Abstract
Since the discovery of mirror neurons (MN) in the monkey there has been a renewed interest in motor theories of cognitive and social development in humans providing evidence for an action observation/execution matching system. It has been proposed that this system plays a fundamental role in the development of complex social and cognitive behaviors such as imitation and action recognition. In this review we discuss what is known about MNs from the work using single-cell recordings in the adult monkey, the evidence for the putative MN system in humans, and the extent to which research using electroencephalography (EEG) methods has contributed to our understanding of the development of these motor systems and their role in the social behaviors postulated by the MN hypothesis.
Keywords: Mirror Neurons, EEG, Infancy, social cognition, imitation, Humans, Rhesus Macaques
Introduction
Since the discovery of mirror neurons (MN) by Rizzolatti and colleagues [17, 22, 29, 65], there has been a renewed interest in motor theories of social development [13, 26, 28, 30, 31, 64]. Prior to the description of MN , theories of the role of motor action on cognition were dependent upon internalizing the motivations, goals, and desires of others’ actions through one’s own actions and interactions with the world [47, 72, 77] and were based on behavioral observation as the neural mechanisms through which information was internalized were poorly understood. The discovery of MNs provided a potential explanatory path for these theories . The proposal put forth by this theory is that when an individual observes an action the same cortical network involved in execution of that action is activated. This matching system has been hypothesized to serve action recognition through the activation of an internal motor knowledge of an action via the visual or auditory description of the action, therefore, because the observer "knows" the outcome based on his or her own motor representation, he/she gains a direct and embodied understanding of the actor’s goal. This has led many to propose that the MN system may underlie the development of complex socio-cognitive and behavioral processes such as action understanding, imitation, and empathy [23, 31, 63, 66].
In this review we first discuss the characteristics of MNs as evidenced by single-cell recordings made in adult rhesus macaques and highlight the hypotheses that have been proposed by this evidence. Because of the invasive nature of single-cell recording, there is limited evidence for MNs in the human motor cortex. However, there is converging evidence from functional imaging (fMRI) electroencephalogram (EEG) and magnetoencephalogram (MEG) studies which strongly supports the existence of a human MN system. Finally, in the latter part of this paper we discuss the issue of development of the MN system and we review current evidence linking a cortical matching mechanism, measured with EEG methods and the activity of MNs, as it is usually assessed with single-cell recordings. Although this relation has never been investigated, we will critically evaluate the evidence of such a link based on the current literature and on the recent developmental work on EEG signal changes in early infancy in relation to action perception and execution.
Mirror Neurons in the Monkey
The incidental discovery of MNs was accomplished through the use of single-cell recordings. By trying to understand at a neurophysiological level how monkeys perform simple grasping actions, di Pellegrino and colleagues [17] noticed that the neurons they were recording from were activated when the monkey observed an experimenter executing grasping actions. The researchers were focusing their study on area F5 of the monkey premotor cortex (see Figure 1), a region dominated by neurons activated during goal-directed hand movements. Their discovery suggested that the premotor cortex was not solely involved in action execution, but also contributed to visual processing of motor actions performed by others suggesting an observation/execution matching system based on the activation of an internal motor representation of actions.
Figure 1.
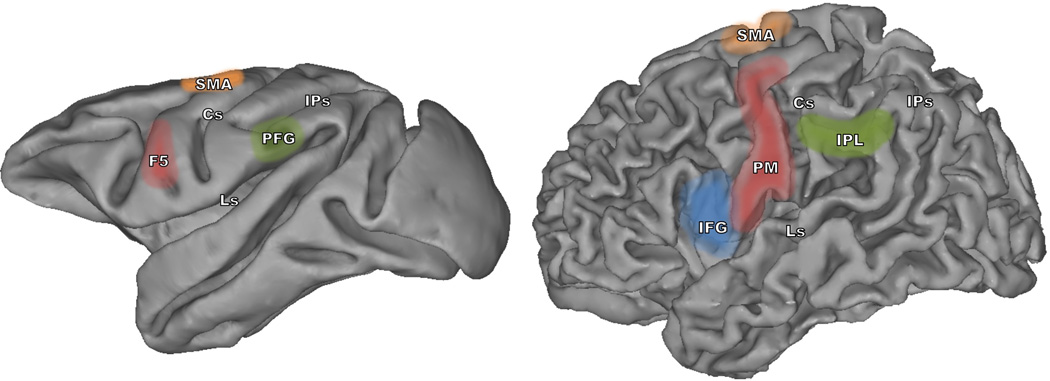
Schematic view of brain regions considered to part of the MN system of humans (left) and monkeys (right). Cortical regions in red and blue identify the crucial nodes of the MN system in the cerebral cortex of humans and monkeys. The regions in green constitute the parietal node of the MN system. F5, rostral part of the ventral premotor cortex in the monkey; IFG, inferior frontal gyrus; PFG, rostral part of the inferior parietal lobule; PM, premotor cortex; SMA, supplementary motor area; IPL, inferior parietal lobule; Cs, central sulcus; Ls, lateral sulcus; IPs, intraparietal sulcus [20].
The primary feature of MNs is their activation during both the execution and observation of actions. One of the main properties of MNs is the congruence between the effective executed and observed action. This pattern of firing activity has been interpreted as providing a functional mapping of the perceptual aspects of the actions of others onto the observer’s motor representation of that action. As a consequence, neural mirroring allows the observer to have a direct, automatic, effortless "understanding" (an understanding from ‘within’) of the actions of others [28, 63, 67].
Subsequent studies reported additional response properties of MNs. For example it was found that some F5 MNs exhibit remarkable generalization properties. Notably, they responded to the same action executed with different effectors, e.g. grasping with the hand or grasping with the mouth [22, 66], to the sound associated with familiar actions [38] and even to partially occluded actions that can be ‘inferred’ only from their initial motion path [75]. Together these findings prompted the idea that MNs can visually describe the goal of an action by exploiting inputs of different sensory information or even when part of the visual information is lacking.
In addition to area F5, neurons with mirror properties were also found in area PF/PFG of the inferior parietal lobule (IPL) [27, 68]. The visual responses of a subset of IPL MNs were investigated and findings suggested that their discharge is modulated by the final goal of the action (placing or eating) in which the grasping was embedded during both observation and execution [6, 27]. Fogassi and colleagues [27] suggested that this pattern of response reflects the monkey's prediction about the final goal, with the possible specific cognitive role of IPL MNs in encoding the intentions of the actor.
In the last decade several other studies on the properties of mirror neurons have been collected from other labs demonstrating that their neuronal discharge can be modulated by several factors such as the viewpoint under which an action is observed [9], the space in which an action is observed (peripersonal or extrapersonal space) [10], and the reward value the target object [8]. Other studies demonstrated that after months of sensorimotor training with a tool some neurons in the premotor and primary motor cortex showed mirror properties specific for the tool [24, 74]. Together these findings suggest that sensorimotor experiences influence MN discharge making this mechanism plastic in relation to individual experiences.
In addition to hand MNs another important class of MNs are those for mouth actions and mouth facial gestures. Ferrari, Gallese, Rizzolatti, & Fogassi [22] identified MNs involved in a number of different mouth actions related to ingestive actions. The most exciting finding was that a subset of the mouth MNs activated for observation of communicative gestures (e.g. lip smacking). This class of MN may have its greatest impact when considering their function in humans, specifically as it relates to their involvement in communication and emotional understanding, which are social behaviors that rely heavily on interpreting oro-facial movements [13]. In fact the capacity to activate shared representations of emotional face expressions may support emotional contagion responses involving motor, somatosensory, and affective components that facilitate the automatic reproduction of the affective states of others’ (see [35] for a review).
As we will discuss in the next section, there is considerable evidence that suggests humans have a MN system that is structurally and functionally similar to that of the monkeys’, however, many of the features of MNs have yet to be characterized in humans and there remains conflicting evidence in some of the domains.
Evidence for Mirror Neurons in Humans
The invasiveness of single-cell recordings has generally precluded its application to research with humans. This has led some scholars to doubt that a similar system could be present in humans. Only one study using single-cell recordings in humans found mirror responses to facial gestures in supplementary motor area (SMA), entorhinal cortex and parahippocampal gyrus [51]. The properties of these neurons have been characterized under only a few experimental conditions making the interpretations of their firing inconclusive and further exploration is required.
Several methods such as functional neuroimaging (fMRI) and changes in sensorimotor alpha (or mu) of the electroencephalogram (EEG) and magnetoencephalogram (MEG) have been successful in identifying neural activity associated with the execution and perception of biologically meaningful stimuli. Functional neuroimaging studies have shown that during the observation and execution of actions a cortical network is activated formed by the posterior part of the inferior frontal gyrus (IFG), the premotor cortex (PM), and the inferior parietal lobe (IPL) [7, 18, 34, 42]. The cytoarchitecture of each of these regions is believed to be the human homologue of the monkey regions associated with the MN circuit (see Figure 1).
A recent meta-analysis on 125 fMRI studies confirmed the results of an increasing number of investigations showing a consistent pattern of activation of the IFG, PM and IPL. These areas are central to processing action observation and execution [49]. The recruitment of other areas such as the cerebellum and the limbic system suggest that additional areas not strictly related to motor functions are probably involved in integrating the affective components accompanying an action.
Electroencephalography as a tool to investigate the MN system
Neuroimaging studies have contributed significantly to identifying the structures involved in the human MN system, but the sensitivity to movement and the cost has limited its broader use, especially in developmental studies with infants and young children. Examining the reactivity of the sensorimotor alpha (or mu) rhythm of the EEG and MEG has expanded our understanding of a number of critical features of the human MN system.
In 1954, Gastaut and Bert [32] were the first to describe the mu rhythm. They observed desynchronization of mu amplitude associated with the onset of films depicting biological motion (e.g. a bike race, boxing, or a funeral), and that the magnitude of desynchronization was related to the amount of identification the participant had with the actor on the screen. Although their descriptions were qualitative in nature, the effect of mu desynchronization to the observation of human motion described by this study has been replicated by several investigations [14, 15, 33, 45].
Pfurtscheller and colleagues [61] identified the characteristics of hand and foot mu rhythm desynchronization in adults performing repetitive movements. They noted a desynchronization in 10Hz prior to onset of motion and then synchronization 2 seconds following movement in lateral central electrodes (C3/4) for the hand and fingers and medial central electrode (Cz) for the foot [4, 61]. The central electrodes are on scalp locations above the sensorimotor cortex and roughly reflect a somatotopic organization.
Indeed a number of studies have shown equivalent desynchronization for execution and observation of hand movements [36, 39, 52, 54]. And studies by Muthukumaraswamy and colleagues showed mu rhythm desynchronization only to goal-directed hand movements, showing modulation of the mu rhythm to the goal, rather than the action itself [52, 54]. However, additional research has shown that non-goal directed actions devoid of a target desynchronize the mu rhythm [4, 36, 61]. Although the goal-directedness of the movement may modulate the mu rhythm, it is not a prerequisite for the suppression effect.
The mu rhythm responds to many of the same types of stimuli that were tested in the monkeys beyond observation/execution matching. Desynchronization of mu has been shown as a result of: (a) action related auditory stimuli [41]; (b) oral ingestive and communicative gestures [53]; (c) positive and negative emotion faces [50]; and (d) abstract motion stimuli, including robotic actions [58] and point-light biological motion [44, 73]. Taken together these data suggest that, in addition to basic observation/execution matching, the mu rhythm may be sensitive to the goals of actions across modalities. The study of the EEG desynchronization during action observation has also been used to investigate the role of experience and learning in modyfing the activity of the mirror system. In humans this has been studied extensively by examining mu activity during actions observed by specialists and non-specialists. For example, studies contrasting dancers versus non-dancers observing dance movements show greater desynchronization in the dancers familiar with the dance movement [60]. Moreover, evidence shows that activation of the action observation/execution matching system improves athletes’ ability to understand the outcome of their sport related actions sooner and more accurately than the non-athletes [1]. These studies parallel those obtained with fMRI investigations showing that the MN areas are more active during the observation of a movement that has been specifically learned during one's own professional training, such as in dancing [11, 12]. Together, these findings suggest that motor experience modifies internal motor representations and the related neural networks involved in processing sensorimotor control.
The assumption that the mu rhythm reflects activation of the MN system has been further supported by studies using both EEG and functional MRI [3]. The results clearly showed that during action observation mu desynchronization correlated with activity in the IPL and other areas associated with the MN system [37], such as the supplementary motor area and the dorsal premotor area. These data suggest that the mu rhythm could be considered a neural signature of the MN system.
The use of these noninvasive methods has provided converging evidence for a human MN system. They also offer an opportunity to measure MN activity in different populations and under different conditions to begin to better understand the role of MNs in typical and atypical development as well as more complex social behaviors such as imitation and empathy.
Development of the MN System
In the last few years MN research has taken several directions but one of the most exciting and inevitable questions that has been raised is how MNs emerge ontogenetically. This represents a formidable challenge in brain research because this knowledge bears empirical and theoretical implications in the way environmental and epigenetic factors contribute to shape neural networks involved in the processing of social information. Furthermore, this issue is even more compelling when considering therapies/preventive interventions targeting the social/cognitive impairments that are associated with MN functioning. The relevance of this topic has also led several scholars to debate the possible function of MNs in imitation and action recognition. It is therefore of utmost importance to monitor the emergence of MNs in the course of infant development and to establish to which extent behavioral and cognitive changes are associated and parallel modifications in their activities.
A growing body of evidence derived by means of behavioral studies (for a review see [13, 26, 40]) and more recently by electrophysiological studies [25], described below, is supporting the presence of a mirror mechanism in the very early stages of development. By means of EEG recordings of the mu rhythm in infancy it is now feasible to track developmental changes of the action observation/execution matching mechanism.
The mu rhythm is characterized by frequencies that fall within the range of the alpha rhythm, however, the alpha rhythm is not static but changes developmentally. Marshall, Bar-Haim, & Fox [43] have shown that the alpha rhythm steadily increases in frequency over infancy and early childhood. Moreover, their data suggest that the mu rhythm follows a similar developmental trajectory with peaks at 5 or 6Hz in 5-month-old infants that increase to 8Hz in 2-year-olds and reach approximately adult frequencies (9 to 10Hz) by 4 years of age. Lepage and Theoret [39] examined mu rhythm activity during the execution and observation of a precision grip in 4- to 11-year-old children. They found desynchronization to both execution and observation condition in 8–13Hz, frequencies similar to that reported in the adult literature, confirming the finding by Marshall and colleagues [43].
Although the development of the mu rhythm is still poorly understood, it is possible that the changes occurring in the course of ontogeny reflect several events occurring in the child’s brain and in the refinement of the sensorimotor system. It is therefore plausible that learning processes may affect the mu rhythm. Based on the MNs’ hypothesized function in action understanding, the system is sensitive to others’ behavior when the motor representations of those behaviors are developed. In fact, behavioral evidence has confirmed that the capacity to understand the actions of others depends on the motor experiences of the infant [69].
Electrophysiological evidence for the association between the developing motor repertoire and action understanding is only beginning to emerge. In a study of 14- to 16- month-old infants van Elk and colleagues [76] recorded EEG while infants observed other infants walking or crawling. They found greater desynchronization in the mu rhythm for observation of crawling compared to walking and the magnitude of desynchronization was significantly correlated with the amount of crawling experience the infants had. While the understanding of the action was not directly assessed, these data show that the mu rhythm is sensitive to the level of experience an infant has had with an action or its goals.
The majority of research examining the mu rhythm has employed reaching behaviors as stimuli and young infants are still developing reaching behaviors [21]. Nevertheless, a number of studies have examined observation of grasping behaviors in infants as young as 8 months [56, 70, 71]. Nystrom and colleagues [56] found desynchronization during the observation of goal-directed grasps in 8 month olds in the 6–8 Hz frequency band, but did not collect data on execution of grasps.
Southgate and colleagues replicated Nystrom and colleagues’ [56] findings in 9 month olds. They also collected EEG data while the infants completed grasps and so they were able to fully characterize the mu rhythm during action execution [70, 71]. The most interesting finding came as a result of their design. In a task similar to Umiltà and colleagues [75] on monkeys, human infants were presented with goal-directed grasps and non-goal directed actions in visible and occluded conditions. They found mu desynchronization occurred for both the occluded and visible goal-directed grasp [70]. Infants of this age easily understand the goal of these grasping actions and desynchronization of the mu rhythm suggests the motor system is involved in extracting the goals of these actions [78].
Attempts at identifying mu rhythm desynchronization to grasping actions in infants younger than 8-months have been inconclusive. In fact, in a study of 6-month-old infants observing grasping behaviors, no desynchronization of the mu rhythm was found [55], providing further evidence that a motor repertoire is required for activation of the MN system.
In the last few years we have conducted a series of studies to investigate, at a behavioral level, newborn macaques’ capacity to match facial gestures. Similarly to human infants [48], we showed that infant rhesus macaques imitate their mothers and other nonconspecifics tongue protrusion and lip smacking and that these behaviors can be observed on the first postnatal days [23, 26]. These imitative behaviors offer an execution paradigm that can be used with infants shortly after birth to understand whether, as it has been proposed [26], a mirror mechanism may underlie such behaviors. Recently, we used this paradigm while collecting EEG data from rhesus infants in the first week of life [25]. We found desynchronization in the 5 – 6Hz frequency band to the execution and observation of lip smacking and tongue protrusion, compared to a non-biological control condition, in electrodes placed approximately over the motor cortex (see Figure 2). These data are the first to show that the motor system is activated equally to early imitative behaviors and facial gesture observation in infant monkeys [25].
Figure 2.
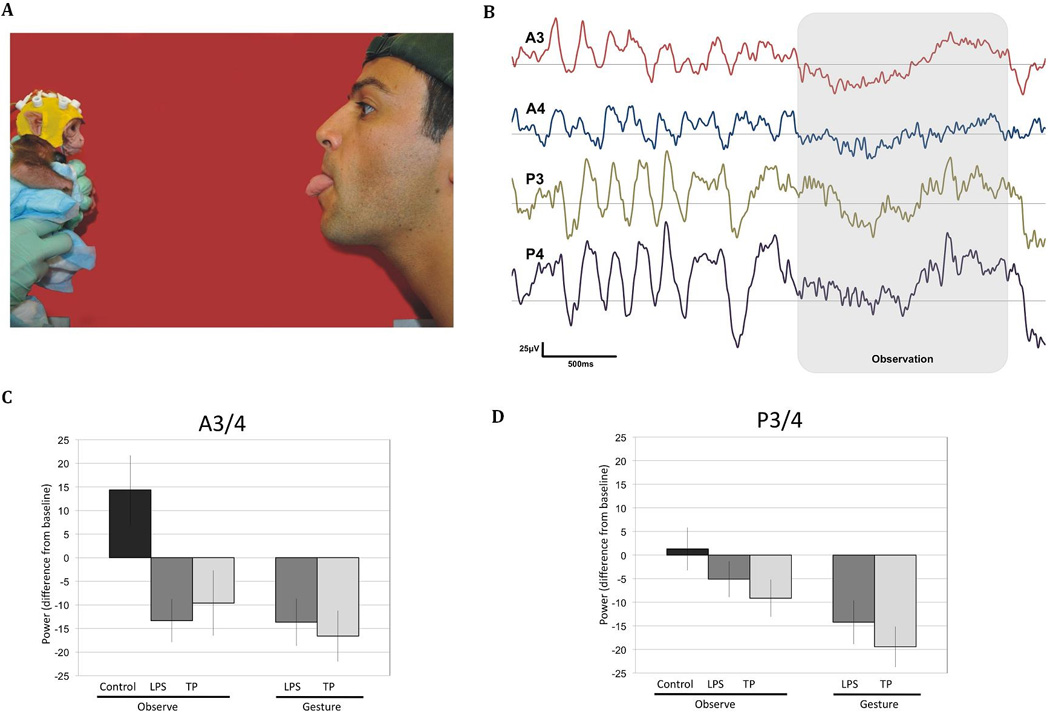
Mu desynchronization in one week-old rhesus macaques to the production and observation of lip smacking (LS) and tongue protrusion (TP). (A) Examples of the settings and procedures used during EEG acquisition. Infants were held by one experimenter while a second experimenter acted as a live stimulus for facial gestures. (B) An example of the EEG from a 3-day-old infant rhesus with desynchronization in the 5 – 6Hz frequency band during gesture lip smacking observation (shaded area) and (B-C) average desynchronization during the observation and gesture conditions in the frontal and posterior electrodes, respectively (from [25]).
The MN System and their Implications in Social Behaviors
The work on the emergence of the MN system in human social cognition has implications for individual differences and for conditions of perturbed social behavior. In particular, autism spectrum disorder (ASD) is marked by deficits in domains that may be directly related to MN function [2, 57].
The evidence from studies with children and adults diagnosed with ASD is conflicting. In the earliest studies, mu desynchronization was observed for execution of repeated releasing and clenching of a fist, but in children and adults with ASD, no desynchronization occurred during observation [57]. A similar effect was observed in children with ASD watching videos of a woman stretching versus balls bouncing [46] and for images of faces expressing the five basic emotions [16]. These studies concluded that children and adults diagnosed with ASD had impairments in the MN system for the observation of actions.
Conversely, Raymaekers and colleagues used the same paradigm as Oberman, et al. [57] and found that children with ASD had similar patterns of mu desynchronization as typically developing children during the observation condition [62]. Similarly, Dinstein and colleagues [19] showed that adults with ASD did not differ from typical adults in activation of the MN circuit during a game of rock-paper-scissors, suggesting that children and adults with ASD do not have impairments in their MN system.
Both sets of studies suffer from methodological problems. First, there were no assessments of the participants’ imitation ability and this may play a role in the degree to which there is MN activation. Bernier, Dawson, Webb, & Murias [5] tested adults with ASD and age matched controls on a number of different hand gestures, facial expression, meaningless hand movements, and actions on objects and scored the fidelity of imitation. They then collected EEG data while they observed, executed, or imitated a precision grip [39, 52]. Compared to the controls, adults with ASD had significantly less mu desynchronization during the observe condition. Importantly, this desynchronization was related to their fidelity scores in the imitation task [5].
Second, familiarity with the stimulus seems to play a role in the activation of the MN system in children with ASD. Oberman, Ramachandran, and Pineda [59] varied the degree to which the hand performing the action was familiar to the participant. When children observed their own hand or a hand of a relative performing the grasp, the magnitude of mu desynchronization was no different than typically developing children; only in the condition in which the hand belonged to a stranger did the children with ASD show no mu desynchronization. Taken together, the results of Oberman, et al. and Bernier, et al. [5, 59] suggest that features of the stimulus or individual differences may impact the degree to which the MN system is activated in children and adults with ASD.
Conclusions
The discovery of MNs in the monkey has led to a great number of studies examining the role of a putative MN system underlying action-perception links in human subjects. It has also led to a great deal of speculation and theorizing (some of it unwarranted) regarding the areas of human cognition and particularly social cognition that may involve this system. It is, however, fair to say that developmental psychologists have debated the underlying mechanisms that may account for the emergence of complex social cognition in the first years of life. No one mechanism or brain system has successfully captured an understanding of the emergence of joint attention, theory of mind and perspective taking, and empathic behaviors that appear over the first years of life. Behavioral approaches that involve imitation, social referencing, and observational learning are important theoretical and conceptual approaches but what the MN system provides, possibly, is the underlying neural system that supports these behavioral processes.
Improved brain technologies have made it possible to examine the MN system in a non-invasive way during observation and execution in infant, child, and adult populations. There are, however, a number of issues that are important to consider before concluding that these findings are analogous to the single cell recordings in the monkey. Among these issues are: 1) timing of the activation. A reading of the monkey MN work suggests that MNs fire at precise times during the execution of a motor action and similarly during a precise moment of observation. Most brain imaging studies either cannot (e.g. functional imaging studies) or have not examined this issue. In order to do this correctly, it is necessary to synchronize the behavior under execution or observation with the ongoing electrophysiology and to examine event related changes. 2) Source localization; while functional MRI provides localization it does not provide the timing necessary. On the other hand, the excellent temporal resolution of EEG has problems with source localization. MEG has both good temporal and spatial resolution but is generally difficult with infant and child populations. Thus, it is not clear that the same MN system, identified in the monkey is being tapped by mu desynchronization in the human. Future studies with finer synchronization and localization should address these issues. 3) The link to social cognition. Until now much of the MN work with monkeys has examined motor behaviors involved in grasping and localization of objects in space. More complex social cognitive behaviors like imitation are just now being reported in non-human primates (see above, [25]). It is important to move the monkey work forward to understand the differences of the MN system between animals and humans in relation to their particular social cognitive abilities.
In sum, there is great promise to understanding both typical and atypical social cognitive development with the MN system. Solid empirical work with appropriate measurement methods will advance our understanding of the links between this motor action perception system and human qualities that make us the social beings we are.
The mu-rhythm is optimal to investigate the mirror neuron system in infancy Studies in monkeys demonstrate that the MNS is functional very early after birth Mu-rhythm changes during ontogeny reflect events related to the sensorimotor system Very likely the MNS is the underlying neural system that supports newborn imitation
Acknowledgements
This research was supported by P01HD064653-01 NIH grant to PFF and NAF, and division of Intramural Research, NICHD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abreu AM, Macaluso E, Azevedo RT, Cesari P, Urgesi C, Aglioti SM. Action anticipation beyond the action observation network: A functional magnetic resonance imaging study in expert basketball players. Eur. J. Neurosci. 2012;35:1646–1654. doi: 10.1111/j.1460-9568.2012.08104.x. [DOI] [PubMed] [Google Scholar]
- 2.APA. Diagnostic and Statistical Manual of Mental Disorders. fourth ed. APA; Washington DC: 2000. [Google Scholar]
- 3.Arnstein D, Cui F, Keysers C, Maurits NM, Gazzola V. μ-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. J. Neurosci. 2011;31:14243–14249. doi: 10.1523/JNEUROSCI.0963-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babiloni C, Carducci F, Cincotti F, Rossini PM, Neuper C, Pfurtscheller G, et al. Human movement-related potentials vs desynchronization of EEG alpha rhythm: A highresolution EEG study. Neuro Image. 1999;10:658–665. doi: 10.1006/nimg.1999.0504. [DOI] [PubMed] [Google Scholar]
- 5.Bernier R, Dawson G, Webb S, Murias M. EEG mu rhythm and imitation impairments in individuals with autism spectrum disorder. Brain. Cogn. 2007;64:228–237. doi: 10.1016/j.bandc.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonini L, Rozzi S, Serventi FU, Simone L, Ferrari PF, Fogassi L. Ventral premotor and inferior parietal cortices make distinct contribution to action organization and intention understanding. Cereb. Cortex. 2010;20:1372–1785. doi: 10.1093/cercor/bhp200. [DOI] [PubMed] [Google Scholar]
- 7.Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. Eur. J. Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- 8.Caggiano V, Fogassi L, Rizzolatti G, Casile A, Giese MA, Their P. Mirror neurons encode the subjective value of an observed action. Proc. Natl. Acad. Sci. U.S.A. 2012;109:11848–11853. doi: 10.1073/pnas.1205553109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caggiano V, Fogassi L, Rizzolatti G, Pomper JK, Thier P, Giese MA, et al. View-based encoding of actions in mirror neurons of area f5 in macaque premotor cortex. Curr. Biol. 2011;21:144–148. doi: 10.1016/j.cub.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Caggiano V, Fogassi L, Rizzolatti G, Thier P, Casile A. Mirror neurons differentially encode the peripersonal and extrapersonal space of monkeys. Science. 2009;324:403–406. doi: 10.1126/science.1166818. [DOI] [PubMed] [Google Scholar]
- 11.Calvo-Merino B, Glaser DE, Grezes J, Passingham RE, Haggard P. Action observation and acquired motor skills: An fMRI study with expert dancers. Cereb. Cortex. 2005;15:1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- 12.Calvo-Merino B, Grezes J, Glaser DE, Passingham RE, Haggard P. Seeing or doing? Influence of visual and motor familiarity in action observation. Curr. Biol. 2006;16:1905–1910. doi: 10.1016/j.cub.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 13.Casile A, Caggiano V, Ferrari PF. The mirror neuron system: A fresh view. Neuroscientist. 2011;17:524–538. doi: 10.1177/1073858410392239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochin S, Barthelemy C, Lejeune B, Roux S, Martineau J. Perception of motion and qEEG activity in human adults. Electroencephalogr. Clin. Neurophysiol. 1998;107:287–295. doi: 10.1016/s0013-4694(98)00071-6. [DOI] [PubMed] [Google Scholar]
- 15.Cochin S, Barthelemy C, Roux S, Martineau J. Electroencephalographic activity during perception of motion in childhood. Eur. J. Neurosci. 2001;13:1791–1796. doi: 10.1046/j.0953-816x.2001.01544.x. [DOI] [PubMed] [Google Scholar]
- 16.Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2005;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: A neurophysiological study. Exp. Brain. Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- 18.Dinstein I, Hasson U, Rubin N, Heeger DJ. Brain areas selective for both observed and executed movements. J. Neurophysiol. 2007;98:1415–1427. doi: 10.1152/jn.00238.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinstein I, Thomas C, Humphreys K, Minshew N, Behrmann M, Heeger DJ. Normal movement selectivity in autism. Neuron. 2010;66:461–469. doi: 10.1016/j.neuron.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Essen DCV. Windows on the brain: The emerging role of atlases and databases in neuro Science. Curr. Opin. Neurobiol. 2002;12:574–579. doi: 10.1016/s0959-4388(02)00361-6. [DOI] [PubMed] [Google Scholar]
- 21.Fagard J. Linked proximal and distal changes in the reaching behavior of 5- to 12- month-old human infants grasping objects of different sizes. Infant. Behav. Dev. 2000;23:317–329. [Google Scholar]
- 22.Ferrari PF, Gallese V, Rizzolatti G, Fogassi L. Mirror neurons responding to observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur. J. Neurosci. 2003;17:1703–1714. doi: 10.1046/j.1460-9568.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- 23.Ferrari PF, Paukner A, Ionica C, Suomi SJ. Reciprocal face-to-face communication between rhesus macaque mothers and their newborn infants. Curr. Biol. 2009;19:1768–1772. doi: 10.1016/j.cub.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrari PF, Rozzi S, Fogassi L. Mirror neurons responding to observation of actions made with tools in monkey ventral premotor cortex. J. Cogn. Neurosci. 2005;17:212–226. doi: 10.1162/0898929053124910. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari PF, Vanderwert RE, Paukner A, Bower S, Suomi SJ, Fox NA. Distinct EEG amplitude suppression to facial gestures as evidence for a mirror mechanism in newborn monkeys. J. Cogn. Neurosci. 2012;24:1165–1172. doi: 10.1162/jocn_a_00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi SJ. Neonatal imitation in rhesus macaques. PLoS. Biol. 2006;4:1501–1508. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: From action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- 28.Gallese V, Eagle MN, Migone P. Intentional attunement: mirror neurons and the neural underpinnings of interpersonal relations. J. Am. Psychoanal. Assoc. 2007;55:131–176. doi: 10.1177/00030651070550010601. [DOI] [PubMed] [Google Scholar]
- 29.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 30.Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends. Cogn. Sci. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- 31.Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends. Cogn. Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Gastaut HJ, Bert J. EEG changes during cinematographic presentation. Electroencephalogr. Clin. Neurophysiol. 1954;6:433–444. doi: 10.1016/0013-4694(54)90058-9. [DOI] [PubMed] [Google Scholar]
- 33.Holtz EM, Doppelmayr M, Klimesch W, Sauseng P. EEG correlates of action observation in humans. Brain. Topogr. 2008;21:93–99. doi: 10.1007/s10548-008-0066-1. [DOI] [PubMed] [Google Scholar]
- 34.Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 35.Iacoboni M. Neurobiology of imitation. Curr. Opin. Neurobiol. 2009;19:661–665. doi: 10.1016/j.conb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Kessler K, Biermann-Ruben K, Jonas M, Siebner HR, Baumer T, Munchau A, et al. Investigating the human mirror neuron system by means of cortical synchronization during the imitation of biological movements. NeuroImage. 2006;33:227–238. doi: 10.1016/j.neuroimage.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Keysers C, Gazzola V. Expanding the mirror: Vicarious activity for actions, emotions, and sensations. Curr. Opin. Neurobiol. 2009;19:666–671. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Kohler E, Keysers C, Umilta MA, Fogassi L, Gallese V, Rizzolatti G. Hearing sounds, understanding actions: Action representation in mirror neurons. Science. 2002;297:846–848. doi: 10.1126/science.1070311. [DOI] [PubMed] [Google Scholar]
- 39.Lepage J-F, Theoret H. EEG evidence for the presence of an action observation-execution matching system in children. Eur. J. Neurosci. 2006;23:2505–2510. doi: 10.1111/j.1460-9568.2006.04769.x. [DOI] [PubMed] [Google Scholar]
- 40.Lepage J-F, Theoret H. The mirror neuron system: Grasping others’ actions from birth? Dev. Sci. 2007;10:513–529. doi: 10.1111/j.1467-7687.2007.00631.x. [DOI] [PubMed] [Google Scholar]
- 41.Lepage J-F, Tremblay S, Nguyen DK, Champoux F, Lassonde M, Theoret H. Action related sounds induce early and late modulations of motor cortex activity. NeuroReport. 2010;21:250–253. doi: 10.1097/WNR.0b013e328334ddcc. [DOI] [PubMed] [Google Scholar]
- 42.Manthey S, Schubotz RI, von Cramon DY. Premotor cortex in observing erroneous action: An fMRI study. Cogn. Brain. Res. 2003;15:296–307. doi: 10.1016/s0926-6410(02)00201-x. [DOI] [PubMed] [Google Scholar]
- 43.Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- 44.Marshall PJ, Shipley TF. Event-related potentials to point-light displays of human actions in five-month-old infants. Dev. Neuropsychol. 2009;34:368–377. doi: 10.1080/87565640902801866. [DOI] [PubMed] [Google Scholar]
- 45.Martineau J, Cochin S. Visual perception in children: human, animal and virtual movement activates different cortical areas. Int. J. Psychophysiol. 2003;51:37–44. doi: 10.1016/s0167-8760(03)00151-x. [DOI] [PubMed] [Google Scholar]
- 46.Martineau J, Cochin S, Magne R, Barthelemy C. Impaired cortical activation in autistic children: Is the mirror neuron system involved? Int. J. Psychophysiol. 2008;68:35–40. doi: 10.1016/j.ijpsycho.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Meltzoff AN. The ‘like me’ framework for recognizing and becoming an intentional agent. Acta. Psychol. 2006;124:26–43. doi: 10.1016/j.actpsy.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- 49.Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci. Biobehav. Rev. 2012;36:341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Moore A, Gorodnitsky I, Pineda J. EEG mu component responses to viewing emotional faces. Behav. Brain Res. 2012;226:309–316. doi: 10.1016/j.bbr.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 51.Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-neuron responses in humans during execution and observation of actions. Curr. Biol. 2010;20:750–756. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muthukumaraswamy SD, Johnson BW. Changes in rolandic mu rhythm during observation of a precision grip. Psychophysiology. 2004;41:152–156. doi: 10.1046/j.1469-8986.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 53.Muthukumaraswamy SD, Johnson BW, Gaetz WC, Cheyne DO. Neural processing of observed oro-facial movements reflects multiple action encoding strategies in the human brain. Brain. Res. 2006;1071:105–112. doi: 10.1016/j.brainres.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 54.Muthukumaraswamy SD, Johnson BW, McNair NA. Mu rhythm modulation during observation of an object-directed grasp. Cogn. Brain. Res. 2004;19:195–201. doi: 10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Nystrom P. The infant mirror neuron system studied with high density EEG. Soc. Neurosci. 2008;3:334–347. doi: 10.1080/17470910701563665. [DOI] [PubMed] [Google Scholar]
- 56.Nystrom P, Ljunghammar T, Rosander K, von Hofsten C. Using mu rhythm perturbations to measure mirror neuron activity in infants. Dev. Sci. 2012;14:327–335. doi: 10.1111/j.1467-7687.2010.00979.x. [DOI] [PubMed] [Google Scholar]
- 57.Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cogn. Brain. Res. 2005;24:190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 58.Oberman LM, McCleery JP, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron activity during the observation of human and robot actions: Toward an analysis of the human qualities of interactive tools. Neurocomputing. 2007;70:2194–2203. [Google Scholar]
- 59.Oberman LM, Ramachandran VS, Pineda JA. Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: The mirror neuron hypothesis. Neuropsychologia. 2008;46:1558–1565. doi: 10.1016/j.neuropsychologia.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 60.Orgs G, Dombrowski J-H, Heil M, Jansen-Osmann P. Expertise in dance modulates alpha/beta event-related desynchronization during action observation. Eur. J. Neurosci. 2008;27:3380–3384. doi: 10.1111/j.1460-9568.2008.06271.x. [DOI] [PubMed] [Google Scholar]
- 61.Pfurtscheller G, Neuper C, Andrew C, Edlinger G. Foot and hand area mu rhythms. Int. J. Psychophysiol. 1997;26:121–135. doi: 10.1016/s0167-8760(97)00760-5. [DOI] [PubMed] [Google Scholar]
- 62.Raymaekers R, Wiersema JR, Roeyers H. EEG study of the mirror neuron system in children with high functioning autism. Brain. Res. 2009;1304:113–121. doi: 10.1016/j.brainres.2009.09.068. [DOI] [PubMed] [Google Scholar]
- 63.Rizzolatti G, Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 64.Rizzolatti G, Fabbri-Destro M. The mirror system and its role in social cognition. Curr. Opin. Neurobiol. 2008;18:179–184. doi: 10.1016/j.conb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 65.Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cogn. Brain. Res. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 66.Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- 67.Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nat. Rev. Neurosci. 2010;11:264–274. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- 68.Rozzi S, Ferrari PF, Bonini L, Rizzolatti G, Fogassi L. Functional organization of inferior parietal lobule convexity in the macaque monkey: Electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. Eur. J. Neurosci. 2008;28:1569–1588. doi: 10.1111/j.1460-9568.2008.06395.x. [DOI] [PubMed] [Google Scholar]
- 69.Sommerville JA, Woodward AL, Needham A. Action experience alters 3-month-old infants’ perception of others’ actions. Cognition. 2005;96:B1–B11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Southgate V, Johnson MH, El Karoui I, Csibra G. Motor system activation reveals infants’ on-line prediction of others’ goals. Psychol. Sci. 2010;21:355–359. doi: 10.1177/0956797610362058. [DOI] [PubMed] [Google Scholar]
- 71.Southgate V, Johnson MH, Osborne T, Csibra G. Predictive motor activation during action observation in human infants. Biol. Lett. 2009;5:769–772. doi: 10.1098/rsbl.2009.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thelen E. Motor development as foundation and future of developmental psychology. Int. J. Behav. Dev. 2000;24:385–397. [Google Scholar]
- 73.Ulloa ER, Pineda JA. Recognition of point-light biological motion: Mu rhythms and mirror neuron activity. Behav. Brain. Res. 2007;183:188–194. doi: 10.1016/j.bbr.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 74.Umiltà MA, Escola L, Intskirveli I, Grammont F, Rochat M, Caruana F, et al. When pliers become fingers in the monkey motor system. Proc. Natl. Acad. Sci. U.S.A. 2008;105:2209–2213. doi: 10.1073/pnas.0705985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Umiltà MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, et al. I know what you are doing: A neurophysiological study. Neuron. 2001;31:155–165. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- 76.van Elk M, van Schie HT, Hunnius S, Vesper C, Bekkering H. You’ll never crawl alone: Neurophysiological evidence for experience-dependent motor resonance in infancy. Neuro Image. 2008;43:808–814. doi: 10.1016/j.neuroimage.2008.07.057. [DOI] [PubMed] [Google Scholar]
- 77.von Hofsten C. Motor development as the development of systems: Comments on the special section. Dev. Psychol. 1998;25:950–953. [Google Scholar]
- 78.Woodward AL. The infant origins of intentional understanding. In: Kail RV, editor. Advances in Child Development and Behavior. Volume 33. Elsevier; Oxford: 2005. pp. 229–262. [DOI] [PubMed] [Google Scholar]


