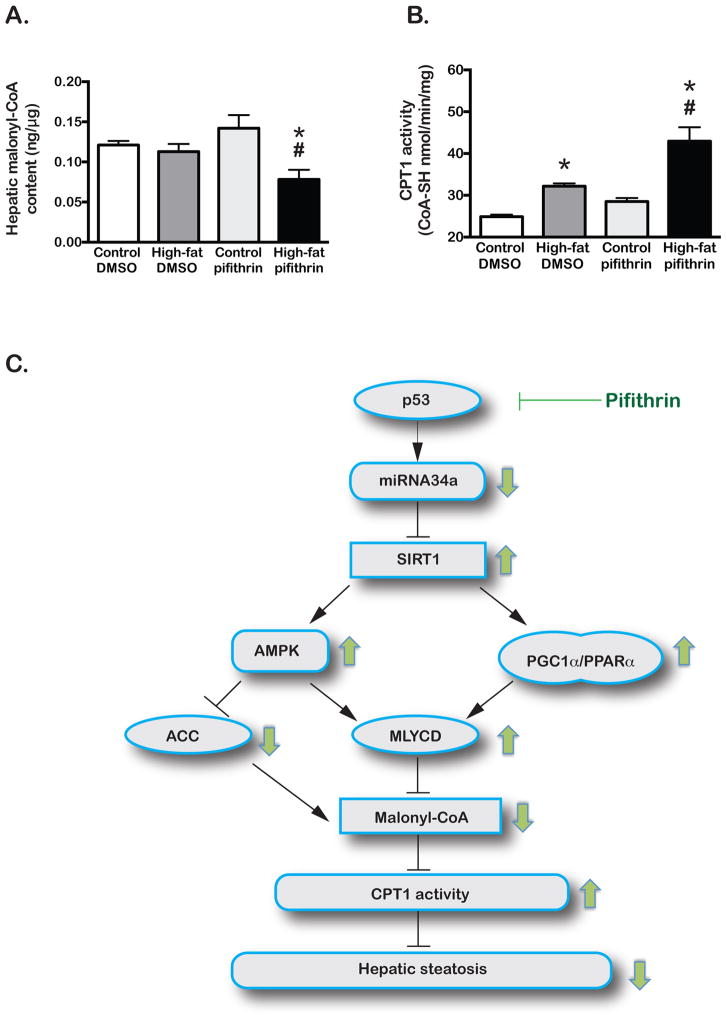
Figure 4. PFT treatment lowers hepatic MCoA thus increases CPT1 activity and promotes fatty acyl-CoA oxidation.
MCoA was determined in liver lysates by ELISA (Fig. 4A). CPT1 activity was determined by a biochemical assay in liver lysates (Fig. 4B). In the proposed model (Fig. 4C), SIRT1 upregulation by PFT administration promotes the transcription and functional activation of MLYCD and inhibits the function of ACC. These events ultimately lead to diminished intrahepatic MCoA content and increased CPT1 activity, allowing more robust β-oxidation of long-chain fatty acyl-CoAs to attenuate hepatic steatosis. Green arrows demonstrate PFT-induced changes in the HFD-fed mice relative to their DMSO-injected counterparts. (*): high-fat vs control (#): pifithrin vs DMSO, p<0.05.
SIRT1 promotes AMPK and PPARα leading to MLYCD activation
Malonyl-CoA is a critically important intermediate in the de novo fatty acid synthesis pathway, as well as an inhibitor of CPT1 that regulates the uptake of long-chain fatty acyl-CoA molecules by the hepatic mitochondria for β-oxidation [31]. The activation of SIRT1/LKB1/AMPK signaling cascade diminishes the intracellular abundance of this critically important metabolite by phosphorylating and inhibiting acetyl-CoA carboxylase (ACC), which generates mCoA from acetyl-CoA. AMPK also phosphorylates and activates MLYCD that degrades mCoA [21]. MLYCD has been shown to be a downstream target of PPARα [17]; the activation of SIRT1/PGC1α/PPARα axis enhances the expression of MLYCD. The transcriptional and functional activation of MLYCD by these two SIRT1- related molecular pathways contribute to decreased intracellular mCoA content. PFT promoted the deacetylation and subsequent cytosolic translocation of LKB1 (Figure 2D&E) that was associated with preserved phosphorylation of AMPK at Thr172 in the HFD-fed mice, which was otherwise suppressed in their DMSO-injected counterparts (Figure 2D&F). Additionally, the inhibitory phosphorylation of ACC at Ser79 was more pronounced in the HFD-fed, PFT-injected mice (Figure 2D&G). PFT administration promoted the deacetylation of PGC1α (Figure 3A) and increased the nuclear abundance of the PGC1α/PPARα regulatory complex in the livers of HFD-fed mice (Figure 3B–D), thus enhanced the expression of MLYCD (Figure 3E). Therefore, PFT administration seemed to promote the hepatic expression and function of MLYCD at the transcriptional and posttranscriptional level in the HFD-fed mice, while it suppressed the function of ACC. Interestingly; other PPARα targets that are involved in fatty acid oxidation were not significantly different between the two HFD groups (data not shown).
Figure 3. PFT administration increases the nuclear abundance of the PGC1α/PPARα complex and promotes MLYCD expression.
The acetylation status of PGC1α was assessed by Western- blotting and the result of densitometry is shown (Fig. 3A). The nuclear abundance of PGC1α and PPARα were assessed by Western-blotting (Fig. 3B) and densitometry was performed (Fig. 3C and 3D, respectively). Nuclear lamin A was used as a loading control. The MLYCD expression was assessed by Western-blotting using whole tissue lysates. A representative Western-blot and the result of MLYCD densitometry are shown in Fig. 3E. MLYCD expression was also assessed by Western-blotting in 800 μM palmitoleic acid-treated human HepaRG cells after 24 hours, in the presence of 25 μM cyclic PFT. A representative Western-blot and densitometry bar graph are shown in Fig. 3F. (*): high-fat (or POA) vs control diet (or control medium) (#): pifithrin vs DMSO, p<0.05.
In vitro pifithrin treatment suppresses steatosis and increases malonyl-CoA decarboxylase expression in human HepaRG cells
Importantly, decreased steatosis after 24 hours of PFT treatment was also demonstrated in 800 μM palmitoleic acid-treated human HepaRG cells (Supplementary Figure 5A&B). Similarly to the in vivo experiment, the blunted triglyceride accumulation was also associated with increased MLYCD expression (Figure 3F)
Pifithrin treatment contributes to diminished hepatic malonyl-CoA levels and increased CPT1 activity
In the HFD-fed mice chemical inhibition of p53 function by PFT inhibited ACC (mCoA synthesizing enzyme) and activated MLYCD (mCoA degrading enzyme). The intracellular decrease of mCoA may have contributed to the diminished steatosis observed in these mice. The intrahepatic mCoA content was assessed and, as demonstrated in Figure 4A, it was found to be significantly lower in the PFT- treated HFD-fed group compared to the DMSO-injected animals on HFD and the controls. Subsequently, the activity of CPT1, which is allosterically inhibited by mCoA, was found to be significantly higher in this group (Figure 4B), as measured by a previously described [32] assay.
The impact of pifithrin on fatty acid synthetizing enzymes
We also considered the possibility that PFT diminished steatosis by inhibiting the SREBP1c-mediated expression of enzymes involved in de novo fatty acid synthesis, since SIRT1 had been previously shown to deacetylate and inhibit the transcriptional activity of SREBP1c [15]. Importantly, as Supplementary Figures 6A–E reveal, HFD was associated with an adaptive mechanism involving decreased SREBP1c transcriptional activity, as evidenced by the marked downregulation of SREBP1c targets such as FAS, SCD1 and ACC. Therefore, there was no additional benefit of PFT on SREBP1c signaling in the HFD-fed animals.
Proposed model linking p53 inhibition to diminished steatosis in the liver
The study presented here suggests that p53 may have a role in regulating hepatic fatty acid metabolism. This protein is dysregulated in the fatty liver [10] and secondary changes that occur during steatosis (e.g., oxidative stress) favor the stabilization and increased transcriptional activity of p53. Inhibition of the transcriptional activity of p53 by PFT suppresses the upregulation of miRNA34a that occurs in the fatty liver [12, 13]. As a consequence, SIRT1 expression increases during high-fat feeding and promotes the transcription and function of the PPARα-target MLYCD, while it inhibits ACC. Therefore, PFT administration initiates an imbalance between the mCoA catabolizing and anabolizing enzymes favoring the degradation of mCoA, and subsequently promoting the activation of CPT1, which in turn increases the uptake and oxidation of long-chain fatty acyl-CoAs in the mitochondria (Figure 4C). The oxidation of these molecules decreases the likelihood of triglyceride accumulation and formation of other toxic lipids (such as diacylglycerol and ceramides). The decreased hepatic lipid load may improve hepatic insulin signaling [33] and cell survival. Inhibition of the transcriptional activity of p53 may enhance hepatic anti-oxidant buffering capacity by decreasing the transcription of the pro-oxidant p66shc and subsequently increasing MnSOD expression [9].
Discussion
This study indicates that p53 may have an important metabolic role in the pathogenesis of NAFLD, since the partial inhibition of the transcriptional activity of p53 by PFT markedly diminished hepatic steatosis in HFD-fed mice. Chemical inhibition of p53 was shown to inhibit miRNA34a upregulation and markedly increased SIRT1 expression and function in HFD-fed mice. The importance of SIRT1 in regulating fatty acid metabolism is highlighted by literature data, demonstrating that global or hepatocyte-specific overexpression of SIRT1 or its activation by resveratrol administration [34] may alleviate hepatic steatosis as well as other associated metabolic abnormalities, such as insulin resistance in various murine models. In this study, PFT-induced hepatic SIRT1 upregulation promoted the activation of the SIRT1/PGC1α/PPARα and the SIRT1/LKB1/AMPK signaling pathways that ultimately led to the hepatic decrease of mCoA, and improved CPT1 activity to enhance mitochondrial oxidation of long-chain fatty acyl-CoAs. Similarly to our findings, a recent work from Tomita et al. [35] also supports the idea that p53 has an important role in regulating hepatic fatty acid metabolism, as they demonstrated that the p53 knock-out mice developed less steatosis and liver injury when fed a methionine-and choline-deficient diet for 8 weeks than their wild-type littermates. Importantly, while Tomita et al. used p53 knock-out mice, our study employed a small molecule to partially inhibit the transcriptional activity of p53 and improve steatosis. While the p53 knock-out mice have been reported to develop early dysplasia associated with fatty liver [2], our approach seems to avoid this problem, as the transcriptional inhibition of p53 leaves the non-transcriptional tumor suppressor functions intact, which may provide efficient protection against tumorigenesis during severe genotoxic stress.
Although SIRT1 activation was only detected in the liver, the fact that PFT efficiently suppressed the HFD-induced weight gain without decreasing food intake suggests that this treatment may have a direct impact on the peripheral adipose tissue. This hypothesis is supported by a recent discovery of genes involved in adipocyte maturation (e.g., StARD4, OSBP) that are under the regulatory control of p53 [36]. It is likely that PFT improves many aspects of the metabolic syndrome in a complex, multi-organ context. It is noteworthy that no adverse effects of this treatment (e.g., aberrant crypt formation or malignancy in the colon) were observed similarly to other studies using PFT [27].
In summary, inhibition of the transcriptional activity of p53 in the livers of mice fed with HFD was successful; it diminished the diet-induced weight gain, hepatic steatosis, oxidative stress and apoptosis. Administration of PFT prevented the HFD-induced upregulation of miRNA34a, increased the expression of SIRT1 and ultimately altered the mCoA metabolism by signaling through AMPK and PPARα. This approach will likely suppress steatosis, oxidative stress and apoptosis not only in NAFLD, but also in ALD, which further suggests common pathological, biochemical and molecular similarities between the two diseases. Further studies may elucidate additional effects of this approach on other organs such as white adipose tissue, as well as characterize long-term effects on more advanced hepatic changes that occur in NAFLD such as steatohepatitis, fibrosis, cirrhosis and development of hepatocellular carcinoma.
Supplementary Material
Acknowledgments
We thank Dr. Murray B. Resnick, Virginia Hovanesian, Paul Monfils, Dr. Jaime Lecker, Andressa Sato Lopes for their technical assistance. We greatly appreciate the help of Dr. Jason T. Machan in the statistical analysis.
Financial support
Supported in part by the 2008 George A. Bray Award for obesity research (Z.D.) and by NIH grants R01CA123544 and R01AA08169 (J.R.W.)
List of abbreviations
- PFT
pifithrin-α p-nitro
- NAFLD
non-alcoholic fatty liver disease
- HFD
high-fat diet
- ALT
alanine aminotransferase
- mCoA
malonyl-CoA
- MLYCD
malonyl-CoA decarboxylase
- LKB1
liver kinase B1
- AMPK
5′ adenosine monophosphate-activated protein kinase
- CPT1
carnitine palmitoyltransferase 1
- ALD
alcoholic liver disease
- NASH
non-alcoholic steatohepatitis
- SIRT1
silent mating type information regulation 2 homolog 1
- SREBP1c
sterol regulatory element-binding protein 1c
- PGC1α
peroxisome proliferator-activated receptor-γ coactivator 1α
- PPARα
peroxisome proliferator-activated receptor-α
- ACC
acetyl-CoA carboxylase
- PUMA
p53 up-regulated modulator of apoptosis
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- ISOL
in situ oligo ligation
- MDA
malondialdehyde
- SCD1
stearoyl-CoA desaturase 1
- FAS
fatty acid synthase
- DNP
dinitrophenyl
- HSL
hormone sensitive lipase
- MnSOD
manganese superoxide dismutase
- StARD4
steroidogenic acute regulatory protein-related lipid transfer domain containing 4
- OSBP
oxysterol-binding protein 1
Footnotes
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lieber CS, Leo MA, Wang X, Decarli LM. Alcohol alters hepatic FoxO1, p53, and mitochondrial SIRT5 deacetylation function. Biochemical and biophysical research communications. 2008;373:246–252. doi: 10.1016/j.bbrc.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Pani G, Fusco S, Colavitti R, Borrello S, Maggiano N, Cravero AA, et al. Abrogation of hepatocyte apoptosis and early appearance of liver dysplasia in ethanol-fed p53-deficient mice. Biochemical and biophysical research communications. 2004;325:97–100. doi: 10.1016/j.bbrc.2004.09.213. [DOI] [PubMed] [Google Scholar]
- 3.Yeon JE, Califano S, Xu J, Wands JR, De La Monte SM. Potential role of PTEN phosphatase in ethanol-impaired survival signaling in the liver. Hepatology. 2003;38:703–714. doi: 10.1053/jhep.2003.50368. [DOI] [PubMed] [Google Scholar]
- 4.Derdak Z, Lang CH, Villegas KA, Tong M, Mark NM, de la Monte SM, et al. Activation of p53 enhances apoptosis and insulin resistance in a rat model of alcoholic liver disease. Journal of hepatology. 2011;54:164–172. doi: 10.1016/j.jhep.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrell GC, Larter CZ, Hou JY, Zhang RH, Yeh MM, Williams J, et al. Apoptosis in experimental NASH is associated with p53 activation and TRAIL receptor expression. Journal of gastroenterology and hepatology. 2009;24:443–452. doi: 10.1111/j.1440-1746.2009.05785.x. [DOI] [PubMed] [Google Scholar]
- 6.Panasiuk A, Dzieciol J, Panasiuk B, Prokopowicz D. Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World journal of gastroenterology: WJG. 2006;12:6198–6202. doi: 10.3748/wjg.v12.i38.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yahagi N, Shimano H, Matsuzaka T, Sekiya M, Najima Y, Okazaki S, et al. p53 involvement in the pathogenesis of fatty liver disease. The Journal of biological chemistry. 2004;279:20571–20575. doi: 10.1074/jbc.M400884200. [DOI] [PubMed] [Google Scholar]
- 8.Derdak Z, Villegas KA, Wands JR. Early growth response-1 transcription factor promotes hepatic fibrosis and steatosis in long-term ethanol-fed Long-Evans rats. Liver Int. 2012;32:761–770. doi: 10.1111/j.1478-3231.2012.02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch OR, Fusco S, Ranieri SC, Maulucci G, Palozza P, Larocca LM, et al. Role of the life span determinant P66(shcA) in ethanol-induced liver damage. Laboratory investigation; a journal of technical methods and pathology. 2008;88:750–760. doi: 10.1038/labinvest.2008.44. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Padhye A, Sharma A, Song G, Miao J, Mo YY, et al. A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. The Journal of biological chemistry. 2010;285:12604–12611. doi: 10.1074/jbc.M109.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermeking H. p53 enters the microRNA world. Cancer Cell. 2007;12:414–418. doi: 10.1016/j.ccr.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, et al. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;48:1810–1820. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pogribny IP, Starlard-Davenport A, Tryndyak VP, Han T, Ross SA, Rusyn I, et al. Difference in expression of hepatic microRNAs miR-29c, miR-34a, miR-155, and miR-200b is associated with strain-specific susceptibility to dietary nonalcoholic steatohepatitis in mice. Laboratory investigation; a journal of technical methods and pathology. 2010;90:1437–1446. doi: 10.1038/labinvest.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, et al. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. The Journal of biological chemistry. 2010;285:33959–33970. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 17.Lee GY, Kim NH, Zhao ZS, Cha BS, Kim YS. Peroxisomal-proliferator-activated receptor alpha activates transcription of the rat hepatic malonyl-CoA decarboxylase gene: a key regulation of malonyl-CoA level. Biochem J. 2004;378:983–990. doi: 10.1042/BJ20031565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. The Journal of biological chemistry. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. The Journal of biological chemistry. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ha J, Daniel S, Broyles SS, Kim KH. Critical phosphorylation sites for acetyl-CoA carboxylase activity. The Journal of biological chemistry. 1994;269:22162–22168. [PubMed] [Google Scholar]
- 21.Saha AK, Schwarsin AJ, Roduit R, Masse F, Kaushik V, Tornheim K, et al. Activation of malonyl-CoA decarboxylase in rat skeletal muscle by contraction and the AMP-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta -D-ribofuranoside. The Journal of biological chemistry. 2000;275:24279–24283. doi: 10.1074/jbc.C000291200. [DOI] [PubMed] [Google Scholar]
- 22.Fulop P, Derdak Z, Sheets A, Sabo E, Berthiaume EP, Resnick MB, et al. Lack of UCP2 reduces Fas-mediated liver injury in ob/ob mice and reveals importance of cell-specific UCP2 expression. Hepatology. 2006;44:592–601. doi: 10.1002/hep.21310. [DOI] [PubMed] [Google Scholar]
- 23.Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, et al. Regulation of PTEN transcription by p53. Mol Cell. 2001;8:317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 24.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 25.Cong WN, Tao RY, Tian JY, Liu GT, Ye F. The establishment of a novel non-alcoholic steatohepatitis model accompanied with obesity and insulin resistance in mice. Life Sci. 2008;82:983–990. doi: 10.1016/j.lfs.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Eipel C, Schuett H, Glawe C, Bordel R, Menger MD, Vollmar B. Pifithrin-alpha induced p53 inhibition does not affect liver regeneration after partial hepatectomy in mice. Journal of hepatology. 2005;43:829–835. doi: 10.1016/j.jhep.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Dinca EB, Lu KV, Sarkaria JN, Pieper RO, Prados MD, Haas-Kogan DA, et al. p53 Small- molecule inhibitor enhances temozolomide cytotoxic activity against intracranial glioblastoma xenografts. Cancer research. 2008;68:10034–10039. doi: 10.1158/0008-5472.CAN-08-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 29.Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 30.Didenko VV, Minchew CL, Shuman S, Baskin DS. Semi-artificial Fluorescent Molecular Machine for DNA Damage Detection. Nano Lett. 2004;4:2461–2466. doi: 10.1021/nl048357e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 32.Karlic H, Lohninger S, Koeck T, Lohninger A. Dietary l-carnitine stimulates carnitine acyltransferases in the liver of aged rats. J Histochem Cytochem. 2002;50:205–212. doi: 10.1177/002215540205000208. [DOI] [PubMed] [Google Scholar]
- 33.Shulman GI. Cellular mechanisms of insulin resistance. The Journal of clinical investigation. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomita K, Teratani T, Suzuki T, Oshikawa T, Yokoyama H, Shimamura K, et al. p53/p66Shc- mediated signaling contributes to the progression of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2012;57:837–843. doi: 10.1016/j.jhep.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, et al. A global map of p53 transcription- factor binding sites in the human genome. Cell. 2006;124:207–219. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.