Abstract
Dietary phosphorus consumption has risen steadily in the United States. Oral phosphorus loading alters key regulatory hormones and impairs vascular endothelial function which may lead to an increase in left ventricular mass (LVM). We investigated the association of dietary phosphorus with LVM in 4,494 participants from the Multi-Ethnic Study of Atherosclerosis, a community-based study of individuals free of known cardiovascular disease. The intake of dietary phosphorus was estimated using a 120-item food frequency questionnaire and the LVM was measured using magnetic resonance imaging. Regression models were used to determine associations of estimated dietary phosphorus with LVM and left ventricular hypertrophy (LVH). Mean estimated dietary phosphorus intake was 1,167 mg/day in men and 1,017 mg/day in women. After adjustment for demographics, dietary sodium, total calories, lifestyle factors, comorbidities, and established LVH risk factors, each quintile increase in the estimated dietary phosphate intake was associated with an estimated 1.1 gram greater LVM. The highest gender-specific dietary phosphorus quintile was associated with an estimated 6.1 gram greater LVM compared to the lowest quintile. Higher dietary phosphorus intake was associated with greater odds of LVH among women, but not men. These associations require confirmation in other studies.
Keywords: Phosphorus, phosphate, diet, consumption, left ventricular mass, left ventricular hypertrophy
INTRODUCTION
Phosphorus is an essential mineral that plays a key role in cell signaling and bone metabolism. The consumption of phosphorus has risen steadily in the United States over past decades and now considerably exceeds the recommended daily intake in men and women, and across all age groups.(1, 2) The increase in dietary phosphorus has been driven, in part, by greater consumption of phosphorus-containing food additives, from which intake alone has nearly doubled over the past decade.(1, 3) Estimating the phosphorus content of such additives is difficult; hence, the size of the increase may be underestimated.(4)
Despite tight hormonal regulation of phosphorus balance, excess dietary phosphorus may adversely impact cardiac structure and function. In controlled feeding studies of healthy volunteers, dietary phosphorus loading stimulates the phosphaturic hormone fibroblast growth factor-23 (FGF-23) and lowers circulating concentrations of 1,25-dihydroxyvitamin D.(5–7) Lower vitamin D and higher FGF-23 concentrations are associated with left ventricular hypertrophy in experimental models and clinical studies.(8–11) Dietary phosphorus loading also acutely impairs flow-mediated vascular dilatation in healthy men.(12) Although serum phosphorus concentrations are generally protected from fluctuations in dietary phosphorus intake, higher serum phosphorus concentrations are associated with greater left ventricular mass (LVM), clinical heart failure, and cardiovascular events in general population studies.(13, 14)
Given plausible effects of dietary phosphorus on ventricular mass, we evaluated associations of dietary phosphorus consumption with LVM, assessed by gold-standard, cardiac magnetic resonance imaging, among 4.494 participants in a community-based, multi-ethnic study population that was free of known cardiovascular disease.
RESULTS
Compared to the 2,320 participants who were excluded due to non-completion of cardiac MRI, missing FFQ, or implausible dietary data, the 4,494 included participants were, on average, younger (61.6 versus 63.2 years), had lower systolic blood pressures (125 versus 129 mm Hg), and were more likely to be White (40.5% versus 35.1%) or Asian (13.9% versus 7.3%).
Overall, men consumed more dietary phosphorus than women (mean 1,167 mg/d; IQR 746–1,435 versus 1,017 mg/d; IQR 634–1,261 mg/d). Subjects who consumed more dietary phosphorus tended to be younger, consumed more alcohol, were more likely to be White or Hispanic, exercised more, and had greater body mass index and lower systolic blood pressures (Table 1). Estimated dietary phosphorus was highly correlated with total kilocalories (Pearson correlation coefficient [PCC] = 0.86), and with dietary protein (PCC = 0.83), and dietary sodium (PCC = 0.61). There was no meaningful relationship between estimated dietary phosphorus intake and the serum phosphorus concentrations among the subset of 947 participants who had serum phosphorus measurements available (Table 1; correlation coefficient −0.055).
Table 1.
Baseline characteristics by sex-specific dietary phosphorus quintile.†
| Dietary phosphorus quintile | |||||
|---|---|---|---|---|---|
| Q1 (N=898) | Q2 (N=898) | Q3 (N=900) | Q4 (N=898) | Q5 (N=898) | |
| Estimated dietary phosphorus (mg/day) men | 270–687 | 688–917 | 918–1166 | 1167–1553 | 1554–5032 |
| Estimated dietary phosphorus (mg/day) women | 251–585 | 586–775 | 776–1009 | 1010–1345 | 1346–4069 |
|
| |||||
| Age | 63.2 ± 10.2 | 62.1 ± 10.1 | 61.8 ± 9.9 | 60.7 ± 10.2 | 60.1 ± 9.9 |
| Male | 433 (48) | 433 (48) | 434 (48) | 433 (48) | 434 (48) |
| Race | |||||
| White | 244 (27) | 342 (38) | 405 (45) | 418 (47) | 411 (46) |
| Black | 242 (27) | 218 (24) | 227 (25) | 208 (23) | 179 (20) |
| Hispanic | 181 (20) | 173 (19) | 166 (18) | 199 (22) | 258 (29) |
| Asian | 231 (26) | 165 (18) | 102 (11) | 73 (8) | 52 (6) |
| Diabetes status | |||||
| Normal | 523 (58) | 535 (60) | 564 (63) | 539 (60) | 556 (62) |
| Impaired glucose | 248 (28) | 251 (28) | 223 (25) | 244 (27) | 230 (26) |
| Diabetes | 125 (14) | 108 (12) | 110 (12) | 113 (13) | 113 (13) |
| Education | |||||
| ≤High school | 183 (20) | 151 (17) | 110 (12) | 131 (15) | 161 (18) |
| Some college | 408 (45) | 411 (46) | 408 (45) | 373 (42) | 411 (46) |
| ≥ College | 307 (34) | 335 (37) | 379 (42) | 392 (44) | 328 (36) |
| Smoking | |||||
| Never | 493 (55) | 476 (53) | 453 (50) | 464 (52) | 433 (48) |
| Former | 307 (34) | 319 (36) | 329 (37) | 326 (36) | 334 (37) |
| Current | 98 (11) | 102 (11) | 115 (13) | 106 (12) | 133 (15) |
| Alcohol use | |||||
| None | 498 (55) | 429 (48) | 427 (47) | 382 (43) | 383 (43) |
| 0–7 drinks per week | 258 (29) | 285 (32) | 297 (33) | 311 (35) | 330 (37) |
| >7 drinks per week | 138 (15) | 174 (19) | 163 (18) | 197 (22) | 178 (20) |
| Physical activity1 | 51.4 ± 52.0 | 55.8 ± 57.8 | 56.4 ± 62.8 | 60.1 ± 61.3 | 66.3 ± 64.3 |
| Body mass index (kg/m2) | 26.8 ± 4.6 | 27.2 ± 4.7 | 27.7 ± 5.0 | 27.8 ± 5.0 | 28.9 ± 5.1 |
| Systolic blood pressure (mm Hg) | 127.0 ± 22 | 125.6 ± 20 | 125.6 ± 22 | 123.3 ± 21 | 124.8 ± 21 |
| Diastolic blood pressure (mm Hg) | 72.0 ± 10 | 71.8 ± 10 | 72.3 ± 11 | 71.1 ± 10 | 71.6 ± 10 |
| Hypertension medication use | 347 (39) | 330 (37) | 319 (35) | 289 (32) | 294 (33) |
| Estimated GFR2 | 79.0 ± 16.2 | 79.2 ± 15.9 | 78.8 ± 17.2 | 79.4 ± 16.1 | 80.6 ± 16.9 |
| Estimated GFR <60 ml/min/1.73m2 | 92 (10) | 85 (10) | 88 (10) | 88 (10) | 68 (8) |
| Serum phosphorus (mg/dL)3 | 3.5 ± 0.5 | 3.5 ± 0.5 | 3.4 ± 0.5 | 3.5 ± 0.5 | 3.4 ± 0.5 |
| Albuminuria (≥30 mg/dL) | 77 (9) | 63 (7) | 94 (10) | 70 (8) | 64 (7) |
| C-reactive protein (mg/L) | 3.6 ± 7.3 | 3.1 ± 4.5 | 3.3 ± 5.0 | 3.6 ± 5.8 | 3.8 ± 5.4 |
| Total calories (kcal) | 949.3 ± 224 | 1255.6 ± 285 | 1562.3 ± 360 | 1918.5 ± 450 | 2701.5 ± 867 |
| Sodium (mg/day) | 1232.4 ± 434 | 1726.2 ± 571 | 2184.2 ± 718 | 2738.8 ± 908 | 4002.8 ± 1632 |
| Percent calories from protein | 15.0 ± 3.8 | 16.0 ± 3.8 | 16.2 ± 3.5 | 16.4 ± 3.3 | 17.3 ± 3.6 |
| Percent calories from fat | 33.9 ± 7.9 | 33.8 ± 7.2 | 34.2 ± 6.7 | 34.6 ± 6.8 | 35.0 ± 6.5 |
All values expressed as mean ± standard deviation or number (percent).
Metabolic equivalent task minutes per week of moderate-vigorous physical activity/100.
Estimated glomerular filtration rate in ml/min/1.73m2.
Serum phosphorus measurements available for a subset of 947 study participants.
The mean and standard deviation LVM for men and women were 168.6 ±36.8 grams and 123.8 ±27.4 grams, respectively. Higher estimated dietary phosphorus intake was associated with greater left ventricular mass (Figure 1 and Table 2). After adjusting for height, weight, age, and race, and weighing model estimates by sex, each 20% greater estimated dietary phosphorus intake was associated with an estimated 0.42 g greater LVM (95% CI 0.14 g, 0.70 g). Progressive adjustment strengthened the association of estimated dietary phosphorus with LVM (Table 2). In the fully adjusted model, each 20% greater estimated dietary phosphorus was associated with an estimated 1.06 g greater LVM (95% CI: 0.50 g, 1.62 g; p-value <0.001). Each 20% greater estimated dietary phosphorus consumption was also associated with a higher mass-to-volume ratio (0.006 g/mL; p-value =0.02), but not left ventricular end diastolic volume or stroke volume in fully adjusted models. Other characteristics that were most strongly associated with LVM include African American race, current smoking, greater body mass, higher systolic blood pressure, anti-hypertensive medication use, and greater urine albumin to creatinine ratio (Supplemental Tables 1 and 2).
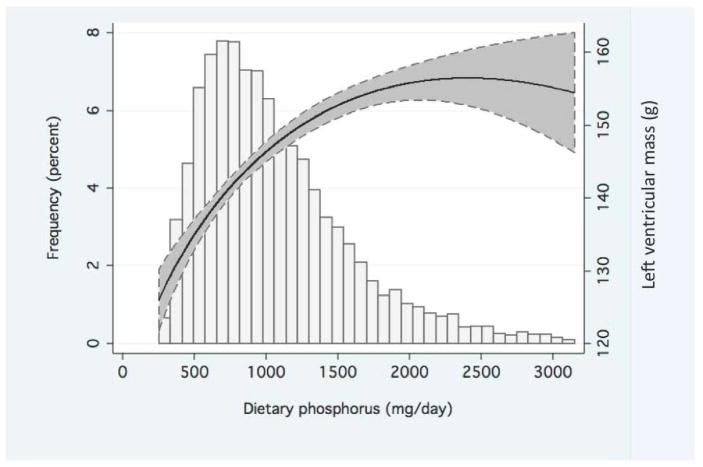
Figure 1. Functional association of left ventricular mass with estimated dietary phosphorus.
In the upper panel, solid and dashed lines depict mean and 95% confidence intervals for left ventricular mass, respectively, by the amount of dietary phosphorus intake. Values were obtained from an unadjusted fractional polynomial linear regression model that included 99% of the study data (dietary phosphorus values <3162 mg/day). In the lower panel, the frequency histogram depicts the distribution of dietary phosphorus consumption across the MESA study population.
Table 2.
Association of continuous estimated dietary phosphorus intake with left ventricular mass.
| Model, adjusted for | N in model | Difference in left ventricular mass (grams)† | |
|---|---|---|---|
| Per 100 mg greater estimated dietary phosphorus (95% CI) | Per 20% greater estimated dietary phosphorus (95% CI) | ||
| Demographic factors1 | 4494 | 0.17 (0.04, 0.30) | 0.42 (0.14, 0.70) |
| Add dietary factors2 | 4494 | 0.23 (−0.01, 0.46) | 0.84 (0.25, 1.43) |
| Add lifestyle factors3 | 4450 | 0.25 (0.01, 0.48) | 0.99 (0.40, 1.59) |
| Add comorbidities4 | 4405 | 0.28 (0.06, 0.50) | 1.06 (0.50, 1.62) |
All model estimates represent weighted averages by sex.
Demographic model adjusted for age, race, height, weight and weight0.5.
Dietary factors model adds total dietary calories and dietary sodium.
Lifestyle factors model adds smoking, alcohol use, education, and moderate-vigorous physical activity.
Comorbidities model adds diabetes status, systolic blood pressure, anti-hypertensive medication use, urinary albumin to creatinine ratio, C-reactive protein, and estimated glomerular filtration rate.
When analyzed according to sex-specific quintiles, higher categories of estimated dietary phosphorus were associated with greater LVM (Table 3). After full adjustment, individuals in the highest sex-specific dietary phosphorus quintile had an estimated 6.2 gram greater LVM (95% CI 2.5, 9.8) compared to those in the lowest quintile. This estimate was comparable to other known LVM risk factors: in the fully adjusted model, each 10-mm Hg greater systolic blood pressure was associated with an estimated 2.7 gram greater LVM (95% CI 2.3 g, 3.1 g), and current smoking was associated with an adjusted 8.4 gram greater LVM (95% CI 6.0g, 10.7g). In contrast, neither total caloric intake nor dietary sodium was associated with LVM.
Table 3.
Association of sex-specific dietary phosphorus quintiles with left ventricular mass.
| Dietary phosphorus quintile | |||||
|---|---|---|---|---|---|
| Q1 (N=898) | Q2 (N=898) | Q3 (N=900) | Q4 (N=898) | Q5 (N=898) | |
| Left ventricular mass (g, mean ± SD) | 139 ± 39 | 143 ± 39 | 146 ± 41 | 148 ± 39 | 151 ± 39 |
| Difference in left ventricular mass, grams (95% CI)† | |||||
| Demographic factors1 | reference | 1.57 (−0.73, 3.88) | 2.46 (0.14, 4.79) | 2.95 (0.60, 5.29) | 3.36 (0.99, 5.72) |
| Add dietary factors2 | reference | 2.17 (−0.31, 4.64) | 3.53 (0.72, 6.35) | 4.40 (1.14, 7.66) | 5.44 (1.34, 9.53) |
| Add lifestyle factors3 | reference | 2.43 (−0.04, 4.90) | 3.98 (1.16, 6.79) | 5.24 (1.98, 8.50) | 6.20 (2.11, 10.30) |
| Add comorbidities4 | reference | 2.66 (0.33, 4.98) | 3.91 (1.26, 6.56) | 5.64 (2.57, 8.72) | 6.13 (2.27, 9.98) |
All model estimates represent weighted averages by sex.
Demographic model adjusted for age, race, height, weight and weight0.5.
Dietary factors model adds total dietary calories and dietary sodium.
Lifestyle factors model adds smoking, alcohol use, education, and moderate-vigorous physical activity.
Comorbidities model adds diabetes status, systolic blood pressure, anti-hypertensive medication use, urinary albumin to creatinine ratio, C-reactive protein, and estimated glomerular filtration rate.
The magnitude of the association between estimated dietary phosphorus and LVM tended to be stronger among women compared to men, particularly for women who were post-menopausal, and for participants who had chronic kidney disease (Table 4); however, these contrasts were not statistically different (all p-values for interaction >0.2). There were too few cases of advanced kidney disease in MESA to permit testing for interaction across the full range of kidney dysfunction. The size of the association between estimated dietary phosphorus and LVM also did not differ appreciably across categories of age, race/ethnicity, or hypertension status.
Table 4.
Association of estimated dietary phosphorus with left ventricular mass by subgroup.
| Subgroup | N | Mean LVM (g) ± SD | Difference in LVM (g) per 20% greater dietary phosphorus (95% CI)† |
|---|---|---|---|
| Overall cohort | 4494 | 145 ± 39 | 1.06 (0.50, 1.62) |
| Men | 2167 | 169 ± 37 | 0.59 (−0.49, 1.67) |
| Women | 2327 | 124 ± 27 | 1.24 (0.58, 1.89) |
| Pre-menopausal women | 355 | 122 ± 25 | 0.42 (−1.27, 2.11) |
| Post-menopausal women | 1,878 | 124 ± 28 | 1.38 (0.65, 2.12) |
| Age 45–64 years | 2467 | 147 ± 39 | 0.97 (0.27, 1.67) |
| Age 65–84 years | 1847 | 142 ± 39 | 1.22 (0.29, 2.16) |
| White | 1820 | 144 ± 38 | 0.89 (0.08, 1.71) |
| Black | 1074 | 158 ± 41 | 0.76 (−0.70, 2.23) |
| Asian | 623 | 124 ± 31 | 0.98 (−0.18, 2.15) |
| Hispanic | 977 | 148 ± 38 | 1.55 (0.34, 2.76) |
| Hypertension1 | 2050 | 153 ± 41 | 1.05 (0.12, 1.98) |
| No Hypertension1 | 2442 | 138 ± 36 | 0.91 (0.22, 1.59) |
| Chronic kidney disease | 421 | 142 ± 40 | 2.01 (0.24, 3.78) |
| No chronic kidney disease | 4061 | 146 ± 39 | 0.99 (0.40, 1.99) |
Except for the covariate representing the subgroup of interest, all models weighed and combined by sex and adjusted for age, race, height, weight and weight0.5, total dietary calories, dietary sodium, smoking, alcohol use, education, moderate-vigorous physical activity, diabetes status, systolic blood pressure, anti-hypertensive medication use, urinary albumin to creatinine ratio, C-reactive protein, and estimated glomerular filtration rate.
Hypertension models exclude adjustment for systolic blood pressure and anti-hypertensive medication use.
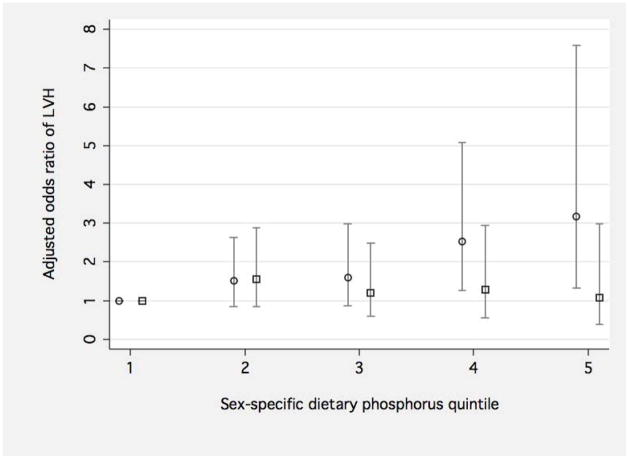
The prevalence of LVH among male and female MESA participants was 7.7% and 9.2%, respectively. Greater estimated dietary phosphorus consumption was associated with progressively higher adjusted odds of LVH among women, but not men (Figure 2).
Figure 2. Adjusted association of sex-specific dietary phosphorus quintile with left ventricular hypertrophy in men and women.
The Y-axis depicts the adjusted the odds ratio of left ventricular hypertrophy for men (squares) and women (circles) according to sex-specific quintiles of dietary phosphorus intake. 95% confidence intervals are presented as gray vertical spikes. Separate logistic regression models for men and women are adjusted for age, race, height, weight and weight0.5, total dietary calories, dietary sodium, smoking, alcohol use, education, moderate-vigorous physical activity, diabetes status, systolic blood pressure, anti-hypertensive medication use, urinary albumin to creatinine ratio, C-reactive protein, and estimated glomerular filtration rate. The unadjusted prevalences of left ventricular hypertrophy for men and women are presented below the figure.
We investigated whether the association of estimated dietary phosphorus with LVM might differ across different foods groups (Table 5; Supplemental Table 3). Our findings did not reveal any significant differences in the primary association according to the type of dietary protein or processed food status.
Table 5.
Association of estimated dietary phosphorus with left ventricular mass within food groups
| Protein type | Processed food type | Examples* | Proportion dietary phosphorus (mg/day) | Difference in LVM (mg)† | 95% CI |
|---|---|---|---|---|---|
| Animal | Processed | Hamburger Fried chicken |
14% (146 mg/day) | +102 | −736, +939 |
| Animal | Unprocessed | Eggs Roast chicken |
16% (169 mg/day) | +82 | −614, +777 |
| Dairy | Processed | Flavored yogurt Ice cream |
5% (54 mg/day) | −153 | −1101, +795 |
| Dairy | Unprocessed | Milk Cottage cheese |
22% (277 mg/day) | +219 | −150, +437 |
| Vegetable | Processed | Fried rice Chili |
6% (66 mg/day) | −19 | −526, +488 |
| Vegetable | Unprocessed | Cold cereal Nuts |
11% (118 mg/day) | +673 | −52, +1398 |
| Non-protein | Processed | Crackers Soda |
9% (96 mg/day) | −975 | −1983, +33 |
| Non-protein | Unprocessed | Fruit Beer |
17% (164 mg/day) | +644 | −389, +1676 |
The complete categorization of all 120-food frequency questionnaire items is presented in supplemental table 3.
LVM = left ventricular mass
Associations per 100 mg greater dietary phosphorus within each food category. N = 4,405 for all models.
All models weighed and combined by sex and adjusted for age, race, height, weight and weight0.5, total dietary calories, dietary sodium, smoking, alcohol use, education, moderate-vigorous physical activity, diabetes status, systolic blood pressure, anti-hypertensive medication use, urinary albumin to creatinine ratio, C-reactive protein, and estimated glomerular filtration rate.
DISCUSSION
In a community-based, multi-ethnic population without clinical cardiovascular disease, higher estimated dietary phosphorus consumption was associated with greater left ventricular mass, measured by cardiac MRI. Associations persisted after adjustment for known LVH risk factors. Higher estimated dietary phosphorus intake was also associated with a greater odds of LVH among women, but not men. If confirmed in other populations these findings suggest the possibility that dietary phosphorus could have adverse cardiovascular consequences in the general population.
An association of dietary phosphorus with left ventricular mass is supported by the hormonal response to dietary phosphorus. In animal models, and some human feeding studies, dietary phosphorus loading stimulates a rise in fibroblast growth factor-23 (FGF-23) and a decline in 1,25-dihydroxyvitamin D (1,25-OH2D).(5, 6, 15) These hormonal changes serve to maintain phosphorus homeostasis: FGF-23 enhances phosphorus excretion through the kidneys and diminished vitamin D activation slows phosphorus absorption through the gut. However, higher FGF-23 and lower 1,25-OH2D concentrations may have adverse effects on the myocardium. Vitamin D potently suppresses the renin-angiotensin system; disruption of the vitamin D system leads to hypertension and LVH in experimental models.(9) In human studies, higher FGF-23 concentrations are associated with greater LVM.(10, 16) While a dietary phosphorus-hormone-LVM hypothesis is intriguing, this theory awaits further study.
Given previous associations of serum phosphorus concentrations with LVM, incident heart failure, and cardiovascular events(13, 14) it is tempting to connect associations for dietary phosphorus through serum phosphorus levels. However, animal models and human studies demonstrate extraordinarily tight regulation of the circulating phosphorus concentration, irrespective of dietary phosphorus intake.(17, 18) Among 15,513 participants in the Third National Health and Nutrition Examination Survey, no association of dietary phosphorus intake with the circulating phosphorus concentration was found across a wide range of phosphorus intake.(19)
Associations of estimated dietary phosphorus with LVM were modestly stronger among women and among participants who had CKD. A possible explanation for sex-specific differences could be a reduction in phosphorus excretion that occurs in post-menopausal women.(20) CKD leads to diminished phosphorus clearance through the kidney, which could enhance relationships of dietary phosphorus intake with adverse outcomes. These sub-group findings require confirmation in other study populations, particularly those that include greater numbers of individuals who have CKD.
Most participants in this study consumed more phosphorus than the 700 mg/day recommended by the Institute of Medicine.(2) Currently, there are no FDA requirements to list the phosphorus content of food products. In addition, standard nutritional software may not account for phosphorus derived from food additives.(4) These factors likely contribute to significant underestimation of dietary phosphorus content in research studies. More rigorous methods are needed to increase precision of dietary phosphorus assessment, potentially validated against repeated 24-hour urine phosphorus collections, which provide a reasonable estimate of total dietary phosphorus intake in the steady state.(6)
An important limitation of our study is potential misclassification of dietary phosphorus consumption using the food frequency questionnaire and NDSR software. This problem is compounded by the increasing use of phosphorus containing additives that are not included in food labels and are therefore missed by current nutritional software.(21) It is likely that such measurement error would occur to a similar degree among participants who had higher versus lower LVM, thereby biasing our results toward the null. A second limitation is the potential for residual confounding, such that individuals who consumed more estimated dietary phosphorus also possessed other characteristics that were linked with LVM, for example consumption of other unhealthy foods or an unhealthy lifestyle. We attempted to address confounding by adjusting for demographics, total calories, dietary sodium, lifestyle factors, and co-morbidities that included established LVH risk factors in a well-characterized cohort; however, confounding remains an important limitation. A third limitation is the cross-sectional study design, obscuring the temporal sequence by which estimated phosphorus intake might influence LVM.
Our study also has some important strengths. Associations were observed in a community-based, multi-ethnic study population, thereby enhancing external validity. LVM was measured using cardiac MRI, which is considered the gold standard for this characteristic.(22) The use of the modified Block FFQ, used in our data collection, has been reproduced and validated in other studies.(23, 24) The study population was free of known cardiovascular disease and analyses were adjusted for well-characterized LVM risk factors, nutritional indices, and lifestyle factors.
In summary, we observed associations of higher estimated dietary phosphorus with greater LVM in a multi-ethnic study population. Important next steps include replication of findings in other study populations, improved dietary phosphorus measurement, and further elucidation of possible mechanisms by which dietary phosphorus might impact LVM.
METHODS
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) was designed to investigate the prevalence, correlates, and progression of subclinical cardiovascular disease among 6,814 men and women aged 45–84 years.(25) Participants were recruited from study sites in Baltimore, MD; Chicago, Ill; St. Paul, MN; Forsyth County, NC; New York, NY; and Los Angeles CA between 2000 and 2002. An informational brochure was mailed to households in targeted areas, households were contacted by telephone, and questionnaires were administered in English, Spanish, Cantonese, or Mandarin to introduce the study and collect eligibility information. The MESA recruited a final study population that was 41% White, 26% African-American, 21% Hispanic, and 12% Asian (primarily Chinese descent). Individuals were excluded from MESA if they had any previous diagnosis of cardiovascular disease, defined by physician-diagnosed heart attack, angina, stroke, transient ischemic attack, heart failure, atrial fibrillation, use of nitroglycerin, or prior angioplasty, coronary artery bypass graft, valve replacement, pacemaker or defibrillator implantation, or any surgery on the heart or arteries. All participants gave informed consent and Institutional Review Board approval was obtained for each site.
For this cross-sectional study, we excluded participants who did not complete the food frequency questionnaire (n=577) or did not undergo cardiac magnetic resonance imaging (MRI; n=1,621). Reasons for not completing cardiac MRI were ineligibility (n=477; usually due to metallic fragment, implant, or device), inability (n=954; usually due to claustrophobia), refusal (n=204), mechanical problems with the scanner (n=27), or unknown (n=68).(26) We also excluded individuals who provided implausible dietary information, defined by total daily caloric intake <600 kcal/day (n=112) or ≥6,000 kcal/day (n=10) based on prior published studies,(27) resulting in a final sample size of 4,494.
Ascertainment of Dietary Phosphorus
Each participant’s usual diet during the previous year was assessed at baseline using a 120-item food frequency questionnaire (FFQ) developed using the validated Block format.(23) The FFQ was adapted from the Insulin Resistance Atherosclerosis Study instrument, in which comparable validity was observed for Whites, African-Americans, and Hispanic persons. It was modified to include foods typically eaten by Chinese persons. This questionnaire has been reproduced and validated in a separate study.(24)
Data from food-specific portions were converted to approximate daily intake amounts of micronutrients in food using the Nutrition Data Systems for Research database (NDSR; Nutrition Coordinating Center (NCC), University of Minnesota).(28) The phosphorus content of each food item was calculated by multiplying the food content in 100 grams of that food item by the serving size (grams) and frequency of consumption (servings/day). To investigate whether potential associations of dietary phosphorus with LVM were dependent on particular foods, we classified each of the 120 food items into 8 groups based on the type of dietary protein (animal, dairy, vegetable, or non-protein) and processed food status (processed versus non-processed). Our study nutritionist (A.K.) created these specific phosphorus food group categories based on National Kidney Foundation’s Nutrition and Diet recommendations for phosphorus intake.(29) The protein and processed food classification scheme for each of the 120-food items is presented in supplemental table 1. To investigate whether dietary phosphorus intake might be related to the serum phosphorus concentration we obtained serum phosphorus measurements that were performed previously among a subset of 947 participants who were included in this study. In a separate validation study of 38 healthy individuals, the correlation coefficient (r) of dietary phosphorus, estimated by FFQ, with 24-hour urine phosphorus was 0.389.(30)
Ascertainment of Left Ventricular Mass and Indices of Cardiac Structure and Function
Cardiac MRI was performed using MRI scanners with 1.5-Tesla magnets. All MRI images were read centrally by readers who were blinded to all study information. A 4-element, phased-array surface coil was placed anteriorly and posteriorly, and electrocardiogram gating and brachial artery blood pressure monitoring were used for all imaging. Left ventricular cine images were produced with a time resolution <50 milliseconds. The intra-class correlation coefficient for LVM was 0.97 (95% confidence interval [CI] 0.96–0.98) meaning that approximately 3% of the total variability was attributed to reader measurement error.(26) Left ventricular hypertrophy (LVH) was defined by a LVM index (LVM indexed to body surface area) greater than 85.3 g/m2 for women or greater than 107.8 g/m2 for men, which corresponds to sex-specific 95th percentile scores among MESA participants without diabetes or hypertension.(31)
We also assessed left ventricular end-diastolic volume (LVEDV) and stroke volume. These indices were calculated from MRI data using commercially available software (MASS 4.2, MEDIS, Leiden, The Netherlands).(32) The ratio of LVM to LVEDV (mass-to-volume ratio) was calculated as LVM divided by LVEDV.
Ascertainment of Other Study Data
MESA investigators ascertained medical histories, including cardiovascular risk factors using standardized questionnaires and determined medication use by the inventory method.(25) Investigators assessed physical activity levels using a survey that queried work, leisure, volunteer, sporting, conditioning, and walking and yard activities. We classified the highest level of education achieved as high school or less, some college, or college or higher. We categorized smoking status as none, current, or former, and we categorized alcohol use as none, 0–7 drinks per week, and >7 drinks per week. We defined diabetes status based on American Diabetic Association 2003 fasting criteria.(33)
MESA investigators measured height and weight during the physical examination period. After a 5-minute rest, MESA study coordinators measured seated blood pressure three times in the right arm at 1-minute intervals using a Dinamap model Pro 100 automated sphygmomanometer (Critikon, Tampa, FL). The average of the last two measurements was used for analysis. Serum levels of creatinine, urine microalbumin, and C-reactive protein (CRP) were measured from blood specimens collected during the MESA baseline examination; serum creatinine measurements were calibrated to the IDMS standard.(34) We defined chronic kidney disease as a glomerular filtration rate <60 mL/min/1.73 m2 using the Modification of Diet in Renal Disease formula.(35) We categorized the urine albumin to creatinine ratio (ACR) as <30 versus ≥30 mg/g.
Statistical Analyses
We evaluated estimated dietary phosphorus intake (mg/day) as a continuous variable and using sex-specific quintiles, given considerable differences in phosphorus consumption between men and women. We log transformed continuous dietary phosphorus due to the approximate log-linear association with LVM. Since LVM was normally distributed in the MESA population we did not transform this outcome variable.
Given known dependence of LVM on height, weight, age, and race,(36) all analyses of LVM included adjustment for these covariates, plus an additional square-root term for weight to best model its relationship with LVM. Given substantial differences in estimated dietary phosphorus consumption and LVM between men and women, and potential interactions of sex with other model covariates (particularly weight), we performed analyses separately among men and women, and when appropriate, report combined point estimates using inverse-variance weighting, which assigns a weighted score based on the standard errors of the coefficients.
We utilized progressive nested linear regression models to estimate cross-sectional associations of estimated dietary phosphorus with LVM after adjustment for relevant confounders. A basic model included height (continuous, meters), weight and weight0.5 (continuous, kilograms), age (continuous, years), and race/ethnicity (White/Black/Asian/Hispanic). A second model added total caloric intake (log-linear, kcal/day), to account for overall differences in energy intake, and dietary sodium (log-linear, mg/day). A third model added smoking (never, former, current), alcohol consumption (none, 0–7, and >7 drinks per day), education (high school or less, some college, college or higher), and amount of moderate-vigorous physical activity (continuous, MET-minutes/week). A fourth model added prevalent diabetes (normal, impaired fasting glucose, diabetes), systolic blood pressure (continuous, mm Hg), use of anti-hypertensive medications, estimated glomerular filtration rate (continuous, mL/min/1.73 m2), urine albumin to creatinine ratio (log-linear, mg/dL), and CRP (log-linear, mg/dL). We also utilized these nested models to determine adjusted associations of estimated dietary phosphorus with LVEDV, stroke volume, and mass-to-volume ratio.
We utilized logistic regression with robust variance estimation(37) to estimate the association of dietary phosphorus with the odds of LVH (yes versus no) after adjustment for covariate sets described above. Because LVH is indexed to body surface area, we excluded height and weight from the logistic regression models. In a first sub-analysis we estimated associations of dietary phosphorus with LVM across categories of age, race, sex, hypertension, and chronic kidney disease status. In a second sub-analysis, we evaluated associations between estimated dietary phosphorus within a set of 8 a-priori defined, mutually exclusive food group categories and LVM. All analyses were conducted using STATA version 11.1 (StataCorp., College Station, TX). Values were considered statistically significant at the two-sided alpha 0.05 level.
Supplementary Material
Acknowledgments
This research was supported by contracts N01-HC-95159 - N01-HC-95166, N01-HC-95169, R01-HL-071739 and R01-HL-072403 from the NHLBI. The authors thank the investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Grant support: National Institutes of Health R01 HL096875-01
Footnotes
DISCLOSURES
The authors report no conflict of interest related to this manuscript.
References
- 1.Calvo MS, Park YK. Changing phosphorus content of the U.S. diet: potential for adverse effects on bone. J Nutr. 1996;126:1168S–1180S. doi: 10.1093/jn/126.suppl_4.1168S. [DOI] [PubMed] [Google Scholar]
- 2.Ross AC Institute of Medicine (U. S.) Dietary reference intakes for calcium and vitamin D. National Academies Press; Washington, DC: 2011. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. [PubMed] [Google Scholar]
- 3.Kemi VE, Rita HJ, Karkkainen MU, Viljakainen HT, et al. Habitual high phosphorus intakes and foods with phosphate additives negatively affect serum parathyroid hormone concentration: a cross-sectional study on healthy premenopausal women. Public Health Nutr. 2009;12:1885–1892. doi: 10.1017/S1368980009004819. [DOI] [PubMed] [Google Scholar]
- 4.Oenning LL, Vogel J, Calvo MS. Accuracy of methods estimating calcium and phosphorus intake in daily diets. J Am Diet Assoc. 1988;88:1076–1080. [PubMed] [Google Scholar]
- 5.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 6.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, et al. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 8.Bodyak N, Ayus JC, Achinger S, Shivalingappa V, et al. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci U S A. 2007;104:16810–16815. doi: 10.1073/pnas.0611202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li YC, Kong J, Wei M, Chen ZF, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirza MA, Larsson A, Melhus H, Lind L, et al. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–551. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Faul C, Amaral AP, Oskouei B, Hu MC, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuto E, Taketani Y, Tanaka R, Harada N, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20:1504–1512. doi: 10.1681/ASN.2008101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhingra R, Gona P, Benjamin EJ, Wang TJ, et al. Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Eur J Heart Fail. 2010;12:812–818. doi: 10.1093/eurjhf/hfq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tonelli M, Sacks F, Pfeffer M, Gao Z, et al. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 15.Perwad F, Azam N, Zhang MY, Yamashita T, et al. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isakova T, Gutierrez O, Shah A, Castaldo L, et al. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J Am Soc Nephrol. 2008;19:615–623. doi: 10.1681/ASN.2007060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berndt T, Thomas LF, Craig TA, Sommer S, et al. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci U S A. 2007;104:11085–11090. doi: 10.1073/pnas.0704446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2009;53:399–407. doi: 10.1053/j.ajkd.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cirillo M, Ciacci C, De Santo NG. Age, renal tubular phosphate reabsorption, and serum phosphate levels in adults. N Engl J Med. 2008;359:864–866. doi: 10.1056/NEJMc0800696. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan CM, Leon JB, Sehgal AR. Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. J Ren Nutr. 2007;17:350–354. doi: 10.1053/j.jrn.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myerson SG, Bellenger NG, Pennell DJ. Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension. 2002;39:750–755. doi: 10.1161/hy0302.104674. [DOI] [PubMed] [Google Scholar]
- 23.Block G, Hartman AM, Dresser CM, Carroll MD, et al. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 24.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, et al. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol. 1999;9:314–324. doi: 10.1016/s1047-2797(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 25.Bild DE, Bluemke DA, Burke GL, Detrano R, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 26.Heckbert SR, Post W, Pearson GD, Arnett DK, et al. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nettleton JA, Polak JF, Tracy R, Burke GL, et al. Dietary patterns and incident cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2009;90:647–654. doi: 10.3945/ajcn.2009.27597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nutrition Data System for Research (NDSR) University of Minnesota; 2011. [Google Scholar]
- 29.Phosphorus and Your CKD Diet. National Kidney Foundation; 2011. [Google Scholar]
- 30.Porrini M, Gentile MG, Fidanza F. Biochemical validation of a self-administered semi-quantitative food-frequency questionnaire. Br J Nutr. 1995;74:323–333. doi: 10.1079/bjn19950138. [DOI] [PubMed] [Google Scholar]
- 31.Edvardsen T, Rosen BD, Pan L, Jerosch-Herold M, et al. Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imaging--the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2006;151:109–114. doi: 10.1016/j.ahj.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Bluemke DA, Kronmal RA, Lima JA, Liu K, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 (Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 34.Myers GL, Miller WG, Coresh J, Fleming J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 35.Levey AS, Bosch JP, Lewis JB, Greene T, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 36.Gardin JM, Wagenknecht LE, Anton-Culver H, Flack J, et al. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation. 1995;92:380–387. doi: 10.1161/01.cir.92.3.380. [DOI] [PubMed] [Google Scholar]
- 37.Hosmer DW, Lemeshow S. Applied logistic regression. 2. xii. Wiley; New York: 2000. p. 373. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.