Abstract
Background Context
Degeneration and injuries of the intervertebral disc result in large alterations in biomechanical behaviors. Repair strategies using biomaterials can be optimized based on biomechanical and biological requirements.
Purpose
To review current literature on 1) effects of degeneration, simulated degeneration, and injury on biomechanics of the intervertebral disc with special attention paid to needle puncture injuries which are a pathway for diagnostics and regenerative therapies; and 2) promising biomaterials for disc repair with a focus on how those biomaterials may promote biomechanical repair.
Study Design/Setting
A narrative review to evaluate the role of biomechanics on disc degeneration and regenerative therapies with a focus on what biomechanical properties need to be repaired and how to evaluate and accomplish such repairs using biomaterials. Model systems for screening of such repair strategies are also briefly described.
Methods
Papers were selected from two main Pubmed searches using keywords: intervertebral AND biomechanics (1823 articles) and intervertebral AND biomaterials (361 articles). Additional keywords (injury, needle puncture, nucleus pressurization, biomaterials, hydrogel, sealant, tissue engineering) were used to narrow articles to the topics most relevant to this review.
Results
Degeneration and acute disc injuries have the capacity to influence nucleus pulposus pressurization and annulus fibrosus integrity, which are necessary for effective disc function, and therefore, require repair. Needle injection injuries are of particular clinical relevance with potential to influence disc biomechanics, cellularity, and metabolism, yet these effects are localized or small, and more research is required to evaluate and reduce potential clinical morbidity using such techniques. NP replacement strategies, such as hydrogels, are required to restore NP pressurization or lost volume. AF repair strategies, including crosslinked hydrogels, fibrous composites, and sealants offer promise for regenerative therapies to restore AF integrity. Tissue engineered intervertebral disc structures, as a single implantable construct, may promote greater tissue integration due to improved repair capacity of vertebral bone.
Conclusions
Intervertebral disc height, neutral zone characteristics and torsional biomechanics are sensitive to specific alterations in nucleus pulposus pressurization and annulus fibrosus integrity, and must be addressed for effective functional repair. Synthetic and natural biomaterials offer promise for NP replacement, AF repair, as an AF sealant, or for whole disc replacement. Meeting mechanical as well as biological compatibility is necessary for the efficacy and longevity of the repair.
Keywords: Intervertebral disc degeneration, needle injection, biomechanics, injury, biomaterials, hydrogels, sealant, tissue engineering
INTRODUCTION
Spinal disorders and degeneration of the intervertebral disc (IVD) are often associated with structural defects that have direct biomechanical consequences and commonly lead to painful or disabling conditions. Structural IVD defects include endplate fracture, radial fissures, and herniation, and are separate from normal aging; such defects are considered markers of impaired disc function, and are more closely related to pain than other features of aging IVDs (1, 2). Bone and soft tissue pathologies of the spine are also inter-related, for example, Schmorl’s nodes are highly associated with severity of disc degeneration (3, 4). These structural defects of IVDs and vertebrae necessarily impact biomechanics and physiology of the spinal system, can lead to further structural failure, and are an essential feature of an accepted definition of intervertebral disc degeneration as an aberrant, cell-mediated response to progressive structural failure (1).
There continues to be a need for early detection of spinal disorders associated with painful conditions and a need for safe and minimally invasive interventions that may mitigate or repair structural defects at earlier stages of degeneration. Discerning symptomatic pathology in the spine from age-associated degeneration in the clinic remains an area of current research to image functional alterations in IVDs (5–9). Surgical procedures range in level of invasiveness, and treatment effectiveness is often related to how specifically the disabling condition can be associated with the observed pathology (10). For example, surgical intervention for herniation and spondylolisthesis offer significant benefit compared to non-operative treatments (10–12). Herniation and discectomy, however may accelerate the progression of IVD degeneration and can alter segmental range of motion, and this accelerated degeneration is at least partly attributed to altered mechanical strain fields in the IVD following removal of disc material (13–15). Structural failure in the IVD is irreversible in adults because of the limited healing potential and the reduced nutritional environment of the IVD; consequently restoring IVD functionality requires repair and tissue engineering strategies for IVD repair offer some promise (16).
Biomechanical measurements are essential to improve the understanding of IVD disorders and repair because altered biomechanics directly affect IVD cell metabolism and also characterize spine integrity and function. The ‘wear and tear’ hypothesis of disc degeneration has historically been accepted as an important contributor to degeneration (17, 18), and exposure to whole body vibrations is associated with degeneration (19). Mechanical loading on the IVD is also known to influence IVD cell metabolism and overloading is capable of inducing degenerative changes (20–22). However, epidemiology studies on twins have demonstrated biomechanics to be of lesser importance than heredity (23), and observations of skipped level degeneration also challenges the paradigm that age and biomechanics are key factors in the etiology of degeneration of the disc (24). Biomechanical testing is also required to characterize functional deterioration of the motion segment or IVD tissues due to degeneration (25, 26) as well as to characterize their functional restoration following repair or regeneration.
The biomechanical properties of the IVD are dependent on the continued biosynthetic activity of IVD cells and their control of degradation in the extracellular matrix. With age or excessive trauma, proteoglycans and collagen type are degraded and there is an associated reduction in the fixed charge density of the NP due to increased activity of catabolic mediators including matrix metalloproteinases and ADAMTs (27, 28) (29, 30). Functionally, the NP becomes dehydrated with a loss of hydrostatic pressurization that can induce delamination, alter compressive stiffness, reduce disc height, and increase the risk of prolapse (29, 30). The gelatinous NP is eventually replaced by fibrocartilaginous tissue, and the disc fails as the AF is unable to withstand the complex compressive and shear forces (31). The pathologic conditions that give rise to disc failure (i.e., trauma, age-associated degeneration) have been implicated as a source of chronic lower back pain, a health care problem with an estimated cost exceeding $100 billion annually (32, 33). IVD disruption may also render the spine mechanically unstable (34), causing deformity or neurologic dysfunction.
The role of biomechanics in IVD degeneration and regenerative therapies, in the context of this paper, involves reviewing the biomechanical changes that occur with injury and degeneration and those biomaterials that offer promise to repair such defects and deterioration. Historical studies on spine biomechanics can be dated to antiquity (35, 36), and many high quality reviews exist on spine biomechanics, mechanobiology, and biomaterials from the last decade (2, 18, 26, 34, 37–41). This is a narrative review focused on IVD injury and repair. The objectives of this paper are: 1) To review current literature on effects of degeneration, simulated degeneration, and injury on biomechanics of the IVD to answer the question – What happens with degeneration & injury and what needs to be repaired?; 2) To review current studies on biomaterials for functional IVD repair to answer the question – how do we repair IVDs using biomaterials?
Papers were selected from two main Pubmed searches on English language journals with no date restrictions (start of database through November 2011) using keywords: intervertebral AND biomechanics (1823 articles) and intervertebral AND biomaterials (361 articles). Additional keywords (injury, needle puncture, nucleus pressurization, biomaterials, hydrogel, sealant, tissue engineering) were used to narrow articles to the topics most relevant to this review. Additional articles were captured using different permutations of these keywords, by additional searches with Google Scholar, and by analyzing the articles obtained including the excellent reviews listed above. Inclusion was predominantly associated with the articles’ relationship to the focused questions of what needs repairing in the IVD and what biomaterials offer promise to achieve such repair? Articles were selected for inclusion in this review by Dr. Iatridis and at least one co-author for biomechanics topics and by Dr. Nicoll and at least one co-author for biomaterials topics. All authors agreed on final article selections.
1. What happens with degeneration & injury and what needs to be repaired? The effects of degeneration, simulated degeneration, and injury on mechanobiology of IVDs
This section first describes the effects of degeneration on IVD biomechanics including in vivo measurements. Next, the effects of simulated IVD injury models on biomechanics are assessed towards a more mechanistic understanding of how surgical and chemical interventions alter IVD biomechanics. There is a particular focus on needle puncture effects due to the high clinical relevance. With this context, we clarify those biomechanical behaviors that require repair following degeneration or injury.
a. Effects of degeneration on IVD mechanics
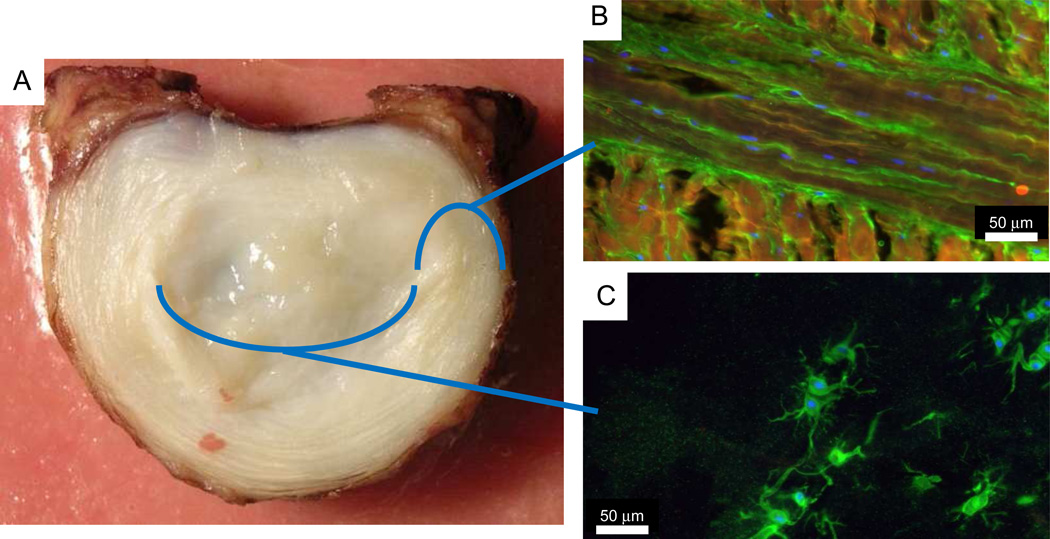
The IVD is comprised of a centrally located nucleus pulposus (NP), which is surrounded on the outer periphery by the annulus fibrosus (AF) and on the superior and inferior surfaces by cartilage and vertebral endplates. The highly organized matrix laid down by relatively few cells in a specific manner, and fibers in the outer AF continue into the longitudinal ligaments and vertebral bodies (42). The NP is a highly hydrated tissue with substantial amounts of glycosaminoglycans (GAGs), and fine collagen type II fibers (Figure 1). Mechanically, the NP behaves isotropically, is capable of producing high swelling pressures, and undergoes marked changes with degeneration (43–47). The AF is an angle-ply laminated structure with high collagen content. The collagen content and types have a strong radial variation being type II in the centrally NP region and type I at the outer AF periphery (31, 48, 49). Mechanically, the AF is highly anisotropic, heterogeneous, and nonlinear, and serves the dual mechanical roles of restraining NP swelling pressures and connecting adjacent vertebrae (47, 50–53). Interconnectivity of microstructural elements of AF is elaborate (54, 55), and while sliding between fine fibers within a ply has been observed (56), there is no evidence for sliding between AF layers (57). The healthy NP and AF tissues are highly hydrated with substantial GAGs, although many structural and biochemical alterations occur with aging and degeneration (58). The cells of both NP and AF contain pericellular matrices (Figure 1) and the orientation of the pericellular matrix varies significantly across the disc, reflecting local collagen matrix architecture (59). The cartilage endplate serves mechanical roles distributing pressurization and inhibiting flow out of the IVD during daily loading (60, 61), and is subjected to alterations with aging and disease (42). Together, all of these components form the IVD and enable mobility of the spine.
Figure 1.
(A) Photograph of intervertebral disc with immunohistochemistry images of (B) annulus fibrosus and (C) nucleus pulposus. Blue: DNA in cells; orange: collagen type I; green: collagen type VI. Immunohistology images obtained in collaboration with Drs Casey Korecki and Marc Levenston.
The spinal motion segment (i.e., vertebra-IVD-vertebra section) exhibits nonlinear responses to load in all six degrees of freedom (compression, anterior-posterior and lateral shear, flexion, bending, and torsion) (62, 63). This response can be divided into a neutral zone, where the disc offers minimal resistance to load, followed by progressive stiffening at larger deformations. In the healthy disc, this behavior allows free mobility within a certain range of motion, along with a strong resistance to spinal column instability (34). Nonlinear, direction-dependent, and viscoelastic behavior can make biomechanical changes from injury or degeneration difficult to quantify and interpret. Neutral zone behavior in particular is highly sensitive to pretension on AF fibers, and is thus dependent on preload and osmotic conditions (63). Consequently, protocol differences must be appreciated when comparing different studies as well as differences in dependent variable measurements. Measurements of the neutral zone are an important part of spine biomechanics literature because they are sensitive to IVD defects and disruption and have a functional impact on stability (34). The definition of neutral zone varies in the literature, and a recent study by Smit et al. provides a concise and objective definition based on a the minimal stiffness region of a sigmoidal curve (64). Since definitions vary through the literature, we consider neutral zone behaviors in this paper to be properties that generally define the low force region of the nonlinear stiffness curve.
Kirkaldy-Willis proposed a hypothesis that degeneration initially increases flexibility resulting in hypermobility in early IVD degeneration that leads to painful limitation of motion and eventually to tissue stiffening and hypomobility in later stages of degeneration (17, 18). This hypothesis has been generally affirmed in cadaveric biomechanics studies of spine motion finding that flexion and bending motions increase to approximately grade IV of degeneration as the segment becomes unstable and decrease in flexibility as the motion segment degenerates more to grade V (25, 65). Torsional range of motion is significantly increased with degenerative level (25, 66). Neutral zone ratio (normalized by total range of motion) also increased with degeneration in all 3 rotational degrees of freedom (67).
Degeneration of the IVD and loss of endplate integrity lead to stress concentrations, loss of NP pressurization and a shift in compressive load carriage from NP to the AF tissues (68, 69). Pressure transducers implanted in the IVD demonstrate hydrostatic behaviors across the healthy NP with the highest compressive stresses in the AF regions, and decreasing only in the outer AF (68). Degeneration reduces NP pressurization and generates stress peaks, particularly in the posterior AF, which is likely associated with damage to the AF or endplate (68). Localized strain measurements in degenerated human IVDs were measured using MRI strain mapping, and exhibited higher compressive axial and tensile radial strains. These findings suggested that depressurization of the NP places more compressive strain on the AF regions of degenerated IVDs (70). Inclusion of posterior elements to the functional spinal unit further enhances nonlinear biomechanical behaviors particularly accentuating coupling between rotation, bending and flexion (71).
There are several sensitive in vivo measurements capable of measuring the integrity of the IVD. The most consistently observed alteration in the structure of the IVD with degeneration is the well known loss of IVD height (68, 72). Measurements of disc height index are used as qualitative or quantitative measures of the extent of degeneration in humans and animal models (69, 73, 74). The loss of IVD height is a very sensitive parameter but it is not specific and can be associated with a number of degeneration-associated changes in the IVD including NP depressurization, endplate damage, and AF injuries, (68, 69, 75–77). Quantitative and functional MRI measurements are being developed for assessment of IVD composition and mechanics with some promising findings that may allow more specific characterization of functional deterioration with injury and degeneration (6, 78). For example, torsional range of motion has been measured to be increased in back pain subjects (66) and seems to be sensitive and specific to AF integrity. New techniques are also being developed to evaluate IVD height in 2 and 3 dimensions as well as to develop important new technologies for measurements of IVD kinematics in vivo during activities of daily life (79–82). An important in vivo human study using advanced imaging techniques confirmed hypomobility in advanced stages of painful degenerative disc disease, particularly at L5-S1, and evidence of hypermobility at levels adjacent to severely degenerated IVDs in flexion, bending and axial rotation in a pattern that further suggests hypermobility prior to hypomobility (81), consistent with the Kirkaldy-Willis hypothesis.
IVD degeneration also induces strong changes to isolated IVD tissues with the most dramatic effects noticed in the nucleus pulposus region. With degeneration of the NP, there is a significantly increased shear modulus, and decrease in swelling pressure, relative energy dissipation (45, 83), and effective aggregate modulus (84). There is a dramatic loss of water content in the NP of the IVD with degeneration which parallels loss of IVD height. Dehydration can occur in the IVD from either loss of NP pressurization or tissue overloading and strongly influences motion segment biomechanics (85) as well as AF and NP tissue mechanics (86, 87). These NP changes are consistent with lost GAG and water contents, and increased fiber content. In the annulus fibrosus, degeneration induces a significantly increased compressive modulus, decreased radial permeability (53), and altered Poisson’s ratio (50, 53, 88). These alterations in the AF may be explained by the loss of water content, tissue compaction, structural remodeling and microfailure that accumulate with degeneration.
b. Effects of simulated degeneration on motion segment mechanics
Compositional and structural changes with human disc degeneration are substantial and include dehydration, loss of GAG, increased collagen crosslinking, as well as focal defects and fissures (58). These compositional and structural alterations manifest in repeatable patterns of altered motion segment biomechanics (26). However, when evaluating human biomechanical patterns, it is important to recognize these reported changes include the combined structural defects of degeneration, damage accumulation throughout life, remodeling changes from attempted repair, and altered behavioral changes associated with aging, degeneration, or pain. Consequently, there is substantial variance that can complicate the interpretation of IVD biomechanics, structure, and composition in humans.
The effects of specific degenerative changes may be simulated using methods including chemical alterations, surgical defects, and simulated injury. These injurious interventions are typically applied to animal models or in vitro systems, and therefore, more specific interventions and comprehensive analyses can be made in systems with known histories and low variability. Techniques for simulating degeneration in the IVD fall into four main categories; biochemical alterations, endplate disruptions, and small and large AF injuries. There is currently no clear consensus in the literature on the exact definitions of small and large AF injuries, and categorizing studies based on the method of injury is difficult due to the large variations in both size and structure of model systems. Rather, it may be more useful to categorize these interventions in the context of the dual role of the AF: containment of NP fluid pressure and attachment between vertebrae. Consequently “small” injuries typically only affect immediately surrounding tissue with the potential to diminish pressure containment ability, while “large” AF injuries disrupt lamellar fibers sufficiently to compromise vertebral connectivity and diminish pressure containment collectively altering spinal column motion. The case of needle puncture injuries are included in both small AF injuries and large AF injuries in this review depending on the extent of that injury compared to the IVD size.
i. Biochemical Alterations
Nonlinearities are a particularly interesting complexity in IVD biomechanics that can create unexpected biomechanical findings and require appreciation for differences in low and high force behaviors as well as differences in biomechanical testing protocols. For example, collagenase digestion of a rat motion segment can cause a paradoxical increase in the axial stiffness due to collagen fiber degradation, which was associated with tissue compaction and increased neutral zone length. This, in turn, caused a shift in deformations to a higher slope region of the nonlinear stiffness curves and consequentially an increase in dynamic stiffness (89). In the same experiment, genipin crosslinking caused a decreased neutral zone length as expected but the stiffness measurements were shifted to a lower slope region of the nonlinear stiffness curve in this force controlled experiment which resulted in a surprising trend of decreased dynamic stiffness. Torsional loading of similarly prepared specimens showed increased stiffness for genipin treatments, decreased stiffness for collagenase digestion, and no change in neutral zone behavior (90), thereby exhibiting nonlinearities but also providing a direct measure of chemical effects on AF stiffness. Chondroitinase digestion reduced dynamic compressive stiffness of rat motion segments that were detected at low equilibrium loads but not at high loads (91). Consequently, detecting effects of matrix degradation on biomechanical behaviors requires appreciation for test protocol but also requires knowledge of how low force behaviors (such as neutral zone and IVD height measurements) are affected differently from high force behaviors.
ii. Endplate disruption and nucleotomy procedures
Endplate disruption and dehydration (induced by creep) in human cadavers reduced IVD height and increased the neutral zone ratio (neutral zone divided by range of motion) in bending modes, suggesting a shift towards instability (69). One might speculate that a similar loss of IVD height and increase in neutral zone ratio may be present in motion segments with Schmorl node defects (3). Nucleotomy produced similar effects as depressurization from endplate damage and significantly affected the radial strain patterns with a loss of radial bulging (15, 92). Nucleotomy also decreased IVD pressure, endplate deformation, and modified loading patterns on the vertebral rim when IVDs were loaded in compression. However, when loaded in shear, which does not pressurize the NP, there were no observed effects of nucleotomy on endplate deformation patterns (93).
iii. Small AF injuries
Needle puncture injuries of the IVD are of particular interest because they occur during diagnostic procedures such as discography and are required for injection of biologics, biomaterials, or other therapeutics. However, needle punctures are also used to simulate herniation with large gauge needles, and have been shown to induce slow, repeatable degenerative changes in small animals (94). Clinical discography using small gauge needle and limited pressurization resulted in accelerated IVD degeneration, disc herniation, loss of disc height and MRI signal intensity, and the development of endplate changes compared to matched-controls (95). Accelerated degenerative changes from discography may be associated with altered biomechanics from the hole, altered biological response from the injection, or an inflammatory cascade due to the injury (40, 94–96), all of which have been investigated in experimental model systems.
Elliott et al. (75) used needle diameter size relative to IVD height to review the literature and perform biomechanical testing that provided an important evaluation of biomechanical effects of puncture injury across species (Figure 2). They found that needle injury has the capacity to affect compression and torsional properties, and that large ratios of needle diameter:IVD height (>40%) resulted in significant effects observed across species, but small ratios <40% had variable and more minor effects. Needle puncture and other small defects that disrupt NP pressurization predominantly affect neutral zone characteristics and other low force behaviors which may be measured with compression/tension tests (75). The 40% biomechanical threshold of injury should be taken cautiously since certain studies have demonstrated sensitivity of puncture injury at much smaller sizes closer to 14% needle diameter:IVD height ratio (77).
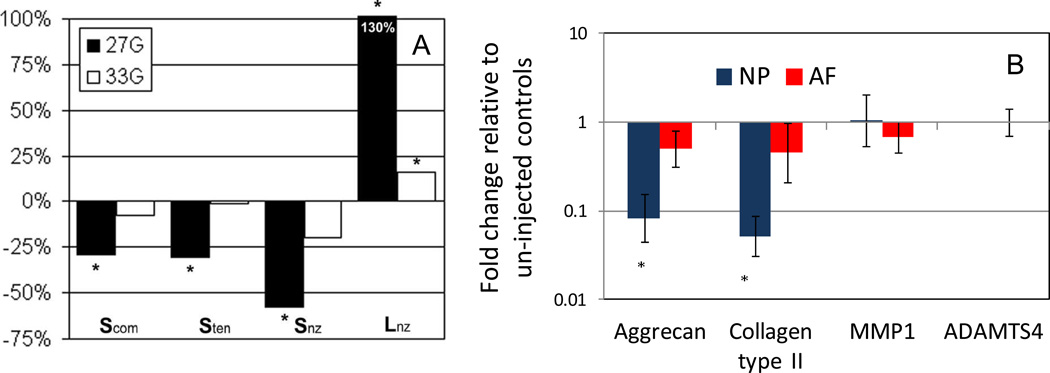
Figure 2.
(A) Effects of needle injuries on changes in IVD mechanical properties compared with preinjection value for compression stiffness (Scom), tensile stiffness (Sten), neutral zone stiffness (Snz), and neutral zone length (Lnz). The largest changes from puncture were reported for the neutral zone characteristics including stiffness and length. Other changes were also reported for compression and tensile stiffness. *Significant difference between pre- an postinjection values, paired t test. From Elliott et al., Spine 2008 and reprinted with permission. (B) Injection (21G needle) of PBS resulted in a significant decrease of anabolic gene expression in the NP region 7 days following injection, as measured by qPCR suggesting co-morbidity of discography could be related to biochemical effects of needle puncture or biological effects of the puncture or injection.
Needle puncture induces localized loss of cell viability adjacent to the puncture hole, evidence of biological remodeling in the NP region (97), and these effects may be amplified by injection. A volume of 100 µL of PBS with 0.1% BSA was slowly injected into the NP of the bovine IVDs in an organ culture model, and resulted in a down-regulation of aggrecan and collagen II mRNA expression at 7 days (Figure 2) providing a specific example of how a minor injury affects the IVD organ response (40).
Needle puncture injury in a bovine organ culture model demonstrated acute biomechanical effects including increased creep and a loss of dynamic stiffness (97). In the bovine model, the needle puncture injury did not seal with NP material (Figure 3), and a lack of needle size dependence in the biomechanical behaviors was suggestive that hydrostatic NP pressurization was more important than displaced NP material (97). It is important to note that a needle of the same size and shape can induce different size and shaped holes on the micro-scale even within the same species. In punctured bovine AF tissues, the shape and size of the puncture injury varied greatly but was typically either a circular hole approximately 100 µm in diameter with an elongated split between fiber bundles or a jagged tear with broken fibers visible stretching more than 500 µm that includes some delamination (98) (Figure 3). The variation in observed damage patterns was the result of how the needle interacted with the particular lamella being imaged, and since there can be more than 20 annular layers, it is possible that the full range of damage types is present in every puncture. While breakage of isolated fiber bundles is insignificant in the context of whole motion segment mechanics, it results in a measurable increase in shearing between adjacent bundles in the vicinity of the puncture under load (Figure 3) (98). Injection of pressurized fluid can induce more damage than needle puncture alone including delaminations and concentric tears (99). When disc pressurization is applied extremely to the point of failure, rate dependent patterns include radial tears through endplate and annular layers (100). Such localized and organ level effects of needle puncture and injection on experimental model systems show many changes, which may help inform potential mechanisms for the observed accelerated degeneration in response to discography (95). While the relevance of these basic science studies on the human clinical situations continues to be elucidated, the potential risks of diagnostic discograms or therapeutic injections must be weighed with the benefits to enable sound clinical judgment (94).
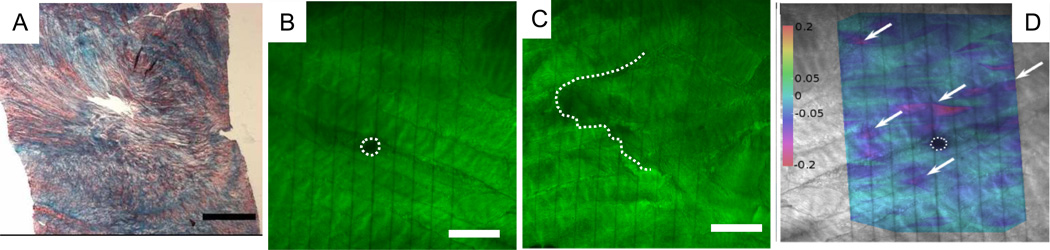
Figure 3.
Effects of needle injuries on annulus fibrosus histology and strain mapping. (A) Needle injuries in bovine AF remained open and unsealed; histology with picosirius red and alcian blue staining. Modified from Korecki et al., 2008 (14G puncture, Scale bar=1 mm). (B–D) Needle injures generally took the form of circular hole or a jagged tears, and this pattern was independent of needle size and shape (21G needle; DTAF staining with confocal imaging. The photobleached parallel vertical lines also were used to calculate shear strain maps and demonstrate discontinuity around a needle puncture and shear strain concentrations. Image modified from Michalek et al., 2010 (Scale bar = 250 mm).
iv. Large AF injuries
There are many models where large injuries have been surgically induced to produce IVD degeneration in animal models (101). It is important to appreciate that large needles in small animal IVDs may be more similar to scalpel injuries in large animal IVDs. However, different species have distinct transitions between the NP and AF regions and also maintain distinct fibrous composition and organization, so that most animal models have important structural differences from human IVDs in addition to the obvious size differences. In contrast to bovine studies, a rat needle puncture study suggested that smaller AF defects became sealed and enabled healing (39). Whether needle holes are able to seal or heal is likely to be both species and age dependent, influenced by how fibrous or gelatinous the NP region remains and how metabolically active the IVD cells are. For large animal IVDs with fibrous NP, like bovine and mature human IVDs, it is likely that holes from needle injury remain unsealed for a longer period than for small animals and perhaps chronically.
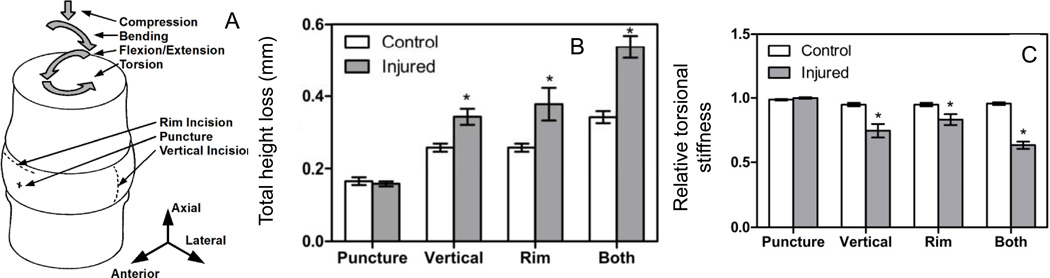
A small defect or localized injury can influence motion segment biomechanics and biology, as previously reviewed, and these injuries have included mid-plane, radial, or concentric tears, rim lesions, and puncture injuries (40). Defects induced with a scalpel provide distinct effects of injury that depend on injury type and severity. A comprehensive biomechanics study investigated the effects of scalpel injuries simulating needle puncture, vertical and rim incisions on IVD pressurization and 6 degree of freedom stiffness behaviors using bovine motion segments (77). This study found that loss of IVD height, torsional stiffness, and nucleus pulposus fluid pressurization are the immediate results of acute anular injury (Figure 4) (77). Specifically, vertical and rim lesions resulted in a loss of IVD height that depended on the extent of disruption with combined injuries (both) having the greatest loss of IVD height during the testing. Torsional stiffness was also sensitive to vertical and rim lesions, with combined injuries resulting in the largest deterioration of torsional stiffness. Together with the literature, we infer that the effect of AF injury on compressive biomechanics is specific to whether the injury has penetrated the entire depth of the AF, and whether there is substantial loss of NP volume or depressurization (40). Torsional behaviors, on the other hand, are sensitive to the extent of injury and consequently the percentage of AF fibers disrupted (40, 76).
Figure 4.
Effects of injury on IVD biomechanics. (A) Surgically-induced injuries are described schematically and include a 21 G needle puncture injury, and 10 mm scalpel injuries to create a vertical incision or rim lesion. Biomechanical changes sensitive to injury included (B) loss of IVD height and (C) reduction in torsional stiffness. Images used with permission from Michalek & Iatridis, Spine J, 2012.
c. What needs to be repaired? Distinguishing effects of NP Pressurization and AF integrity: Sensitive parameters to small and large injuries
IVD degeneration involves complex and interacting changes in composition, structure and biomechanics that can be difficult to interpret and detect. The long-time constants for viscoelastic behaviors of the motion segment makes detection of biomechanical effects of injuries protocol specific. Nonlinear motion segment biomechanics indicate that mechanical behaviors depend on direction of loading and axial preload (63, 102). IVDs are also remarkably elegant laminated composite structures that are effective at inhibiting crack propagation and ‘masking’ or minimizing the effects of damage accumulation on IVD motion segment behaviors (31) so that mechanical function can be maintained throughout many decades of life. Furthermore, genetic variation as well as accumulation of injuries and remodeling through decades of life adds to large experimental variation in human subjects.
IVD injury affects torsional and axial biomechanics differently (76); both of these parameters are sensitive to small and large injuries and can be useful to distinguish NP pressurization and AF integrity effects (40, 75, 77). In an effort to simplify the biomechanical changes with injury and degeneration, we suggest that it is a priority to design tests that are able to characterize how NP pressurization and AF integrity may be influenced by injury and repair (Figure 5).
Figure 5.
Biomechanical effects of IVD injury including the mechanisms for biomechanical changes, sensitive biomechanical tests, observed effects on mechanical behaviors, and defects that are likely to be detectable from that test. Loss of NP pressurization or volume can happen at early or minor stages of injury and degeneration with axial loading being a sensitive biomechanical test. Loss of AF integrity occurs with larger injuries or more advanced stages of degeneration and also encompasses those changes observed with loss of NP pressurization. Torsional testing tends to be sensitive and specific for loss of AF integrity. Axial and torsion testing can enable characterization of both of these essential functions.
A loss of NP pressurization is often found before there is a large loss of AF integrity, and may occur by needle puncture, small penetrating AF injuries, loss of NP GAGs, mild alterations in AF structure that induce laxity as well as endplate damage. Low force biomechanical behaviors including IVD height loss, variations of neutral zone characteristics (length, slope), and measurements of low force tension and compression behaviors tend to be sensitive to losses of NP pressurization. IVD height loss is perhaps the most sensitive variable affected by IVD injury and degeneration, although a repeatable baseline load condition must be made to account for diurnal variations in vivo or other swelling effects in vitro. Alterations in neutral zone characteristics are a low force biomechanical test that is also sensitive to NP pressurization, and includes neutral zone length, neutral zone slope, or neutral zone ratio. The choice of measuring IVD height or neutral zone properties is typically based on convenience, with IVD height being among the simplest sensitive in vivo measurements, and neutral zone changes being easier to characterize through in vitro motion segment biomechanical testing and providing more detailed functional information.
More severe injuries have a larger effect on AF integrity in addition to loss of NP pressurization. Larger injuries affecting AF integrity involve large AF defects, fissures or injuries, herniation, discectomy, or moderate and severe IVD degeneration. AF integrity can be evaluated in torsion testing, and the sensitive parameters include loss of torsional stiffness or increased range of motion, and the effects are generally proportional to the size of the injury (40, 75, 77). Torsion properties are commonly measured in vitro (26, 63, 102), and the sensitivity of torsional properties to painful human IVD conditions has been measured in vivo (66, 81).
Combined assessments of NP pressurization and AF integrity are sensitive to injury and degeneration, and can therefore be used as an organizing principle to characterize and evaluate functional biomechanical repair using tissue engineered materials. Attempts at repair can be focused on restoring NP pressurization and volume which may be evaluated with axial testing as well as restoring AF integrity which may be evaluated using torsion testing. These two biomechanical objectives provide distinct challenges to enable restoration of proper biomechanical function of the IVD. Additional testing such as full nonlinear tension/compression behaviors as well as tests in other degrees of freedom (bending, flexion, extension, or shears) remain important for full biomechanical characterization, but may not be fully necessary as an initial screening for repair feasibility.
Biomechanical damage can be observed mechanically with relatively small defect sizes, yet, microscale damage has been observed with even very small needles (98). Additional data support the hypothesis that biologic remodeling precedes damage to the IVD structure (103), and that repeated injuries induce persistent inflammation (104). We suggest, therefore, that while motion segment testing is a necessary component in determining effectiveness of IVD repair, it is not be sufficient to fully define successful repair techniques.
d. Conclusions part 1: What must be repaired following injury or degeneration?
IVD tissue and motion segment biomechanics are complex involving nonlinear, direction-dependent, and viscoelastic behaviors such that interpretation of biomechanical results requires an appreciation for test protocol, species, and load history.
Human IVD degeneration influences IVD height, neutral zone characteristics and torsional biomechanics with generally repeatable patterns that can be partially masked by large human variations.
Needle injection injuries are of great clinical relevance with the potential to affect macro-scale and micro-scale biomechanics, cellularity and gene expression responses. However, the extent of such injection injuries on clinical decision-making requires further research.
Small and large IVD injuries result in organ level changes affecting IVD biomechanics and cell response.
Small IVD defects including needle puncture influence NP pressurization and these most specifically affect IVD height measurements and neutral zone characterizations.
Large IVD defects involve a loss of AF integrity, which may be characterized well using torsion tests.
IVD injury, degeneration, and repair influence NP pressurization and AF integrity and can be evaluated with measurements of IVD height, neutral zone characteristics, and torsional behaviors as an initial screening.
Biological measurements are very sensitive to IVD injury and repair so that biomechanical testing of the motion segment is necessary but not sufficient to fully characterize IVD repair.
2. How do we repair IVDs? Biomaterials for repair of the IVD
a. Biomaterials for repair
A variety of surgical procedures have been developed to treat ailments stemming from IVD degeneration, all of which involve stabilization and fusion of adjacent motion segments. Such procedures result in loss of motion of the cervical spine at the surgical site and abnormally excessive local stresses (105). In some instances, the deleterious consequences of intervertebral fusion include persistent pain, abnormal spinal contour, and accelerated degeneration of adjacent segments (106).
The use of prosthetic devices has also been proposed for IVD repair (107, 108). Thus far, attempts to develop artificial replacements for spinal segments have not been entirely successful. These failures appear to be due to the difficulty in developing durable prostheses of sufficient size and suitable mechanical properties. There is also an inability to replicate the wide range of motion permitted by the normal spine (105). In addition, problems of bony fixation and anchorage of the devices to the host spine as well as potential inflammatory responses to prosthetic wear debris are all difficulties that must be overcome (109). The most widely implanted total disc replacement is the SB Charite III (DePuy Spine), which has been approved by the Food and Drug Administration for treatment of single-level insults at the L4-5 or L5-S1 levels. Although, these prostheses have shown fair to good short-term results, the long-term outcomes are less promising, often requiring posterior fusions or removal of the implants due to subsidence, formation of wear debris or degeneration of adjacent discs (110, 111).
Autologous and allogeneic grafts have been routinely used for the repair of connective tissue defects. Autogenous grafts have limited application because of a scarce supply of donor tissue and donor site morbidity (112, 113). Allograft tissue, on the other hand, is more readily available, but the risk of disease transmission and immunological responses to alloantigens present difficulties. Despite these intrinsic drawbacks, canine models have been employed to analyze the effectiveness of intervertebral disc allografting and autograft transfer for the restoration of physiologic structural integrity and function. Autograft intervertebral discs have been shown to be structurally sound, but exhibit abnormal morphology and metabolic functioning, while allografted discs display evidence of progressive degeneration shortly after transplantation (105, 114). Similar results were found using a bipedal animal model in which fresh frozen discs allografted in rhesus monkeys survived but resulted in severe disc degeneration 24 months after transplantation (115).
All of the current therapies for IVD degeneration attempt to alleviate pain rather than fully restore tissue functional properties. A promising alternative may involve the use of biomaterials methodologies to fabricate constructs for IVD repair. To date, experimental approaches have largely focused on replacement of individual components of the disc (i.e., NP or AF), while only a few studies have attempted to engineer composite disc analogs that recapitulate the structure and function of the native tissue. The success of these various approaches has primarily been evaluated based on tissue composition and organization. However, mechanical properties are essential outcomes that need to be considered to assess satisfactory performance relative to the intact disc. In particular, the biomaterials must be able to restore NP pressurization and AF integrity as well as withstand physiologic loading conditions chronically to successfully be translated to the clinic. The mechanical design criteria for IVD tissue engineering have been reviewed elsewhere (41), as have the importance of cell sourcing and soluble factors for the development of IVD tissue constructs (16, 116–118). This section of this review focuses on select synthetic and natural materials employed for IVD replacement, with emphasis on mechanical compatibility of biomaterials intended for restoration of the NP, AF and for whole disc equivalents.
Design criteria for mechanical repair include mechanical similarity to native IVD, biocompatibility, and ability to withstand fatigue loading found with chronic implantation. To achieve the longevity goals, it can be preferable to have a cell seeded degradable material that enables cells to build replacement tissue. Biomaterials can be designed for several purposes, including NP replacement, AF replacement, sealants to allow AF repair and to enhance replacement tissue integration, and whole IVD replacements.
b. NP replacement
As an alternative to spinal fusion or total disc replacement, NP replacement devices in various stages of clinical development have been explored for the treatment of early stage IVD degeneration. These approaches include the use of injectable, in situ curable materials such as a recombinant silk-elastin protein hydrogels (NuCore™, Spine Wave, Shelton, CT) and water-in-oil emulsions containing hydroxypropyl methylcellulose and barium glass (DiscCell™, Gentis, Wayne, PA) (119–121). Preformed devices with a defined size and shape are also under development, such as NeuDisc™ (Replication Medical Inc, New Brunswick, NJ), which is comprised of hydrolyzed polyacrylonitrile copolymer reinforced with a dacron mesh (119–121). These NP replacements allow immediate restoration of IVD height, redistribute loads to the remaining structures of the IVD, and require a less invasive surgical procedure than fusion, however such implants run the risk of extrusion, and complications with the endplate response to the devices have been reported (119). Partial disc replacements do not incorporate cells to aid in the regenerative process, thus limiting their potential for biological repair.
Tissue engineering strategies may provide viable NP replacement therapies as an alternative to invasive surgical procedures for alleviating back pain and restoring both the structural and mechanical function of the IVD (117, 122). Experimentally, many polymeric materials, both synthetic and natural, have been explored to create NP tissue replacements. Several properties of native NP tissue have been characterized as benchmarks for creating a successful engineered NP tissue replacement. This includes an extracellular matrix (ECM) composed of mainly collagen type II and GAG, where the GAG to collagen ratio is approximately 27:1 (123). With respect to functional mechanics, the human NP tested in unconfined compression has an equilibrium Young’s modulus (Ey) of ~5 kPa, a Poisson’s ratio of 0.62 and a percent relaxation of ~65% (124). In confined compression, the human NP has a swelling pressure of ~0.14 MPa, an effective aggregate modulus (HAeff) of ~1 MPa, and a permeability (ka) of ~0.9 × 10−15 m4/Ns (5). In torsional shear, the complex shear modulus (|G*|) ranges between 7.4–19.8 kPa and the phase shift (δ) was found to be between 23–30° (45). Despite the availability of these baseline criteria, few studies have attempted to directly compare the properties of engineered NP materials to those of the native tissue.
Synthetic Materials
A variety of synthetic polymeric materials have been investigated as potential NP substitutes since their properties are well controlled, with minimal variation between batches. Such materials include polyvinyl alcohol (PVA) and poly (L-lactic acid) (PLLA) (125–127). Newer approaches include the use of poly(N-isopropylacrylamide) (PNIPAAm), a thermoresponsive polymer that has been copolymerized with polyethylene glycol (PEG) to produce hydrogels capable of in situ gel formation (128, 129). The unconfined compressive modulus of these gels ranges from 50–200 kPa depending on PEG content and molecular weight (128–130). PVA cryogels have been fabricated to control swelling dimensions using freeze-thaw cycles (FTC), and can be tailored to produce unconfined compressive moduli (2–5 kPa) similar to the human NP by varying the number cycles and the PVA concentration (127). Although these various synthetic polymeric materials provide consistent mechanical properties, they do not necessarily present the native hydrogel format (i.e., PLLA foams) or the negatively-charged swollen network (i.e., PVA, PNIPAAm-PEG) of the actual NP, creating the need for more biomimetic alternatives.
Natural Materials
Biopolymers have been extensively studied for NP tissue engineering, as these natural materials are biocompatible and can easily encapsulate cells to create a functional tissue construct. Such biomaterials include alginate, agarose, hyaluronan, collagen, chitosan, and carboxymethylcellulose (CMC) either alone or in combination. Most of these are used in a hydrated or hydrogel-like form, which has been proposed as the ideal format for NP replacements due to the similarity with the native tissue (131).
Alginate, a natural polysaccharide derived from brown algae, has been widely employed for culturing NP cells and as a replacement scaffold (132–135). Ionic crosslinking of alginate may be achieved in the presence of divalent cations, such as calcium, to produce a crosslinked network. Acellular ionically crosslinked alginate gels tested in unconfined compression have a modulus ranging between ~5–13 kPa (124). Although, ionically crosslinked alginate has been used successfully to encapsulate disc cells, alginate hydrogels have been shown to lose mechanical integrity over time, likely resulting from ion diffusion or depletion of calcium by incorporated cells, leading to a breakdown of the ionic crosslinks (136). The formation of more stable covalent crosslinks between alginate chains may be accomplished by modifying the polymer backbone with functional methacrylate groups. For example, photocrosslinked alginate hydrogels may be produced by free radical polymerization of methacrylated alginate in the presence of a photoinitiator and upon exposure to ultraviolet light (137, 138). Recently, photocrosslinked alginate was shown to maintain viability of encapsulated bovine NP cells, promote phenotypic matrix accumulation, and sustain structural and mechanical properties in vitro and in vivo (137, 138). In vivo studies demonstrated an increase in the Ey from 1.24 ± 0.09 kPa to 4.31 ± 1.39 kPa, (137) within the range reported for the human NP (124).
Carboxymethylcellulose, a negatively-charged, water-soluble polysaccharide derivative of cellulose, is a biocompatible material that is commercially available in high-purity forms, making it an attractive option for biomedical applications, such as drug delivery (139). Recently, photocrosslinked CMC hydrogels produced by methacrylation of CMC (as with alginate), have been shown to promote NP-like tissue formation (i.e., collagen type II and GAG production) and functional mechanical properties (Figure 6A) (140, 141). Specifically, bovine NP cell-laden hydrogels exhibited an Ey of ~18kPa when cultured in chemically defined medium supplemented with TGF-β3. In addition, these hydrogels maintained an equilibrium weight swelling ratio of 22.44 ± 0.71, comparable to that of native bovine NP (19.94 ± 3.09) (140). The closely matched swelling ratio is particularly noteworthy since it is important for pressurization and restoration of disc height.
Figure 6.
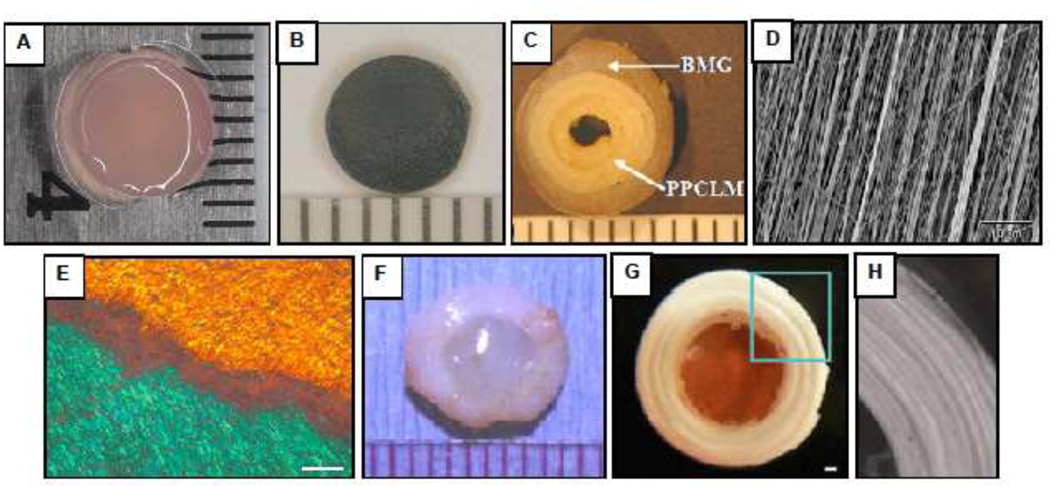
Selected biomaterials for IVD repair. (A) Photocrosslinked carboxymethylcellulose hydrogel with encapsulated NP cells (B) Fibrin-Genipin adhesive for AF fissures (C) Biphasic scaffold for AF repair consisting of concentric sheets of poly(polycaprolactone triol malate) (PPCLM) surrounded by a ring of demineralized bone matrix gelatin (BMG) (Wan et al., 2008). (D) Scanning electron micrograph of an electrospun polycarbonate polyurethane nanofibrous scaffold for AF replacement (Yeganegi et al., 2010). (E) Polarized light micrograph of an electrospun poly(e-caprolactone) (PCL) nanofibrous scaffold oriented in opposing bilayers (+30°/−30°) seeded with mesenchymal stem cells which elaborate aligned intra-lamellar collagen that recapitulates the gross fiber orientation of the native AF (Nerukar et al., 2009). Scale: 200 µm (F) Composite whole disc equivalent comprised of NP cells encapsulated in an alginate hydrogel surrounded by a reinforced poly(glycolic acid) mesh seeded with AF cells (Mizuno et al., 2006). (G) Disc-like angle-ply structure constructed from PCL nanofibers oriented at +30°/−30° to mimic the AF with a central agarose hydrogel core serving as an NP analog (Nerukar et al., 2010). Scale: 1 mm. (H) Higher magnification view of AF region outlined in panel G. Published images reprinted with permission from Elsevier (C, D, F), Nature Publishing Group (E) and Wolters Kluwer (G, H).
Hyaluronic acid (HA) is a polysaccharide naturally found in the ECM of many connective tissues. Although HA has been modified with methacrylate groups and photocrosslinked in a similar manner to alginate and CMC, it has not been employed for engineering of NP tissue (142, 143). However, other methods of forming crosslinked HA networks have been reported using additional chemistries. For example, HA was covalently bonded to polyethylene glycol-g-chitosan using a mixture of ethyl-3-[3-dimethylamino] propyl carbodiimide and N-hydroxysuccinimide. These hydrogels had an unconfined compressive modulus ranging between ~6–22 kPa and a Poisson’s ratio of ~0.5 (124). More recently, oxidized HA was crosslinked with adipic acid dihydrizide (ADH) to form injectable hydrogels with rheological properties close to native NP values (144). The gels exhibited a complex shear modulus of ~5–30 kPa and a phase shift of 1–17°, depending on the ADH concentration.
Not surprisingly, collagen-based materials have also been utilized for engineering NP-like tissue. Scaffolds comprised of collagen type I and HA seeded with bovine NP cells displayed significant matrix accumulation, particularly of GAGs, although functional properties were not assessed (145). Composites of enzymatically crosslinked atelocollagen type II, aggrecan and hyaluronan were found to maintain a stable structure, and when tested in confined compression, exhibited a compressive stress ranging from ~5–20 kPa (146). Finally, dense collagen type I gels intended for NP replacement were tested in dynamic shear and found to have a complex shear modulus that increased from ~0.1 to 10 kPa as a function of increasing collagen content (147).
Overall, many of the materials developed for NP replacement display mechanical properties comparable to the native NP. In particular, the unconfined compressive and dynamic shear properties closely match those of human tissue. However, additional characterization of the confined compressive properties and swelling pressure are important for ultimate validation of construct efficacy. Moreover, few studies have evaluated these candidate materials in vivo for biocompatibility, tissue formation or restoration of function using relevant injury or human model systems. The fate of these constructs in the challenging mechanical and physiological environment of the both the healthy subject and the physiologically-compromised patient must be further characterized and validated.
c. Sealant/adhesive
A particular challenge facing NP replacement is the opening left in the AF by the procedure. Failure to secure the incision site may result in extrusion of the NP material (148) and subsequent loss of disc material and height. This combination of displaced NP material and loss of AF integrity has been shown to increase the risk of disc herniation under bending (149). Repair of AF incisions is thus a high priority for successful NP replacement therapies (150). To date, most methods for AF repair have not been successful. In-vivo testing of various suture techniques failed to restore healthy biomechanics better than no repair (151). Plug devices intended to retain NP fluid pressurization have failed due to mismatched compliance between the implant and surrounding tissue (152). Recently developed fibrin-genipin hydrogel adhesives with tuneable mechanical properties (Figure 6B) (153) may have the potential to fill these gaps without inducing stress concentrations in the surrounding tissue. An optimal solution for AF closure will likely require both a bioactive adhesive for bridging microscale gaps along with a higher strength anchor to support macroscale loads. Combinations of surface adhesives and sutures have already shown more promising high cycle fatigue performance than either method alone (154).
d. AF replacement
As with the NP, a wide range of materials have been investigated for repair of the AF. However, the complex, oriented lamellar structure of the AF imparts unique properties that are not easily matched using fabricated biomaterials. The tensile modulus of a single lamella ranges from 28–140 MPa in the fiber direction and is 0.22 MPa in the transverse direction (155, 156). For multiple lamellae, the tensile moduli in the linear region of the stress-strain curve in the circumferential, axial and radial directions range between 5–45 MPa, 0.8 MPa and 0.47 MPa, respectively (51, 157, 158), while the failure stress has been reported to vary from 1–9 MPa (157, 159). The shear properties of the human AF have also been measured, with a |G*| of 75–200 kPa and a δ of 17–20° (160, 161). In confined compression, the swelling pressure has been determined to be 0.11–0.14 MPa and the aggregate modulus is 0.27–0.75 MPa (53, 86, 162). It is important to note that all of these properties are dependent on location within the disc (i.e., anterior versus posterior, inner versus outer annulus) and testing parameters (i.e., strain rate), which explains the wide distribution of values reported for many of the outcome measures.
To date, there have been no materials that can fully match the mechanical properties of the native AF, although several promising scaffolds have been found to support the growth of AF cells. These include porous matrices derived from natural materials such as silk (163), collagen/GAGs (164), and atelocollagen (165). Such scaffolds promote AF cell proliferation and characteristic ECM elaboration, but have not been characterized for mechanical functionality. A synthetic porous scaffold fabricated from biodegradable poly(1,8-octanediol malate) has also been shown to maintain AF cells in culture (166). These scaffolds were found to have a failure strength in tension of ~7–15 MPa, close to the values reported for the human AF (157, 159), which was dependent on the post-polymerization reaction time. A variation of this strategy was employed to engineer biphasic multi-lamellar constructs for AF repair using concentric sheets of porous polymer foams composed of poly(polycaprolactone triol malate) (PPCLM) seeded with rabbit chondrocytes to approximate the inner AF, which was further surrounded by ring-shaped bone matrix gelatin to mimic the outer AF, rich in collagen type I (167) (Figure 6C). The tensile failure strength of these composite materials was ~3.4 MPa, significantly greater than that for PPCLM alone (0.6 MPa), approaching the value of the rabbit AF (~7 MPa) and within the range of the human AF (1–9 MPa) (157, 159). Nevertheless, the various porous foam scaffolds do not approximate the fibrous format of the native tissue.
In an effort to model the fibrous nature of the AF, hybrid alginate/chitosan fibers (40–100 µm diameter) were produced using a wet-spinning and lyophilization technique (168). Although the scaffolds were capable of maintaining AF cell growth, the cells formed into clusters at later time points. Also, the scale of the fibers was much greater than that of the collagenous fibers (100–150 nm diameter) that comprise the natural tissue (169). More recent attempts to match the architecture and mechanical requirements of the native AF have employed electrospinning techniques, in which polymer solutions are drawn through a voltage gradient, forming jets that are collected layer-by-layer on a grounded target surface to create nanofiber meshes with fiber diameters in the range of 200–500 nm (170–172). The fiber alignment may be controlled by collecting samples onto a rotating mandrel. Electrospun polycarbonate polyurethane (PU) nanofiber meshes were fabricated in a unidirectional alignment and surface-modified with an anionic oligomer (Figure 6D) which allowed for improved AF cell attachment and collagen accumulation (171). Acellular PU scaffolds exhibited an initial tensile modulus of 46 MPa and a tensile strength of 14 MPa, which decreased over 4 weeks due to polymer degradation (172). Similar values were reported for aligned nanofibrous poly(-caprolactone) (PCL) scaffolds seeded with bovine AF cells (170). AF cells oriented along the nanofiber direction and secreted matrix that resulted in an increase in tensile modulus from ~20 MPa to ~50MPa after 8 weeks. A follow-up study utilized electrospinning to engineer elegant multi-lamellar materials which recapitulated the ± 30° angle-ply collagen architecture of the native AF in opposing bilayer constructs (173) (Figure 6E). Bovine marrow-derived mesenchymal stromal cells (MSCs) were seeded on the scaffolds for 10 weeks and the uniaxial tensile modulus of bilayer constructs with opposing fiber orientation reached ~14.5 MPa, close to the human AF (51, 157, 158) and greater than that for scaffolds with parallel bilayer fiber directionality (~10 MPa). The findings also indicated that inter-lamellar shearing may be responsible for the improved tensile mechanics of the laminates.
The majority of research efforts focused on the development of scaffolds for AF replacement have been able to demonstrate sufficient cell adhesion, proliferation and ECM production, while a few studies have successfully achieved the multi-scale hierarchical structure and tensile properties comparable to the natural tissue. Nevertheless, the compressive and shear properties have been largely ignored as have in vivo evaluation of the materials. In addition, it may be difficult to implement a stand-alone AF repair strategy without accounting for integration with other structures in the disc (i.e., NP) or joint (i.e., cartilage endplate). Thus, it may be necessary to rely on multi-component biomaterials for whole IVD replacement in severe cases of disc degeneration.
e. Whole IVD replacement
AF and other IVD materials can mimic natural forms which can further our understanding of IVD mechanics (173, 174). Additional challenges in the integration of IVD structures to vertebrae (16) exist, and lessons from development can help drive how biomaterials are made and incorporated into the IVD for improved understanding of degeneration processes and repair opportunities (175, 176). The complex interactions of many cell types is an essential process driving the process of development from the notochord to IVD and vertebral patterning (177). The incorporation of multiple cell types and materials with disparate structural and functional properties into a single implantable construct presents significant challenges to engineering a composite IVD replacement. However, a few groups have attempted to engineer whole IVD equivalents using various combinations of cells and scaffold materials. The first reported strategy involved the assembly of a cylindrical disc comprised of an alginate core with ovine NP cells that was surrounded by a confining PGA/PLA mesh seeded with AF cells (178, 179) (Figure 6F). The constructs were implanted subcutaneously in athymic nude mice over 16 weeks. Progressive matrix elaboration was detected over time, although the GAG and collagen content were lower than native values, but regions of distinct tissue formation were evident. The increased ECM production gave rise to improved functional properties, as the equilibrium compressive modulus increased from ~10 kPa at 0 weeks to ~49 kPa at 16 weeks. Using identical testing methods, the compressive modulus of native ovine IVD tissue was measured to be ~65 kPa for the AF and ~41 kPa for the NP. Although the PGA/PLA mesh component of the construct did not approximate the fiber orientation or scale of the natural tissue, which may have contributed to the inability to match native IVD benchmarks, this work represents a significant advance in IVD tissue engineering.
Most recently, multi-lamellar IVD analogs that mimic the structural angle-ply organization of the AF were fabricated using nanofibrous PCL scaffolds with alternating ±30° fiber angles and a central agarose hydrogel core (180) (Figure 6G–H). Bovine MSCs were incorporated into both regions of the constructs, which were evaluated during 6 weeks of in vitro culture. Cells seeded in both the AF and NP regions adopted distinct morphologies that mirror those observed in native tissue, and the matrix deposited in the AF region was organized into an angle-ply configuration. After 6 weeks, the equilibrium Young’s modulus increased over 2-fold to 45 kPa. Despite these promising results, long-term compressive and shear properties need to be assessed and diffusional limitations may be difficult to overcome when designing clinically relevant sizes and geometries of such IVD replacements. In addition, insertion and stable integration of whole disc equivalents in the joint space presents a significant hurdle for clinical translation.
The first study to evaluate engineered composite IVDs in an animal model was described by Bowles et al. using an aligned collagen gel surrounding a central alginate NP analog in the rat caudal spine (181). The engineering constructs maintained disc height (83 ±13% at 6 months) comparable to reimplanted native discs in the caudal disc space. In addition, mechanical testing of intact motion segments measured an equilibrium modulus (235 ± 51 kPa) and hydaulic permeability (1.3 ± 0.3 × 10−14 m2/Pa•s) that were not significantly different from native motion segments. Although these findings are quite promising, the size of the rat disc and the loads experienced in the rat caudal disc space are quite different from those of the human lumbar and cervical spine. Moreover, only the axial compressive properties were determined in this study. Torsional and shear properties would be important for evaluating whether such composites are capable of restoring the full range of movement in the spine.
f. Implantation and evaluation of effective repair strategies
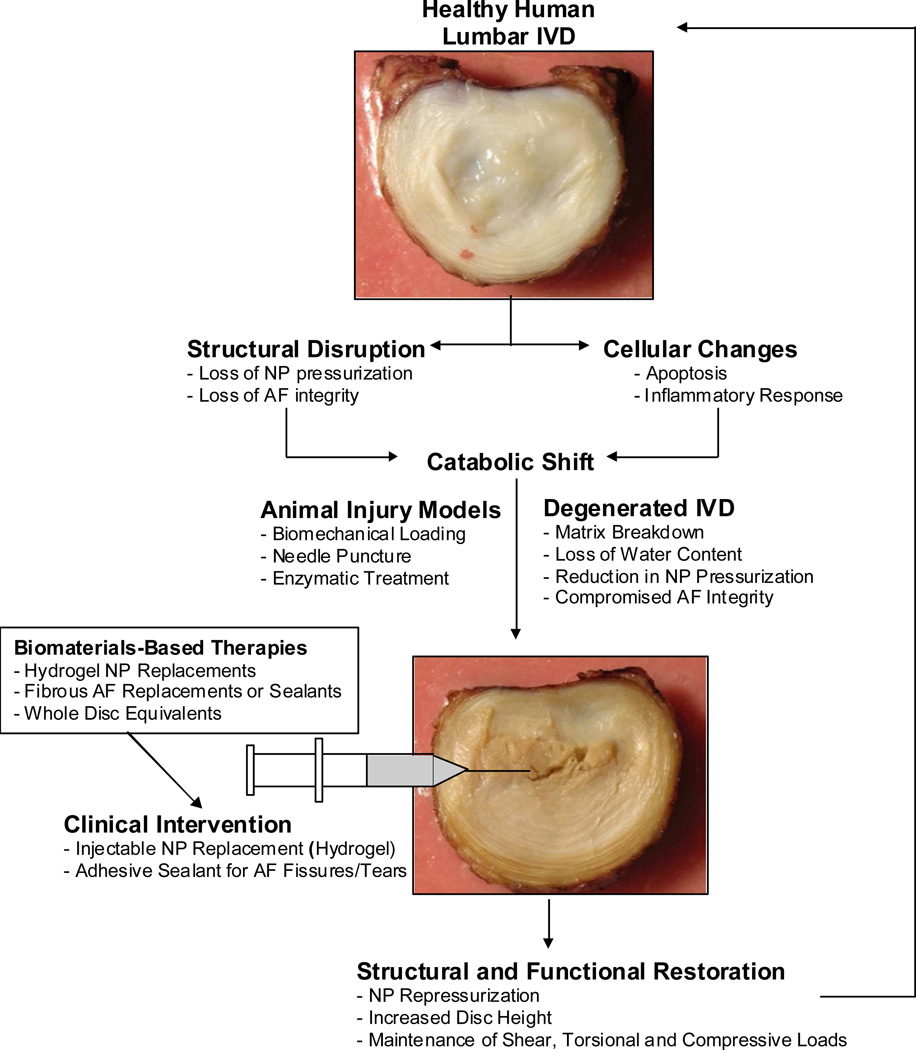
Injuries and degeneration of the IVD involve many structural and cellular changes that lead to increases in proinflammatory cytokines, a catabolic shift in IVD metabolism, and progress towards a cascade of degeneration. The IVD does not effectively repair without intervention, and consequently biomaterials can be used for both structural and functional restoration (Figure 7). Ideally, an intervention will be early and implemented via a minimally invasive procedure by injection or a small incision. Given the difficulties associated with implanting whole IVD replacements in the spinal column, the use of injectable NP hydrogel materials in combination with AF sealants may represent a more near-term treatment strategy for restoring NP pressurization and AF integrity. Such a biomaterial intervention has the capacity to restore structure and function.
Figure 7.
General strategy for repair of injured and degenerated IVDs using biomaterials to restore structure and function.
The degenerative cascade found in injured and degenerated human IVDs can be simulated in different model systems. There are many reviews on important animal models for evaluating intervertebral disc repair strategies (2, 182–186). The vastly different sizes and cellularity of different animal models of the IVD creates differences in nutritional pathways, biomechanics, and metabolism from the human condition. Small and large animal models are helpful for assessment of varying aspects of biomechanics and biocompatibility of biomaterials. The harsh physicochemical environment of human IVDs (16, 187) and biomechanical demands of the human motivate an important role for use of large animal models to evaluate feasibility and efficacy of biomaterials and tissue engineering approaches. Human IVDs have a fibrous NP, lose notochordal phenotype at very young ages, and develop fissures and structural defects with advancing degeneration. Bovine caudal IVDs are a readily available large animal IVD with a fibrous NP, and structure, composition, and metabolic rates that are similar to human lumbar IVDs (188). Porcine IVDs are another readily available large animal IVD with many biologic similarities to humans, although their retention of notochordal cells until an advanced age (189) suggests that porcine IVDs are likely to heal more readily than human IVDs. While biomechanics of bovine caudal IVDs and lumbar IVDS of other large animal species differs from that of human IVDs (77, 190), evaluations of biomaterials in large animal IVDs can inform how effectively biomaterials can restore NP pressurization and AF integrity, and can be used to evaluate performance under fatigue loading conditions. Animal models are important for biomechanical evaluations because of their greater availability as compared to intact human IVDs.
A pathway for development and evaluation of regenerative medicine therapies for IVD treatments, that closely follows the pre-market approval process of the Food and Drug Administration, was described that involves establishing efficacy and safety of regenerative treatments using models of increasing complexity, starting with cell culture, small animals, and eventually clinical trials (191). Appropriate evaluation of biomaterials should follow a similar pathway spanning from in vitro testing to clinical trials. In vitro biomechanics and cell culture studies are important for rapid screening of multiple conditions in order to optimize and evaluate biomaterial formulations. Certain human cell specific questions can be answered with human cell culture studies. The high ethical and economic costs of large animal studies has also promoted the development of many organ culture systems that offer promise for screening the feasibility of biomaterials for implantation into IVDs of large animals (103, 192, 193). Such organ culture models can be used to evaluate efficacy of biomaterial and tissue engineering approaches to treat disc degeneration, such as the implantation of biomaterials alone or cell-seeded scaffolds (182). Organ culture has the added benefit that it can analyze interacting factors such as biomechanical loading and nutrition (192). Such organ culture models may be an important intermediary step between cell culture and live animal testing as they provide a living ‘ex vivo’ or ‘in situ’ environment that can reduce the number of live animal studies should the material prove ineffectual (182). The use of large animal and human IVDs in organ culture may also provide a quicker transition to treating the human condition. In vivo testing is required for biocompatibility, efficacy and safety studies, as well as pain mitigation studies. While screening studies can be performed on small animal models, large animal studies and eventual clinical trials must be the final steps to evaluate the most promising biomaterial repairs.
In vivo, ex vivo, and in vitro experiments will all be important to screen and evaluate these newly developed biomaterials for compatibility, function and integration with neighboring tissues. Models of increasing complexity allow these promising biomaterials to be evaluated for biological and biomechanical compatibility and efficacy. For motion segment mechanics, we highlight the sensitivity of low force axial behaviors and torsional behaviors to assess injury and extent of repair of the AF and NP, and recommend using axial and torsional loading as a starting place for evaluating biomechanical injuries and repairs on the whole IVD level.
g. Conclusions 2: Biomaterials for repair of the IVD
Biomaterials offer much promise for repair of the nucleus pulposus, annulus fibrosus and whole intervertebral disc.
Biomechanical compatibility with native IVD tissues, structural organization, and compositional measurements are all important for assessing potential of biomaterials for IVD repair.
Synthetic and natural biomaterials offer promise for NP replacement, as an AF sealant, for AF repair, or for whole IVD replacement.
Several biomaterials for NP replacement are able to match unconfined compression and shear moduli of native NP tissue, yet further biomaterial refinement will require evaluation of and matching of confined compression and swelling pressure behaviors.
Biomaterials for AF replacement have demonstrated sufficient cell adhesion, proliferation and matrix production. While fibrous AF replacements show promise, they are only able to partially recapitulate the multi-scale hierarchical structure and tensile properties comparable to the natural tissue.
Challenges integrating biomaterials with native IVD tissues have led to development of whole composite IVD replacement structures as a single implantable construct that may more easily address tissue integration.
Difficulties associated with insertion and integration of whole IVD equivalents in the joint space may require the use of injectable NP hydrogel replacements in combination with AF sealants as a more near-term treatment strategy for restoring NP pressurization and AF integrity.
Evaluation of developing biomaterials will require screening with models of increasing complexity, including in vitro testing, small animal models, organ culture and in vivo testing of large animal IVDs, and eventual clinical trials.
Acknowledgements
This work was made possible by funding from the NIH (R01AR051146, R01AR057397 & HL007944), and AO Foundation (JCI & SN). We gratefully acknowledge technical assistance from Drs. Casey Korecki and Marc Levenston for providing immunohistochemistry in Figure 1. We also gratefully acknowledge Dr. Devina Purmessur for providing data on PBS injection into IVD organ culture models in Figure 2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31(18):2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 2.Lotz JC, Ulrich JA. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J Bone Joint Surg Am. 2006;88(Suppl 2):76–82. doi: 10.2106/JBJS.E.01448. [DOI] [PubMed] [Google Scholar]
- 3.Mok FP, Samartzis D, Karppinen J, Luk KD, Fong DY, Cheung KM. ISSLS prize winner: Prevalence, determinants, and association of Schmorl nodes of the lumbar spine with disc degeneration: a population-based study of 2449 individuals. Spine (Phila Pa 1976) 2010;35(21):1944–1952. doi: 10.1097/BRS.0b013e3181d534f3. [DOI] [PubMed] [Google Scholar]
- 4.Williams FM, Manek NJ, Sambrook PN, Spector TD, Macgregor AJ. Schmorl's nodes: common, highly heritable, and related to lumbar disc disease. Arthritis Rheum. 2007;57(5):855–860. doi: 10.1002/art.22789. [DOI] [PubMed] [Google Scholar]
- 5.Johannessen W, Auerbach JD, Wheaton AJ, et al. Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine (Phila Pa 1976) 2006;31(11):1253–1257. doi: 10.1097/01.brs.0000217708.54880.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mwale F, Iatridis JC, Antoniou J. Quantitative MRI as a diagnostic tool of intervertebral disc matrix composition and integrity. Eur Spine J. 2008;17(Suppl 4):432–440. doi: 10.1007/s00586-008-0744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajasekaran S, Venkatadass K, Naresh Babu J, Ganesh K, Shetty AP. Pharmacological enhancement of disc diffusion and differentiation of healthy, ageing and degenerated discs : Results from in-vivo serial post-contrast MRI studies in 365 human lumbar discs. Eur Spine J. 2008;17(5):626–643. doi: 10.1007/s00586-008-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Videman T, Gibbons LE, Battie MC. Age- and pathology-specific measures of disc degeneration. Spine (Phila Pa 1976) 2008;33(25):2781–2788. doi: 10.1097/BRS.0b013e31817e1d11. [DOI] [PubMed] [Google Scholar]
- 9.Wilson DC, Niosi CA, Zhu QA, Oxland TR, Wilson DR. Accuracy and repeatability of a new method for measuring facet loads in the lumbar spine. J Biomech. 2006;39(2):348–353. doi: 10.1016/j.jbiomech.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Pearson A, Lurie J, Tosteson T, et al. Who should have surgery for an intervertebral disc herniation? Comparative effectiveness evidence from the spine patient outcomes research trial. Spine (Phila Pa 1976) 2012;37(2):140–149. doi: 10.1097/BRS.0b013e3182276b2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am. 2009;91(6):1295–1304. doi: 10.2106/JBJS.H.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT) Spine (Phila Pa 1976) 2008;33(25):2789–2800. doi: 10.1097/BRS.0b013e31818ed8f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KT, Park SW, Kim YB. Disc height and segmental motion as risk factors for recurrent lumbar disc herniation. Spine (Phila Pa 1976) 2009;34(24):2674–2678. doi: 10.1097/BRS.0b013e3181b4aaac. [DOI] [PubMed] [Google Scholar]
- 14.McGirt MJ, Eustacchio S, Varga P, et al. A prospective cohort study of close interval computed tomography and magnetic resonance imaging after primary lumbar discectomy: factors associated with recurrent disc herniation and disc height loss. Spine (Phila Pa 1976) 2009;34(19):2044–2051. doi: 10.1097/BRS.0b013e3181b34a9a. [DOI] [PubMed] [Google Scholar]
- 15.O'Connell GD, Malhotra NR, Vresilovic EJ, Elliott DM. The Effect of Discectomy and the Dependence on Degeneration of Human Intervertebral Disc Strain in Axial Compression. Spine (Phila Pa 1976) 2011;36(21):1765–1771. doi: 10.1097/BRS.0b013e318216752f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kandel R, Roberts S, Urban JP. Tissue engineering and the intervertebral disc: the challenges. Eur Spine J. 2008;17(Suppl 4):480–491. doi: 10.1007/s00586-008-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yong-Hing K, Kirkaldy-Willis WH. The pathophysiology of degenerative disease of the lumbar spine. Orthop Clin North Am. 1983;14(3):491–504. [PubMed] [Google Scholar]
- 18.Stokes IA, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine. 2004;29(23):2724–2732. doi: 10.1097/01.brs.0000146049.52152.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuisma M, Karppinen J, Haapea M, et al. Are the determinants of vertebral endplate changes and severe disc degeneration in the lumbar spine the same? A magnetic resonance imaging study in middle-aged male workers. BMC Musculoskelet Disord. 2008;9:51. doi: 10.1186/1471-2474-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iatridis JC, MacLean JJ, Roughley PJ, Alini M. Effects of mechanical loading on intervertebral disc metabolism in vivo. J Bone Joint Surg Am. 2006;88(Suppl 2):41–46. doi: 10.2106/JBJS.E.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23(23):2493–2506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 22.Wuertz K, Godburn K, MacLean JJ, et al. In vivo remodeling of intervertebral discs in response to short- and long-term dynamic compression. J Orthop Res. 2009;27(9):1235–1242. doi: 10.1002/jor.20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battie MC, Videman T, Kaprio J, et al. The Twin Spine Study: contributions to a changing view of disc degeneration. Spine J. 2009;9(1):47–59. doi: 10.1016/j.spinee.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Cheung KM, Samartzis D, Karppinen J, et al. Intervertebral disc degeneration: new insights based on "skipped" level disc pathology. Arthritis Rheum. 2010;62(8):2392–2400. doi: 10.1002/art.27523. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara A, Lim TH, An HS, et al. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine (Phila Pa 1976) 2000;25(23):3036–3044. doi: 10.1097/00007632-200012010-00011. [DOI] [PubMed] [Google Scholar]
- 26.Niosi CA, Oxland TR. Degenerative mechanics of the lumbar spine. Spine J. 2004;4(6 Suppl):202S–208S. doi: 10.1016/j.spinee.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Roughley PJ, Mort JS. Identification of human intervertebral disc stromelysin and its involvement in matrix degradation. J Orthop Res. 1991;9(4):568–575. doi: 10.1002/jor.1100090413. [DOI] [PubMed] [Google Scholar]
- 28.Pockert AJ, Richardson SM, Le Maitre CL, et al. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009;60(2):482–491. doi: 10.1002/art.24291. [DOI] [PubMed] [Google Scholar]
- 29.Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98(4):996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohshima H, Tsuji H, Hirano N, Ishihara H, Katoh Y, Yamada H. Water diffusion pathway, swelling pressure, and biomechanical properties of the intervertebral disc during compression load. Spine (Phila Pa 1976) 1989;14(11):1234–1244. doi: 10.1097/00007632-198911000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Iatridis JC, ap Gwynn I. Mechanisms for mechanical damage in the intervertebral disc annulus fibrosus. J Biomech. 2004;37(8):1165–1175. doi: 10.1016/j.jbiomech.2003.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The Burden of Musculoskeletal Diseases in the United States. Rosemont, IL: Bone and Joint Decade: American Academy of Orthopaedic Surgeons; 2008. Available from: http://www.boneandjointburden.org/ [Google Scholar]
- 33.Jensen MC, Kelly AP, Brant-Zawadzki MN. MRI of degenerative disease of the lumbar spine. Magn Reson Q. 1994;10(3):173–190. [PubMed] [Google Scholar]
- 34.Panjabi MM. Clinical spinal instability and low back pain. J Electromyogr Kinesiol. 2003;13(4):371–379. doi: 10.1016/s1050-6411(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 35.Naderi S, Andalkar N, Benzel EC. History of spine biomechanics: part II--from the Renaissance to the 20th century. Neurosurgery. 2007;60(2):392–403. doi: 10.1227/01.NEU.0000249263.80579.F9. discussion-4. [DOI] [PubMed] [Google Scholar]
- 36.Naderi S, Andalkar N, Benzel EC. History of spine biomechanics: part I--the pre-Greco-Roman, Greco- Roman, and medieval roots of spine biomechanics. Neurosurgery. 2007;60(2):382–390. doi: 10.1227/01.NEU.0000249276.94933.8D. discussion 90-1. [DOI] [PubMed] [Google Scholar]
- 37.Ferguson SJ, Steffen T. Biomechanics of the aging spine. Eur Spine J. 2003;12(Suppl 2):S97–S103. doi: 10.1007/s00586-003-0621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grad S, Alini M, Eglin D, et al. Cells and biomaterials for intervertebral disc regeneration. In: Athanasiou KA, editor. Synthesis lectures on tissue engineering. Morgan & Claypool: 2010. http://www.morganclaypool.com/toc/tis/1/1#lecturesAvailableOnline. [Google Scholar]
- 39.Hsieh AH, Hwang D, Ryan DA, Freeman AK, Kim H. Degenerative anular changes induced by puncture are associated with insufficiency of disc biomechanical function. Spine. 2009;34(10):998–1005. doi: 10.1097/BRS.0b013e31819c09c4. [DOI] [PubMed] [Google Scholar]
- 40.Iatridis JC, Michalek AJ, Purmessur D, Korecki CL. Localized Intervertebral Disc Injury Leads to Organ Level Changes in Structure, Cellularity, and Biosynthesis. Cell Mol Bioeng. 2009;2(3):437–447. doi: 10.1007/s12195-009-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nerurkar NL, Elliott DM, Mauck RL. Mechanical design criteria for intervertebral disc tissue engineering. J Biomech. 2010;43(6):1017–1030. doi: 10.1016/j.jbiomech.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 43.Iatridis JC, Laible JP, Krag MH. Influence of fixed charge density magnitude and distribution on the intervertebral disc: applications of a poroelastic and chemical electric (PEACE) model. J Biomech Eng. 2003;125(1):12–24. doi: 10.1115/1.1537190. [DOI] [PubMed] [Google Scholar]
- 44.Iatridis JC, MacLean JJ, O'Brien M, Stokes IA. Measurements of proteoglycan and water content distribution in human lumbar intervertebral discs. Spine (Phila Pa 1976) 2007;32(14):1493–1497. doi: 10.1097/BRS.0b013e318067dd3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iatridis JC, Setton LA, Weidenbaum M, Mow VC. Alterations in the mechanical behavior of the human lumbar nucleus pulposus with degeneration and aging. J Orthop Res. 1997;15(2):318–322. doi: 10.1002/jor.1100150224. [DOI] [PubMed] [Google Scholar]
- 46.Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine (Phila Pa 1976) 1988;13(2):179–187. doi: 10.1097/00007632-198802000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Yao H, Justiz MA, Flagler D, Gu WY. Effects of swelling pressure and hydraulic permeability on dynamic compressive behavior of lumbar annulus fibrosus. Ann Biomed Eng. 2002;30(10):1234–1241. doi: 10.1114/1.1523920. [DOI] [PubMed] [Google Scholar]
- 48.Brickley-Parsons D, Glimcher MJ. Is the chemistry of collagen in intervertebral discs an expression of Wolff's Law? A study of the human lumbar spine. Spine. 1984;9(2):148–163. doi: 10.1097/00007632-198403000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect Tissue Res. 1989;23(1):75–88. doi: 10.3109/03008208909103905. [DOI] [PubMed] [Google Scholar]
- 50.Acaroglu ER, Iatridis JC, Setton LA, Foster RJ, Mow VC, Weidenbaum M. Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine. 1995;20(24):2690–2701. doi: 10.1097/00007632-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 51.Elliott DM, Setton LA. Anisotropic and inhomogeneous tensile behavior of the human anulus fibrosus: experimental measurement and material model predictions. J Biomech Eng. 2001;123(3):256–263. doi: 10.1115/1.1374202. [DOI] [PubMed] [Google Scholar]
- 52.Gu WY, Mao XG, Rawlins BA, et al. Streaming potential of human lumbar anulus fibrosus is anisotropic and affected by disc degeneration. J Biomech. 1999;32(11):1177–1182. doi: 10.1016/s0021-9290(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 53.Iatridis JC, Setton LA, Foster RJ, Rawlins BA, Weidenbaum M, Mow VC. Degeneration affects the anisotropic and nonlinear behaviors of human anulus fibrosus in compression. J Biomech. 1998;31(6):535–544. doi: 10.1016/s0021-9290(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 54.Schollum ML, Robertson PA, Broom ND. ISSLS prize winner: microstructure and mechanical disruption of the lumbar disc annulus: part I: a microscopic investigation of the translamellar bridging network. Spine (Phila Pa 1976) 2008;33(25):2702–2710. doi: 10.1097/BRS.0b013e31817bb92c. [DOI] [PubMed] [Google Scholar]
- 55.Veres SP, Robertson PA, Broom ND. ISSLS prize winner: microstructure and mechanical disruption of the lumbar disc annulus: part II: how the annulus fails under hydrostatic pressure. Spine (Phila Pa 1976) 2008;33(25):2711–2720. doi: 10.1097/BRS.0b013e31817bb906. [DOI] [PubMed] [Google Scholar]
- 56.Bruehlmann SB, Matyas JR, Duncan NA. ISSLS prize winner: Collagen fibril sliding governs cell mechanics in the anulus fibrosus: an in situ confocal microscopy study of bovine discs. Spine (Phila Pa 1976) 2004;29(23):2612–2620. doi: 10.1097/01.brs.0000146465.05972.56. [DOI] [PubMed] [Google Scholar]
- 57.Michalek AJ, Buckley MR, Bonassar LJ, Cohen I, Iatridis JC. Measurement of local strains in intervertebral disc anulus fibrosus tissue under dynamic shear: contributions of matrix fiber orientation and elastin content. J Biomech. 2009;42(14):2279–2285. doi: 10.1016/j.jbiomech.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29(23):2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 59.Cao L, Guilak F, Setton LA. Three-dimensional morphology of the pericellular matrix of intervertebral disc cells in the rat. J Anat. 2007;211(4):444–452. doi: 10.1111/j.1469-7580.2007.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ayotte DC, Ito K, Tepic S. Direction-dependent resistance to flow in the endplate of the intervertebral disc: an ex vivo study. J Orthop Res. 2001;19(6):1073–1077. doi: 10.1016/S0736-0266(01)00038-9. [DOI] [PubMed] [Google Scholar]
- 61.Setton LA, Zhu W, Weidenbaum M, Ratcliffe A, Mow VC. Compressive properties of the cartilaginous end-plate of the baboon lumbar spine. J Orthop Res. 1993;11(2):228–239. doi: 10.1002/jor.1100110210. [DOI] [PubMed] [Google Scholar]
- 62.Costi JJ, Stokes IA, Gardner-Morse MG, Iatridis JC. Frequency-dependent behavior of the intervertebral disc in response to each of six degree of freedom dynamic loading: solid phase and fluid phase contributions. Spine. 2008;33(16):1731–1738. doi: 10.1097/BRS.0b013e31817bb116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gardner-Morse MG, Stokes IA. Physiological axial compressive preloads increase motion segment stiffness, linearity and hysteresis in all six degrees of freedom for small displacements about the neutral posture. J Orthop Res. 2003;21(3):547–552. doi: 10.1016/S0736-0266(02)00199-7. [DOI] [PubMed] [Google Scholar]
- 64.Smit TH, van Tunen MS, van der Veen AJ, Kingma I, van Dieen JH. Quantifying intervertebral disc mechanics: a new definition of the neutral zone. BMC Musculoskelet Disord. 2011;12:38. doi: 10.1186/1471-2474-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Niosi CA, Zhu QA, Wilson DC, Keynan O, Wilson DR, Oxland TR. Biomechanical characterization of the three-dimensional kinematic behaviour of the Dynesys dynamic stabilization system: an in vitro study. Eur Spine J. 2006;15(6):913–922. doi: 10.1007/s00586-005-0948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haughton VM, Rogers B, Meyerand ME, Resnick DK. Measuring the axial rotation of lumbar vertebrae in vivo with MR imaging. AJNR Am J Neuroradiol. 2002;23(7):1110–1116. [PMC free article] [PubMed] [Google Scholar]
- 67.Mimura M, Panjabi MM, Oxland TR, Crisco JJ, Yamamoto I, Vasavada A. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine (Phila Pa 1976) 1994;19(12):1371–1380. doi: 10.1097/00007632-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 68.Adams MA, McNally DS, Dolan P. 'Stress' distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br. 1996;78(6):965–972. doi: 10.1302/0301-620x78b6.1287. [DOI] [PubMed] [Google Scholar]
- 69.Zhao F, Pollintine P, Hole BD, Dolan P, Adams MA. Discogenic origins of spinal instability. Spine (Phila Pa 1976) 2005;30(23):2621–2630. doi: 10.1097/01.brs.0000188203.71182.c0. [DOI] [PubMed] [Google Scholar]
- 70.O'Connell GD, Vresilovic EJ, Elliott DM. Human intervertebral disc internal strain in compression: The effect of disc region, loading position, and degeneration. J Orthop Res. 2011;29(4):547–555. doi: 10.1002/jor.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cholewicki J, Crisco JJ, 3rd, Oxland TR, Yamamoto I, Panjabi M M. Effects of posture and structure on three-dimensional coupled rotations in the lumbar spine. A biomechanical analysis. Spine (Phila Pa 1976) 1996;21(21):2421–2428. doi: 10.1097/00007632-199611010-00003. [DOI] [PubMed] [Google Scholar]
- 72.Benneker LM, Heini PF, Anderson SE, Alini M, Ito K. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur Spine J. 2005;14(1):27–35. doi: 10.1007/s00586-004-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976) 2005;30(1):5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 74.Sobajima S, Shimer AL, Chadderdon RC, et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005;5(1):14–23. doi: 10.1016/j.spinee.2004.05.251. [DOI] [PubMed] [Google Scholar]
- 75.Elliott DM, Yerramalli CS, Beckstein JC, Boxberger JI, Johannessen W, Vresilovic EJ. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine. 2008;33(6):588–596. doi: 10.1097/BRS.0b013e318166e0a2. [DOI] [PubMed] [Google Scholar]
- 76.Michalek AJ, Funabashi KL, Iatridis JC. Needle puncture injury of the rat intervertebral disc affects torsional and compressive biomechanics differently. Eur Spine J. 2010;19(12):2110–2116. doi: 10.1007/s00586-010-1473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Michalek AJ, Iatridis JC. Height and torsional stiffness are most sensitive to annular injury in large animal intervertebral discs. Spine J. 2011 doi: 10.1016/j.spinee.2012.04.001. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borthakur A, Maurer PM, Fenty M, et al. T1rho MRI and Discography Pressure as Novel Biomarkers for Disc Degeneration and Low Back Pain. Spine (Phila Pa 1976) 2011 doi: 10.1097/BRS.0b013e31820287bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anderst WJ, Vaidya R, Tashman S. A technique to measure three-dimensional in vivo rotation of fused and adjacent lumbar vertebrae. Spine J. 2008;8(6):991–997. doi: 10.1016/j.spinee.2007.07.390. [DOI] [PubMed] [Google Scholar]
- 80.McDonald CP, Bachison CC, Chang V, Bartol SW, Bey MJ. Three -dimensional dynamic in vivo motion of the cervical spine: assessment of measurement accuracy and preliminary findings. Spine J. 2010;10(6):497–504. doi: 10.1016/j.spinee.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 81.Passias PG, Wang S, Kozanek M, et al. Segmental lumbar rotation in patients with discogenic low back pain during functional weight-bearing activities. J Bone Joint Surg Am. 2011;93(1):29–37. doi: 10.2106/JBJS.I.01348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamaguchi T, Baw WC, Inoue N, et al. Intervertebral Disc Height Measurement: Comparison of Two7 dimensional and Three-dimensional Methods. Trans Orthop Res Soc. 2011;36:0173. [Google Scholar]
- 83.Iatridis JC, Weidenbaum M, Setton LA, Mow VC. Is the nucleus pulposus a solid or a fluid? Mechanical behaviors of the nucleus pulposus of the human intervertebral disc. Spine (Phila Pa 1976) 1996;21(10):1174–1184. doi: 10.1097/00007632-199605150-00009. [DOI] [PubMed] [Google Scholar]
- 84.Johannessen W, Elliott DM. Effects of degeneration on the biphasic material properties of human nucleus pulposus in confined compression. Spine. 2005;30(24):E724–E729. doi: 10.1097/01.brs.0000192236.92867.15. [DOI] [PubMed] [Google Scholar]
- 85.McMillan DW, Garbutt G, Adams MA. Effect of sustained loading on the water content of intervertebral discs: implications for disc metabolism. Ann Rheum Dis. 1996;55(12):880–887. doi: 10.1136/ard.55.12.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perie D, Korda D, Iatridis JC. Confined compression experiments on bovine nucleus pulposus and annulus fibrosus: sensitivity of the experiment in the determination of compressive modulus and hydraulic permeability. J Biomech. 2005;38(11):2164–2171. doi: 10.1016/j.jbiomech.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 87.Perie DS, Maclean JJ, Owen JP, Iatridis JC. Correlating material properties with tissue composition in enzymatically digested bovine annulus fibrosus and nucleus pulposus tissue. Ann Biomed Eng. 2006;34(5):769–777. doi: 10.1007/s10439-006-9091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gu WY, Mao XG, Foster RJ, Weidenbaum M, Mow VC, Rawlins BA. The anisotropic hydraulic permeability of human lumbar anulus fibrosus. Influence of age, degeneration, direction, and water content. Spine (Phila Pa 1976) 1999;24(23):2449–2455. doi: 10.1097/00007632-199912010-00005. [DOI] [PubMed] [Google Scholar]
- 89.Barbir A, Michalek AJ, Abbott RD, Iatridis JC. Effects of enzymatic digestion on compressive properties of rat intervertebral discs. J Biomech. 2010;43(6):1067–1073. doi: 10.1016/j.jbiomech.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barbir A, Godburn KE, Michalek AJ, Lai A, Monsey RD, Iatridis JC. Effects of Torsion on Intervertebral Disc Gene Expression and Biomechanics, Using a Rat Tail Model. Spine (Phila Pa 1976) 2011 doi: 10.1097/BRS.0b013e3181d9b58b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boxberger JI, Orlansky AS, Sen S, Elliott DM. Reduced nucleus pulposus glycosaminoglycan content alters intervertebral disc dynamic viscoelastic mechanics. J Biomech. 2009;42(12):1941–1946. doi: 10.1016/j.jbiomech.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seroussi RE, Krag MH, Muller DL, Pope MH. Internal deformations of intact and denucleated human lumbar discs subjected to compression, flexion, and extension loads. J Orthop Res. 1989;7(1):122–131. doi: 10.1002/jor.1100070117. [DOI] [PubMed] [Google Scholar]
- 93.Frei H, Oxland TR, Rathonyi GC, Nolte LP. The effect of nucleotomy on lumbar spine mechanics in compression and shear loading. Spine (Phila Pa 1976) 2001;26(19):2080–2089. doi: 10.1097/00007632-200110010-00007. [DOI] [PubMed] [Google Scholar]
- 94.Kang JD. Does a needle puncture into the annulus fibrosus cause disc degeneration? Spine J. 2010;10(12):1106–1107. doi: 10.1016/j.spinee.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 95.Carragee EJ, Don AS, Hurwitz EL, Cuellar JM, Carrino JA, Herzog R. 2009 ISSLS Prize Winner: Does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine (Phila Pa 1976) 2009;34(21):2338–2345. doi: 10.1097/BRS.0b013e3181ab5432. [DOI] [PubMed] [Google Scholar]
- 96.Nassr A, Lee JY, Bashir RS, et al. Does incorrect level needle localization during anterior cervical discectomy and fusion lead to accelerated disc degeneration? Spine (Phila Pa 1976) 2009;34(2):189–192. doi: 10.1097/BRS.0b013e3181913872. [DOI] [PubMed] [Google Scholar]
- 97.Korecki CL, Costi JJ, Iatridis JC. Needle puncture injury affects intervertebral disc mechanics and biology in an organ culture model. Spine. 2008;33(3):235–241. doi: 10.1097/BRS.0b013e3181624504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Michalek AJ, Buckley MR, Bonassar LJ, Cohen I, Iatridis JC. The effects of needle puncture injury on microscale shear strain in the intervertebral disc annulus fibrosus. Spine J. 2010;10(12):1098–1105. doi: 10.1016/j.spinee.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fazzalari NL, Costi JJ, Hearn TC, et al. Mechanical and pathologic consequences of induced concentric anular tears in an ovine model. Spine. 2001;26(23):2575–2581. doi: 10.1097/00007632-200112010-00010. [DOI] [PubMed] [Google Scholar]
- 100.Veres SP, Robertson PA, Broom ND. ISSLS prize winner: how loading rate influences disc failure mechanics: a microstructural assessment of internal disruption. Spine (Phila Pa 1976) 2010;35(21):1897–1908. doi: 10.1097/BRS.0b013e3181d9b69e. [DOI] [PubMed] [Google Scholar]
- 101.Lotz JC. Animal models of intervertebral disc degeneration: lessons learned. Spine (Phila Pa 1976) 2004;29(23):2742–2750. doi: 10.1097/01.brs.0000146498.04628.f9. [DOI] [PubMed] [Google Scholar]
- 102.Wilke HJ, Rohlmann A, Neller S, Graichen F, Claes L, Bergmann G. ISSLS prize winner: A novel approach to determine trunk muscle forces during flexion and extension: a comparison of data from an in vitro experiment and in vivo measurements. Spine (Phila Pa 1976) 2003;28(23):2585–2593. doi: 10.1097/01.BRS.0000096673.16363.C7. [DOI] [PubMed] [Google Scholar]
- 103.Korecki CL, MacLean JJ, Iatridis JC. Dynamic compression effects on intervertebral disc mechanics and biology. Spine. 2008;33(13):1403–1409. doi: 10.1097/BRS.0b013e318175cae7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ulrich JA, Liebenberg EC, Thuillier DU, Lotz JC. ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine (Phila Pa 1976) 2007;32(25):2812–2819. doi: 10.1097/BRS.0b013e31815b9850. [DOI] [PubMed] [Google Scholar]
- 105.Frick SL, Hanley EN, Jr, Meyer RA, Jr, Ramp WK, Chapman TM. Lumbar intervertebral disc transfer. A canine study. Spine (Phila Pa 1976) 1994;19(16):1826–1834. doi: 10.1097/00007632-199408150-00006. discussion 34-5. [DOI] [PubMed] [Google Scholar]
- 106.Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine (Phila Pa 1976) 1988;13(3):375–377. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 107.Hedman TP, Kostuik JP, Fernie GR, Hellier WG. Design of an intervertebral disc prosthesis. Spine (Phila Pa 1976) 1991;16(6 Suppl):S256–S260. doi: 10.1097/00007632-199106001-00016. [DOI] [PubMed] [Google Scholar]
- 108.Lee CK, Langrana NA, Parsons JR, Zimmerman MC. Development of a prosthetic intervertebral disc. Spine (Phila Pa 1976) 1991;16(6 Suppl):S253–S255. doi: 10.1097/00007632-199106001-00015. [DOI] [PubMed] [Google Scholar]
- 109.Schmiedberg SK, Chang DH, Frondoza CG, Valdevit AD, Kostuik JP. Isolation and characterization of metallic wear debris from a dynamic intervertebral disc prosthesis. J Biomed Mater Res. 1994;28(11):1277–1288. doi: 10.1002/jbm.820281105. [DOI] [PubMed] [Google Scholar]
- 110.Punt IM, Visser VM, van Rhijn LW, et al. Complications and reoperations of the SB Charite lumbar disc prosthesis: experience in 75 patients. Eur Spine J. 2008;17(1):36–43. doi: 10.1007/s00586-007-0506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Ooij A, Oner FC, Verbout AJ. Complications of artificial disc replacement: a report of 27 patients with the SB Charite disc. J Spinal Disord Tech. 2003;16(4):369–383. doi: 10.1097/00024720-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 112.Luk KD, Ruan DK. Intervertebral disc transplantation: a biological approach to motion preservation. Eur Spine J. 2008;17(Suppl 4):504–510. doi: 10.1007/s00586-008-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vaccaro AR, Chiba K, Heller JG, et al. Bone grafting alternatives in spinal surgery. Spine J. 2002;2(3):206–215. doi: 10.1016/s1529-9430(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 114.Katsuura A, Hukuda S. Experimental study of intervertebral disc allografting in the dog. Spine (Phila Pa 1976) 1994;19(21):2426–2432. doi: 10.1097/00007632-199411000-00011. [DOI] [PubMed] [Google Scholar]
- 115.Luk KD, Ruan DK, Lu DS, Fei ZQ. Fresh frozen intervertebral disc allografting in a bipedal animal model. Spine (Phila Pa 1976) 2003;28(9):864–869. doi: 10.1097/01.BRS.0000058710.01729.29. discussion 70. [DOI] [PubMed] [Google Scholar]
- 116.Acosta FL, Jr, Lotz J, Ames CP. The potential role of mesenchymal stem cell therapy for intervertebral disc degeneration: a critical overview. Neurosurg Focus. 2005;19(3):E4. doi: 10.3171/foc.2005.19.3.5. [DOI] [PubMed] [Google Scholar]
- 117.O'Halloran DM, Pandit AS. Tissue-engineering approach to regenerating the intervertebral disc. Tissue Eng. 2007;13(8):1927–1954. doi: 10.1089/ten.2005.0608. [DOI] [PubMed] [Google Scholar]
- 118.Sakai D. Future perspectives of cell-based therapy for intervertebral disc disease. Eur Spine J. 2008;17(Suppl 4):452–458. doi: 10.1007/s00586-008-0743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carl A, Ledet E, Yuan H, Sharan A. New developments in nucleus pulposus replacement technology. Spine J. 2004;4(6 Suppl):325S–329S. doi: 10.1016/j.spinee.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 120.Coric D, Mummaneni PV. Nucleus replacement technologies. J Neurosurg Spine. 2008;8(2):115–120. doi: 10.3171/SPI/2008/8/2/115. [DOI] [PubMed] [Google Scholar]
- 121.Goins ML, Wimberley DW, Yuan PS, Fitzhenry LN, Vaccaro AR. Nucleus pulposus replacement: an emerging technology. Spine J. 2005;5(6 Suppl):317S–324S. doi: 10.1016/j.spinee.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 122.Risbud MV, Albert TJ, Guttapalli A, et al. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine. 2004;29(23):2627–2632. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 123.Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58–63. doi: 10.22203/ecm.v008a06. discussion-4. [DOI] [PubMed] [Google Scholar]
- 124.Cloyd JM, Malhotra NR, Weng L, Chen W, Mauck RL, Elliott DM. Material properties in unconfined compression of human nucleus pulposus, injectable hyaluronic acid-based hydrogels and tissue engineering scaffolds. Eur Spine J. 2007;16(11):1892–1898. doi: 10.1007/s00586-007-0443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Allen MJ, Schoonmaker JE, Bauer TW, Williams PF, Higham PA, Yuan HA. Preclinical evaluation of a poly (vinyl alcohol) hydrogel implant as a replacement for the nucleus pulposus. Spine (Phila Pa 1976) 2004;29(5):515–523. doi: 10.1097/01.brs.0000113871.67305.38. [DOI] [PubMed] [Google Scholar]
- 126.Richardson SM, Curran JM, Chen R, et al. The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-L-lactic acid (PLLA) scaffolds. Biomaterials. 2006;27(22):4069–4078. doi: 10.1016/j.biomaterials.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 127.Wang BH, Campbell G. Formulations of polyvinyl alcohol cryogel that mimic the biomechanical properties of soft tissues in the natural lumbar intervertebral disc. Spine (Phila Pa 1976) 2009;34(25):2745–2753. doi: 10.1097/BRS.0b013e3181b4abf5. [DOI] [PubMed] [Google Scholar]
- 128.Thomas JD, Fussell G, Sarkar S, Lowman AM, Marcolongo M. Synthesis and recovery characteristics of branched and grafted PNIPAAm-PEG hydrogels for the development of an injectable load-bearing nucleus pulposus replacement. Acta Biomater. 2010;6(4):1319–1328. doi: 10.1016/j.actbio.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 129.Vernengo J, Fussell GW, Smith NG, Lowman AM. Evaluation of novel injectable hydrogels for nucleus pulposus replacement. J Biomed Mater Res B Appl Biomater. 2008;84(1):64–69. doi: 10.1002/jbm.b.30844. [DOI] [PubMed] [Google Scholar]
- 130.Vernengo J, Fussell GW, Smith NG, Lowman AM. Synthesis and characterization of injectable bioadhesive hydrogels for nucleus pulposus replacement and repair of the damaged intervertebral disc. J Biomed Mater Res B Appl Biomater. 2010;93(2):309–317. doi: 10.1002/jbm.b.31547. [DOI] [PubMed] [Google Scholar]
- 131.Yang X, Li X. Nucleus pulposus tissue engineering: a brief review. Eur Spine J. 2009;18(11):1564–1572. doi: 10.1007/s00586-009-1092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chiba K, Andersson GB, Masuda K, Thonar EJ. Metabolism of the extracellular matrix formed by intervertebral disc cells cultured in alginate. Spine. 1997;22(24):2885–2893. doi: 10.1097/00007632-199712150-00011. [DOI] [PubMed] [Google Scholar]
- 133.Maldonado BA, Oegema TR., Jr Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992;10(5):677–690. doi: 10.1002/jor.1100100510. [DOI] [PubMed] [Google Scholar]
- 134.Melrose J, Smith S, Ghosh P, Taylor TK. Differential expression of proteoglycan epitopes and growth characteristics of intervertebral disc cells grown in alginate bead culture. Cells Tissues Organs. 2001;168(3):137–146. doi: 10.1159/000047829. [DOI] [PubMed] [Google Scholar]
- 135.Wang JY, Baer AE, Kraus VB, Setton LA. Intervertebral disc cells exhibit differences in gene expression in alginate and monolayer culture. Spine (Phila Pa 1976) 2001;26(16):1747–1751. doi: 10.1097/00007632-200108150-00003. discussion 52. [DOI] [PubMed] [Google Scholar]
- 136.Baer AE, Wang JY, Kraus VB, Setton LA. Collagen gene expression and mechanical properties of intervertebral disc cell-alginate cultures. J Orthop Res. 2001;19(1):2–10. doi: 10.1016/S0736-0266(00)00003-6. [DOI] [PubMed] [Google Scholar]
- 137.Chou AI, Akintoye SO, Nicoll SB. Photo-crosslinked alginate hydrogels support enhanced matrix accumulation by nucleus pulposus cells in vivo. Osteoarthritis Cartilage. 2009;17(10):1377–1384. doi: 10.1016/j.joca.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chou AI, Nicoll SB. Characterization of photocrosslinked alg inate hydrogels for nucleus pulposus cell encapsulation. J Biomed Mater Res A. 2009;91(1):187–194. doi: 10.1002/jbm.a.32191. [DOI] [PubMed] [Google Scholar]
- 139.Ogushi Y, Sakai S, Kawakami K. Synthesis of enzymatically-gellable carboxymethylcellulose for biomedical applications. J Biosci Bioeng. 2007;104(1):30–33. doi: 10.1263/jbb.104.30. [DOI] [PubMed] [Google Scholar]
- 140.Reza AT, Nicoll SB. Serum-free, chemically defined medium with TGF-beta(3) enhances functional properties of nucleus pulposus cell-laden carboxymethylcellulose hydrogel constructs. Biotechnol Bioeng. 2010;105(2):384–395. doi: 10.1002/bit.22545. [DOI] [PubMed] [Google Scholar]
- 141.Reza AT, Nicoll SB. Characterization of novel photocrosslinked carboxymethylcellulose hydrogels for encapsulation of nucleus pulposus cells. Acta Biomater. 2010;6(1):179–186. doi: 10.1016/j.actbio.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 142.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6(1):386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Erickson IE, Huang AH, Sengupta S, Kestle S, Burdick JA, Mauck RL. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage. 2009;17(12):1639–1648. doi: 10.1016/j.joca.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Su WY, Chen YC, Lin FH. Injectable oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for nucleus pulposus regeneration. Acta Biomater. 2010;6(8):3044–3055. doi: 10.1016/j.actbio.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 145.Alini M, Li W, Markovic P, Aebi M, Spiro RC, Roughley PJ. The potential and limitations of a cell18 seeded collagen/hyaluronan scaffold to engineer an intervertebral disc-like matrix. Spine (Phila Pa 1976) 2003;28(5):446–454. doi: 10.1097/01.BRS.0000048672.34459.31. discussion 53. [DOI] [PubMed] [Google Scholar]
- 146.Halloran DO, Grad S, Stoddart M, Dockery P, Alini M, Pandit AS. An injectable cross-linked scaffold for nucleus pulposus regeneration. Biomaterials. 2008;29(4):438–447. doi: 10.1016/j.biomaterials.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 147.Bron JL, Koenderink GH, Everts V, Smit TH. Rheological characterization of the nucleus pulposus and dense collagen scaffolds intended for functional replacement. J Orthop Res. 2009;27(5):620–626. doi: 10.1002/jor.20789. [DOI] [PubMed] [Google Scholar]
- 148.Wilke HJ, Heuer F, Neidlinger-Wilke C, Claes L. Is a collagen scaffold for a tissue engineered nucleus replacement capable of restoring disc height and stability in an animal model? Eur Spine J. 2006;15(Suppl 3):S433–S438. doi: 10.1007/s00586-006-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brinckmann P, Porter RW. A laboratory model of lumbar disc protrusion. Fissure and fragment. Spine. 1994;19(2):228–235. doi: 10.1097/00007632-199401001-00019. [DOI] [PubMed] [Google Scholar]
- 150.Bron JL, Helder MN, Meisel HJ, Van Royen BJ, Smit TH. Repair, regenerative and supportive therapies of the annulus fibrosus: achievements and challenges. Eur Spine J. 2009;18(3):301–313. doi: 10.1007/s00586-008-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ahlgren BD, Lui W, Herkowitz HN, Panjabi MM, Guiboux JP. Effect of anular repair on the healing strength of the intervertebral disc: a sheep model. Spine (Phila Pa 1976) 2000;25(17):2165–2170. doi: 10.1097/00007632-200009010-00004. [DOI] [PubMed] [Google Scholar]
- 152.Bron JL, van der Veen AJ, Helder MN, van Royen BJ, Smit TH. Biomechanical and in vivo evaluation of experimental closure devices of the annulus fibrosus designed for a goat nucleus replacement model. Eur Spine J. 2010;19(8):1347–1355. doi: 10.1007/s00586-010-1384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schek RM, Michalek AJ, Iatridis JC. Genipin-crosslinked fibrin hydrogels as a potential adhesive to augment intervertebral disc annulus repair. Eur Cell Mater. 2011;21:373–383. doi: 10.22203/ecm.v021a28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Heuer F, Ulrich S, Claes L, Wilke HJ. Biomechanical evaluation of conventional anulus fibrosus closure methods required for nucleus replacement. Laboratory investigation. J Neurosurg Spine. 2008;9(3):307–313. doi: 10.3171/SPI/2008/9/9/307. [DOI] [PubMed] [Google Scholar]
- 155.Holzapfel GA, Schulze-Bauer CA, Feigl G, Regitnig P. Single lamellar mechanics of the human lumbar anulus fibrosus. Biomech Model Mechanobiol. 2005;3(3):125–140. doi: 10.1007/s10237-004-0053-8. [DOI] [PubMed] [Google Scholar]
- 156.Skaggs DL, Weidenbaum M, Iatridis JC, Ratcliffe A, Mow VC. Regional variation in tensile properties and biochemical composition of the human lumbar anulus fibrosus. Spine (Phila Pa 1976) 1994;19(12):1310–1319. doi: 10.1097/00007632-199406000-00002. [DOI] [PubMed] [Google Scholar]
- 157.Ebara S, Iatridis JC, Setton LA, Foster RJ, Mow VC, Weidenbaum M. Tensile properties of nondegenerate human lumbar anulus fibrosus. Spine (Phila Pa 1976) 1996;21(4):452–461. doi: 10.1097/00007632-199602150-00009. [DOI] [PubMed] [Google Scholar]
- 158.Fujita Y, Duncan NA, Lotz JC. Radial tensile properties of the lumbar annulus fibrosus are site and degeneration dependent. J Orthop Res. 1997;15(6):814–819. doi: 10.1002/jor.1100150605. [DOI] [PubMed] [Google Scholar]
- 159.Green TP, Adams MA, Dolan P. Tensile properties of the annulus fibrosus II. Ultimate tensile strength and fatigue life. Eur Spine J. 1993;2(4):209–214. doi: 10.1007/BF00299448. [DOI] [PubMed] [Google Scholar]
- 160.Fujita Y, Wagner DR, Biviji AA, Duncan NA, Lotz JC. Anisotropic shear behavior of the annulus fibrosus: effect of harvest site and tissue prestrain. Med Eng Phys. 2000;22(5):349–357. doi: 10.1016/s1350-4533(00)00053-9. [DOI] [PubMed] [Google Scholar]
- 161.Iatridis JC, Kumar S, Foster RJ, Weidenbaum M, Mow VC. Shear mechanical properties of human lumbar annulus fibrosus. J Orthop Res. 1999;17(5):732–737. doi: 10.1002/jor.1100170517. [DOI] [PubMed] [Google Scholar]
- 162.Best BA, Guilak F, Setton LA, et al. Compressive mechanical properties of the human anulus fibrosus and their relationship to biochemical composition. Spine (Phila Pa 1976) 1994;19(2):212–221. doi: 10.1097/00007632-199401001-00017. [DOI] [PubMed] [Google Scholar]
- 163.Chang G, Kim HJ, Kaplan D, Vunjak-Novakovic G, Kandel RA. Porous silk scaffolds can be used for tissue engineering annulus fibrosus. Eur Spine J. 2007;16(11):1848–1857. doi: 10.1007/s00586-007-0364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Saad L, Spector M. Effects of collagen type on the behavior of adult canine annulus fibrosus cells in collagen-glycosaminoglycan scaffolds. J Biomed Mater Res A. 2004;71(2):233–241. doi: 10.1002/jbm.a.30150. [DOI] [PubMed] [Google Scholar]
- 165.Sato M, Asazuma T, Ishihara M, et al. An atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS-scaffold) for the culture of annulus fibrosus cells from an intervertebral disc. J Biomed Mater Res A. 2003;64(2):248–256. doi: 10.1002/jbm.a.10287. [DOI] [PubMed] [Google Scholar]
- 166.Wan Y, Feng G, Shen FH, Balian G, Laurencin CT, Li X. Novel biodegradable poly(1,8-octanediol malate) for annulus fibrosus regeneration. Macromol Biosci. 2007;7(11):1217–1224. doi: 10.1002/mabi.200700053. [DOI] [PubMed] [Google Scholar]
- 167.Wan Y, Feng G, Shen FH, Laurencin CT, Li X. Biphasic scaffold for annulus fibrosus tissue regeneration. Biomaterials. 2008;29(6):643–652. doi: 10.1016/j.biomaterials.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 168.Shao X, Hunter CJ. Developing an alginate/chitosan hybrid fiber scaffold for annulus fibrosus cells. J Biomed Mater Res A. 2007;82(3):701–710. doi: 10.1002/jbm.a.31030. [DOI] [PubMed] [Google Scholar]
- 169.Baer E, Cassidy JJ, Hiltner A. Hierarchical structure of collagen composite systems lessons from biology. In: Glasser WG, Hatakeyama H, editors. Viscoelasticity of Biomaterials: ACS Symposium Series. 1992. pp. 2–23. [Google Scholar]
- 170.Nerurkar NL, Mauck RL, Elliott DM. ISSLS prize winner: integrating theoretical and experimental methods for functional tissue engineering of the annulus fibrosus. Spine (Phila Pa 1976) 2008;33(25):2691–2701. doi: 10.1097/BRS.0b013e31818e61f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yang L, Kandel RA, Chang G, Santerre JP. Polar surface chemistry of nanofibrous polyurethane scaffold affects annulus fibrosus cell attachment and early matrix accumulation. J Biomed Mater Res A. 2009;91(4):1089–1099. doi: 10.1002/jbm.a.32331. [DOI] [PubMed] [Google Scholar]
- 172.Yeganegi M, Kandel RA, Santerre JP. Characterization of a biodegradable electrospun polyurethane nanofiber scaffold: Mechanical properties and cytotoxicity. Acta Biomater. 2010;6(10):3847–3855. doi: 10.1016/j.actbio.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 173.Nerurkar NL, Baker BM, Sen S, Wible EE, Elliott DM, Mauck RL. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat Mater. 2009;8(12):986–992. doi: 10.1038/nmat2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Iatridis JC. Tissue engineering: Function follows form. Nat Mater. 2009;8(12):923–924. doi: 10.1038/nmat2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Choi KS, Harfe BD. Hedgehog signaling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1007566108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Smith LJ, Nerurkar NL, Choi KS, Harfe BD, Elliott DM. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech. 4(1):31–41. doi: 10.1242/dmm.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Choi BH, Woo JI, Min BH, Park SR. Low-intensity ultrasound stimulates the viability and matrix gene expression of human articular chondrocytes in alginate bead culture. J Biomed Mater Res A. 2006;79(4):858–864. doi: 10.1002/jbm.a.30816. [DOI] [PubMed] [Google Scholar]
- 178.Mizuno H, Roy AK, Vacanti CA, Kojima K, Ueda M, Bonassar LJ. Tissue-engineered composites of anulus fibrosus and nucleus pulposus for intervertebral disc replacement. Spine (Phila Pa 1976) 2004;29(12):1290–1297. doi: 10.1097/01.brs.0000128264.46510.27. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 179.Mizuno H, Roy AK, Zaporojan V, Vacanti CA, Ueda M, Bonassar LJ. Biomechanical and biochemical characterization of composite tissue-engineered intervertebral discs. Biomaterials. 2006;27(3):362–370. doi: 10.1016/j.biomaterials.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 180.Nerurkar NL, Sen S, Huang AH, Elliott DM, Mauck RL. Engineered disc-like angle-ply structures for intervertebral disc replacement. Spine (Phila Pa 1976) 2010;35(8):867–873. doi: 10.1097/BRS.0b013e3181d74414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bowles RD, Gebhard HH, Hartl R, Bonassar LJ. Tissue-engineered intervertebral discs produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proc Natl Acad Sci U S A. 2011;108(32):13106–13111. doi: 10.1073/pnas.1107094108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17(1):2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.An HS, Masuda K. Relevance of in vitro and in vivo models for intervertebral disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):88–94. doi: 10.2106/JBJS.E.01272. [DOI] [PubMed] [Google Scholar]
- 184.Larson JW, 3rd, Levicoff EA, Gilbertson LG, Kang JD. Biologic modification of animal models of intervertebral disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):83–87. doi: 10.2106/JBJS.F.00043. [DOI] [PubMed] [Google Scholar]
- 185.Leung VY, Chan D, Cheung KM. Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future direction. Eur Spine J. 2006;15(Suppl 3):S406–S413. doi: 10.1007/s00586-006-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Masuda K, Oegema TR, Jr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. 2004;29(23):2757–2769. doi: 10.1097/01.brs.0000146048.14946.af. [DOI] [PubMed] [Google Scholar]
- 187.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29(23):2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 188.Demers CN, Antoniou J, Mwale F. Value and limitations of using the bovine tail as a model for the human lumbar spine. Spine. 2004;29(24):2793–2799. doi: 10.1097/01.brs.0000147744.74215.b0. [DOI] [PubMed] [Google Scholar]
- 189.Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205(5):357–362. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Beckstein JC, Sen S, Schaer TP, Vresilovic EJ, Elliott DM. Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine (Phila Pa 1976) 2008;33(6):E166–E173. doi: 10.1097/BRS.0b013e318166e001. [DOI] [PubMed] [Google Scholar]
- 191.Masuda K, Lotz JC. New challenges for intervertebral disc treatment using regenerative medicine. Tissue Eng Part B Rev. 2010;16(1):147–158. doi: 10.1089/ten.teb.2009.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Junger S, Gantenbein-Ritter B, Lezuo P, Alini M, Ferguson SJ, Ito K. Effect of limited nutrition on in situ intervertebral disc cells under simulated-physiological loading. Spine (Phila Pa 1976) 2009;34(12):1264–1271. doi: 10.1097/BRS.0b013e3181a0193d. [DOI] [PubMed] [Google Scholar]
- 193.Haglund LA, Moir J, Beckman L, et al. Development of a Bioreactor for Axially Loaded Intervertebral Disc Organ Culture. Tissue Eng Part C Methods. 2011;17(10):1011–1019. doi: 10.1089/ten.TEC.2011.0025. [DOI] [PubMed] [Google Scholar]