Abstract
The ER aminopeptidase associated with antigen processing, ERAAP (or ERAP1), is essential for trimming peptides that are presented by MHC class I molecules. ERAP1 is inhibited by human cytomegalovirus, and ERAP1 polymorphisms are associated with autoimmune diseases. How the immune system detects ERAAP dysfunction, however, is unknown. We have shown previously that ERAAP-deficient cells present an immunogenic pMHC I repertoire, that elicits CD8+ T cell response in WT mice. Additionally, we discovered that the WT CD8+ T cells recognized novel peptides presented by non-classical, or MHC class Ib, molecules on ERAAP-deficient cells. The MHC Ib restricted WT CD8 T cells eliminated ERAAP-deficient cells in vitro and in vivo. We identified the FL9 peptide, presented by Qa-1b, a MHC class Ib molecule exclusively on ERAAP-deficient cells. Remarkably, T cells specific for the FL9-Qa-1b complex were frequent in naïve WT mice, and had an antigen-experienced phenotype. Thus, novel non-classical pQa-1b complexes direct cytotoxic T cells to target cells with defective peptide processing in the endoplasmic reticulum. Here, we discuss the implications of our findings, and the possible roles of pMHC Ib-specific T cells in immune surveillance for ERAAP dysfunction.
Introduction
MHC class I molecules present peptides on the cell surface. These peptide-MHC I complexes, referred to as pMHC I, represent the cellular state at any given time. CD8 T cells and NK cells constantly monitor pMHC I complexes on the cell surface, and are alerted and activated by changes in the steady-state repertoire of surface pMHC I.
The peptides presented by MHC I molecules are generated by the concerted action of multiple cellular components, called the antigen processing pathway. Because of the importance of this pathway for immune surveillance, many components of the antigen processing pathway are targeted for inhibition in virally infected or transformed cells. As a counter measure, it is critical that the immune system detect defects in the antigen processing pathway. It is becoming increasingly clear that dysfunction of various cellular mediators of antigen processing results in the alteration of the cellular pMHC I repertoire. These dysfunction-induced changes in the pMHC I repertoire activate CD8 T cell, and NK cell responses, leading the to the elimination of cells with dysregulated antigen processing.
ERAAP (or ERAP1), the ER aminopeptidase associated with antigen processing, is an ER-resident aminopeptidase that is critical for trimming N-terminally extended precursors of peptides presented by MHC I. The loss of ERAAP function causes expression of novel, immunogenic pMHC I on the cell surface, a large fraction of which are longer than, and N-terminally extended compared to, their wild type counterparts (Hammer, Gonzalez et al. 2007). In addition, many peptides that were apparently destroyed by ERAAP are also presented in its absence (Hammer, Gonzalez et al. 2007). Alterations of ERAAP function are also associated with autoimmune disease as well as poor cancer prognosis, and ERAAP expression is downregulated by viral infection. Here we briefly summarize the discovery and implications of immune monitoring mechanisms for ERAAP function.
Current Status
After the discovery of ERAAP, we, and others, generated mice genetically deficient in ERAAP (ERAAP-KO) (Blanchard and Shastri 2008). Compared to their wild-type (WT) counterparts, ERAAP-deficient mice were found to express moderately lower levels of classical, or MHC class Ia, molecules on the cells surface. The pMHC I on the surface of ERAAP-KO cells were also less stable relative to WT cells. Certain endogenous antigens were poorly presented by ERAAP-KO cells, while presentation of other antigens was enhanced or unaffected, suggesting a selective effect on generation of pMHC I. While the overall number of CD8 T cells appeared normal in ERAAP-KO mice, the immunodominance hierarchy of certain cellular and viral antigens was greatly altered, again suggesting that pMHC I complexes have differential requirements for ERAAP function.
We also discovered that ERAAP-deficiency results in a dramatically altered and highly immunogenic pMHC I repertoire (Hammer, Gonzalez et al. 2007). WT mice immunized with ERAAP-KO cells mounted a robust immune response against ERAAP-KO cells, and vice versa. When we analyzed the WT anti-ERAAP-KO T cell response further, we found that a large fraction of these T cells responded to MHC Ia-deficient antigen presenting cells (APCs) (Nagarajan, Gonzalez et al. 2012), demonstrating that the immunogenic pMHC I presented by ERAAP-KO cells included peptides presented by non-classical, or MHC Ib molecules. We found that WT mice, previously primed with ERAAP-KO cells, rejected MHC Ia- and MHC Ib-expressing ERAAP-KO target cells (Nagarajan, Gonzalez et al. 2012). Thus, T cell mediated-immune surveillance for altered pMHC I complexes leads to the elimination of ERAAP-deficient cells.
We generated a pMHC Ib-specific T cell hybridoma—BEko8Z— from WT anti-ERAAP-KO T cell lines (Nagarajan, Gonzalez et al. 2012). BEko8Z recognized a peptide presented by the MHC class Ib molecule Qa-1b. We used expression cloning to identify the FYAEATPML (FL9) peptide derived from the Fam49b gene that was presented by the Qa-1 molecule to BEko8Z T cells. Interestingly, the nine residue long FL9 peptide was not detected in ERAAP+ WT cells, suggesting that the FL9 peptide was generated only in the absence of ERAAP.
Analysis of WT anti-ERAAP-KO T cell populations with the Qa-1-FL9 tetramer showed that CD8+tetramer+ T cells were present at a high frequency. Remarkably, Qa-1-FL9 tetramer-negative cells were also found to recognize ERAAP-KO APCs that expressed only MHC Ib molecules, suggesting that there might be additional pMHC Ib presented in the absence of ERAAP (Nagarajan, Gonzalez et al. 2012).
We isolated a distinct and abundant population of Qa1-FL9 specific T cells, referred to as QFL T cells in naïve WT mice. The majority of QFL T cells were antigen-experienced in naïve WT mice. Strikingly, QFL T cells did not require Qa-1 expression for their development, and did not express markers of antigen experience in mice lacking Qa-1. Also, unusual for pMHC Ib-specific T cells, QFL T cells formed a stable pool of memory T cells after exposure to ERAAP-deficient cells (Nagarajan, Gonzalez et al. 2012).
In the following section, we discuss the implications of these findings for mechanisms of antigen processing and immune surveillance
Future perspectives
Mechanisms of altered peptide presentation in ERAAP-KO cells
Why is the FL9 peptide presented by Qa-1 molecule only in the absence of ERAAP? One possibility is that the FL9 peptide is not available for presentation due to its destruction by ERAAP in WT cells. In the absence of ERAAP, FL9 is spared and thus available for presentation by Qa-1. The consensus motif for peptides presented by Qa-1 is presently unknown. It is therefore uncertain whether the FL9 peptide matches the Qa-1 binding motif and whether FL9 is particularly susceptible to destruction by ERAAP. It is also possible that the FL9 peptide represents the N-terminally extended precursor that is normally cleaved by ERAAP to generate a shorter product for presentation by Qa-1 in WT cells.
Regardless of the nature of proteolytic intermediates, the exclusive presentation of the FL9 peptide in ERAAP-deficient cells suggests that peptides may compete for loading MHC I in the ER. It may be that a large number of peptides require ERAAP for their generation, and are effectively absent in ERAAP-deficient cells. The loss of these peptides would reduce competition for PLC components and MHC I molecules, leading to the presentation of FL9-like peptides that would not have been presented in the presence of ERAAP. The competition model also suggests that to favor FL9 presentation, peptides presented by Qa-1 in WT cells would be reduced in the absence of ERAAP.
In addition to the presentation of the novel FL9 peptide by the Qa-1 MHC Ib molecule, it is clear that ERAAP-deficiency also alters the presentation of peptides by MHC Ia molecules. A fraction of CD8 T cells elicited by immunizing WT mice with ERAAP-deficient cells fails to respond to cells lacking MHC Ia molecules (Hammer, Gonzalez et al. 2007). Analysis of the peptides presented by MHC Ia molecules by mass spectrometry has shown that absence of ERAAP changes the overall composition of the peptide repertoire (Blanchard, Kanaseki et al. 2010). Novel peptides were detected in WT and ERAAP-KO cells, with the latter presenting peptides with a bias towards longer N-terminal extensions. Which of these peptides presented by MHC Ia molecules elicit reciprocal CD8 T cell responses is not known. Likewise, the relative role of MHC Ia versus MHC Ib restricted CD8 T cells in the recognition and elimination of ERAAP-deficient cells in vivo remains to be established.
The significance and function of anti - ERAAP-KO specific CD8+ T cells
The CD8+ T cells specific for the Qa-1-FL9 complex, and referred to as QFL T cells, have several unusual properties. In addition to their relative abundance, QFL T cells express markers of antigen experience in naïve WT mice. The expression of these markers is dependent on the presence of Qa-1 (Nagarajan, Gonzalez et al. 2012; Nagarajan, Gonzalez et al. 2012), and presumably exposure to a stimulatory peptide-Qa-1 complex. However, because the Qa-1-FL9 complex is expressed only when ERAAP in inhibited or absent, where and when do QFL T cells encounter a stimulatory pMHC Ib in naïve ERAAP-sufficient mice?
We discuss two intriguing possibilities here. One is that ERAAP function may be transiently inhibited in WT cells, perhaps due to transformation, the action of commensal microbes, or to localized inflammation. Human ERAP1 can be inhibited by the action of human cytomegalovirus (Kim, Lee et al. 2011), suggesting that chronic viral infection may also lead to reduced ERAAP function. This impairment of ERAAP function during “routine” organism function may lead the expression of the Qa-1-FL9 complex and the activation—or priming of QFL cells. This hypothesis is consistent with a role for these T cells in immune surveillance.
Another possibility is that these T cells develop by a unique pathway, which imprints them with certain characteristics as has been suggested for other MHC Ib-restricted T cell populations. Innate-like T cells, like invariant NKT or MAIT cells, are MHC Ib-restricted, and undergo a different program of thymic selection than conventional MHC Ia-specific T cells. However, the observation that QFL T cells develop in Qa-1-deficient mice, but do not have an antigen-experienced phenotype, distinguishes QFL T cells from these other MHC Ib-specific T cells. Another highly homologous MHC Ib molecule, perhaps in the closely related H2-T locus may be able to induce positive selection of QFL T cells, while recognition of the Qa-1-FL9 complex might be necessary for activation of these T cells post-development. The analysis of these pathways will advance our understanding of pMHC Ib-specific T cell development and selection.
Another open question is the function of QFL T cells. We have shown that these T cells detect and expand in response to a challenge with ERAAP-deficient cells. We also showed that the expression of the Qa-1-FL9 complex is sufficient for killing target cells. These observations suggest that QFL T cells are effectors of immune surveillance, patrolling for the presence of ERAAP-deficient cells. However, do these T cells also have regulatory functions, analogous to other Qa-1-restricted suppressor T cells? Do QFL T cells have a particular tissue-specificity, like the other innate-like T cells, which might correlate with their functions? Are there states of physiological dysregulation in which these cells may be induced?
Future applications of pMHC Ib-mediated immune surveillance
As discussed here, the MHC Ib molecules, such as Qa-1, play an important role in immune surveillance for defects in antigen processing. These findings are exciting because MHC Ib molecules are essentially non-polymorphic and highly conserved across species. Thus understanding the mechanisms that generate pMHC Ib and the effectors cells that recognize and respond to these ligands could lead to a universal pMHC Ib-mediated immune surveillance.
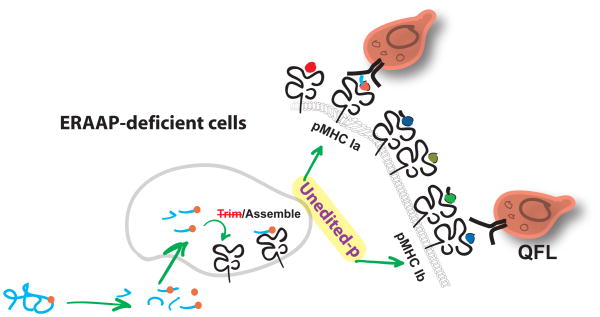
Figure 1.
Immune surveillance for ERAAP dysfunction Peptide trimming is impaired in cells that lack ERAAP. Altered peptide-MHC I complexes are exported to the surface of these cells, forming an immunogenic pMHC I repertoire. WT CD8 T cells detect novel pMHC I complexes on ERAAP-deficient cells, presented by both classical MHC class Ia, and non-classical MHC class Ib, molecules. WT mice naturally have a large number of T cells, called QFL T cells, specific to the Qa-1-FL9 complex, expressed only on ERAAP-deficient cells. QFL T cells, and other pMHC I-specific T cells participate in immune surveillance for ERAAP dysfunction.
Highlights.
Current state of knowledge on the molecular and immunological consequences of deficiency in ERAAP.
Future perspectives.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blanchard N, Kanaseki T, et al. Endoplasmic reticulum aminopeptidase associated with antigen processing defines the composition and structure of MHC class I peptide repertoire in normal and virus-infected cells. Journal of immunology. 2010;184(6):3033–3042. doi: 10.4049/jimmunol.0903712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard N, Shastri N. Coping with loss of perfection in the MHC class I peptide repertoire. Curr Opin Immunol. 2008;(20):82–88. doi: 10.1016/j.coi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer GE, Gonzalez F, et al. In the absence of aminopeptidase ERAAP, MHC class I molecules present many unstable and highly immunogenic peptides. Nature immunology. 2007;8(1):101–108. doi: 10.1038/ni1409. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee S, et al. Human cytomegalovirus microRNA miR-US4-1 inhibits CD8(+) T cell responses by targeting the aminopeptidase ERAP1. Nature Immunology. 2011;12(10):984–991. doi: 10.1038/ni.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan NA, Gonzalez F, et al. Nonclassical MHC class Ib-restricted cytotoxic T cells monitor antigen processing in the endoplasmic reticulum. Nature immunology. 2012;13(6):579–586. doi: 10.1038/ni.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]