Abstract
CD1 molecules are Major Histocompatibility Complex (MHC) class I-like proteins that present diverse lipid antigens to T cells. Most of our understanding of CD1 lipid presentation and T cell recognition has come from study of the invariant Natural Killer T cell recognition of CD1d. However, in addition to CD1d, humans possess three additional CD1 molecules: CD1a, CD1b and CD1c, referred to as the Group 1 CD1s. The lack of an appropriate murine molecule to probe the function and disease relevance of these molecules has hindered understanding their precise immunological role, despite their pivotal role in human immunity. In this perspective, we discuss the progress of functional and molecular studies of CD1c. CD1c has been shown to specifically present lipids from Mycobacterium tuberculosis and other related pathogenic mycobacteria. αβ T cells reactive to these lipids presented in the context of CD1c have been characterized and upon stimulation secrete IFN-γ, an important cytokine in tuberculosis disease clearance. Other ligands characterized for CD1c include PI and PC, a lipopeptide with a dodecameric peptide moiety and sulfatides. These structurally and chemically diverse ligands suggest that CD1c has the capacity to present a wide repertoire of antigens to reactive T cells. Indeed, a substantial percentage (~2%) of the circulating αβ T cell population is reactive to CD1c presenting endogenous antigens, suggesting that this particular Group 1 molecule may play an important role in the human immune response.
Introduction
CD1c belongs to the family of Major Histocompatibility Complex (MHC) class I like proteins that serve to present lipid-based antigens to T cells. Located outside of the MHC region on chromosome 1 in humans and chromosome 3 in mouse, the CD1 genes are essentially monomorphic, unlike the highly polymorphic MHC classical class I genes that encode the peptide-presenting classical MHC molecules. In humans there are five CD1 molecules that have been grouped, based on their genomic organization, sequence homology and cellular functions into Group 1 (CD1a, CD1b, and CD1c) and Group 2 (CD1d) (Brigl and Brenner, 2004). Both Group 1 and Group 2 CD1 molecules have been shown to present lipid antigens on the cell surface in humans, whereas CD1e is found predominantly intracellularly and is thought to perform chaperone functions. Two CD1D genes are present in the mouse, however mice lack the Group 1 and CD1E genes likely due to a deletion of the region surrounding the Group 1 genes via a chromosomal translocation. Representatives of both the Group 1 and Group 2 CD1 molecules have been characterized in other species such as guinea pigs, rabbits, pigs, cows, dogs and sheep, therefore the Group 1 deletion appears to be specific to the rodent lineage (Brigl and Brenner, 2004). The lack of Group 1 CD1 molecules represent a distinct difference in lipid antigen presentation potential between humans and mice and thus poses a challenge when extrapolating CD1 function from the mouse model. CD1c, the focus of this perspective, is found in multiple copies in the guinea pig and as a single copy in the dog but has not been characterized in other species. Our understanding of the role of CD1c in human immunity is becoming better-understood thanks to structural information, characterization of CD1c specific T cells and humanized-mouse models where CD1c and the T cells that respond to it can be more easily manipulated. Our current understanding of the structure and function of CD1 molecules, CD1c in particular, is discussed below.
Current Status
Intracellular trafficking and cellular expression profiles of CD1c compared to the other CD1 isoforms
CD1c and the other CD1 molecules are synthesized in the endoplasmic reticulum where they are loaded with endogenous lipids and then trafficked to the cell surface via the secretory pathway (Brigl and Brenner, 2004). Internalization of the CD1 molecules then occurs, and their pathway through the endosomal compartment is dictated by specific sequence motifs in their cytoplasmic tails. CD1c is unique in that it broadly traffics through all compartments, but the other CD1 isoforms vary in the depth of their trafficking. CD1a does not contain a clear sorting motif and thus is trafficked to early recycling endosomes, whereas CD1b primarily surveys late endosomes and lysosomes. CD1d sorts through early and late endosomes but not in recycling endosomes and is only partially localized in lysosomes. After surveying their respective compartments, where lipid transfer is presumed to occur, the CD1 molecules return to the cell surface where they present their newly loaded lipid antigens. The trafficking pattern of each CD1 type is a key parameter in what lipid antigens they are exposed to, both in proximity and via facilitated transfer through lipid chaperone molecules. Thus, different CD1 isoforms will be exposed to a different repertoire of lipid antigens depending on how they are trafficked. In addition, biochemical parameters such as pH (due to differential acidification of the endosomal compartments) will also have profound affects on lipid loading and protein stability. Other features, such as protein structure, also shape the lipid repertoire presented by each CD1 isoform and are discussed in more detail below.
The unique expression and regulation patterns of the Group 1 CD1 isoforms are another key feature that distinguishes human CD1 molecules from that of the mouse model (Brigl and Brenner, 2004). CD1d, the only CD1 isoform in mouse, is expressed broadly on professional antigen presenting cells (APCs), immature and mature thymocytes and peripheral T cells, in the liver and on the gastrointestinal epithelium. Human CD1d is also broadly expressed; it is detected at low levels on most monocytes, highly expressed on circulating and splenic B cells and also found on thymocytes, epithelial cells, parenchymal cells and vascular smooth muscle in the gut and liver. CD1d expression, however, is characteristically low and does not appear to be up-regulated on mature professional APCs. The Group 1 CD1 molecules, CD1c in particular, are characteristic markers for dendritic cells and other professional APCs. CD1c is found on Langerhans cells, and is unique in its expression on subsets of B cells, including lymph node mantle zones and germinal centers, in splenic marginal B zones and on certain circulating B cells in fetal and adult human blood. CD1c, in combination with the other CD1 family members, likely coordinate to present chemically and structurally divergent classes of self and foreign lipids to T cells, and this coordination may play key roles in combatting specific infections or diseases. The study of the structural and biochemical features of lipid presentation by CD1c as well as how these complexes are recognized by CD1c specific T cells will be key steps forward in our understanding of the coordinated functioning of these molecules in the immune response. Furthermore, the established role for CD1c presentation of lipids from pathogenic mycobacteria (M. tuberculosis for example, see below) makes this a potential candidate for vaccine development against important human diseases such as tuberculosis.
CD1c lipid presentation and its role in disease
Lipid presentation by CD1 molecules typically involves lipids with two fatty acid chains. These come in the form of phospholipids (like the self lipids phosphatidylcholine (PC) and phosphatidylinositol (PI)) and glycolipids (both self and foreign forms, typically differing in the linkage of their sugar head group). β-linked carbohydrates are found in both eukaryotes and prokaryotes, however α-linked glycolipids are not found in mammals; this linkage is therefore an ideal sensor for bacterial infection (Brigl and Brenner, 2004). Most of what is known about CD1 lipid presentation has been derived from work on mouse and human CD1d, mainly due to their restriction by invariant Natural Killer T cells (iNKT) (Bendelac et al., 2007). This work has shown that subtle modifications in the length and/or saturation of the fatty acid chains can have profound affects on the effector functions of the reactive T cells, and can influence disease outcome in animal models of multiple sclerosis, rheumatoid arthritis and inflammatory bowel disease. This approach is now being extended to the Group 1 CD1s, and our understanding of what range of lipid antigens these molecules present is improving. Work that we and others have done have demonstrated that CD1c can bind and in some cases present to T cells glycolipids classically presented by CD1d, such as α-galactosylceramide (α-GalCer), GT1b, sulfatides as well as others. Endogenous lipids for CD1c have recently been characterized and include different isoforms of PC and PI (Haig et al., 2011). In addition, through very elegant studies, two unique classes of lipid antigens have been defined for CD1c: 1) those derived from pathogenic bacteria strains such as Mycobacterium tuberculosis and M. avium that contain methylated (isoprenoid) mono-alkyl chains (such as phosphodolichols and phosphomycoketides) (see (Brigl and Brenner, 2004)) and 2) N-terminally acylated lipopeptides, such as those seen in myristoylated proteins (Van Rhijn et al., 2009). Both of these classes have only one hydrocarbon tail and thus suggest that CD1c may preferentially bind lipids of this type.
αβ T cells specific for CD1c presenting the mycobacterial antigens discussed above have been characterized from healthy human donors, suggesting that these reactive T cells are at a detectable frequency in previously unexposed individuals (see (Brigl and Brenner, 2004)). Furthermore αβ T cells from immunized, PPD+ (skin test) healthy human donors reacted strongly against the lipid fraction from M. tuberculosis. Both CD8+ and CD8−/CD4− (double negative or “DN”) αβ T cells have been characterized that are specific for M. tuberculosis mycoketides. The CD8-1 (CD8+) T cell line was the first established and has been widely used to study the mycobacterial response mediated by CD1c. This cell line, as well as two DN lines (DN2 and DN6) are stimulated (as measured by proliferation, IFN-γ secretion and specific lysis) strongly by mannosyl-β1-phosphomycoketide (MPM) and mannosyl phosphodolichol (MPD). Interestingly, these cells were not reactive to a related mannosyl phosphoprenol, which only differ from MPD in the saturation of the α-isoprene unit. Nor were they reactive to phosphodolichols from mammals that have substantially longer polyprenol chains (C90-C100). It is this key length difference that likely inhibits mammalian dolichol presentation in CD1c and prevents autoimmune recognition of this otherwise highly conserved lipid type. αβ T cells reactive to CD1c presenting an undefined lipid antigen from Mycobacterium leprae have also been characterized, suggesting that CD1c mediates immune recognition of diverse mycobacterial species. An important effector function of these CD1c reactive αβ T cells is IFN-γ secretion, which, in the case of M. tuberculosis, is key in the destruction of sequestered intracellular mycobacteria through stimulating endosomal maturation. The involvement of CD1c in immune recognition of one of the world’s most significant human pathogens makes it an ideal candidate for vaccine targeting. By understanding the molecular mechanisms of how this molecule presents mycobacterial lipids and how reactive αβ T cells recognize this complex, candidate synthetic molecules can be generated and screened for potential clinical applications.
CD1c restricted T cells have also been shown to respond to N terminally acylated peptides similar in sequence to that of the myristoylated NEF protein from HIV and thus may play an important role in recognition of bacterial or viral lipoprotein antigens. It is currently unknown how or if other lipopeptide antigens can be presented by CD1c and whether they are involved in αβ T cell recognition of viruses or bacteria. Our crystal structure of CD1c (Scharf et al., 2010) revealed unique features of CD1c that likely allows this molecule to present lipopeptide antigens, discussed in more detail below.
Structural features of CD1c that distinguishes it from the other CD1 isoforms
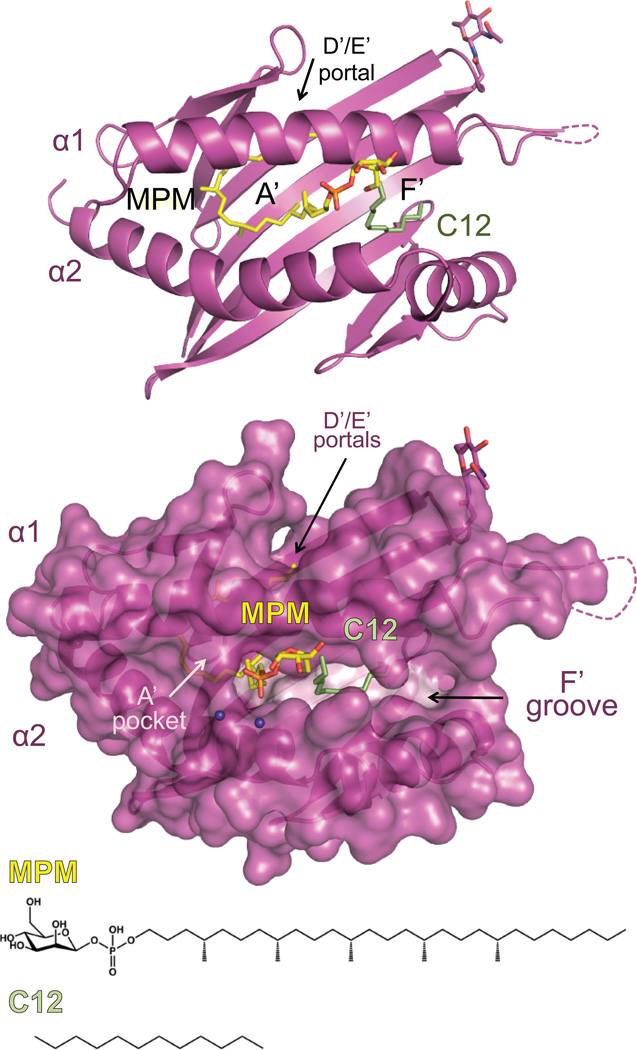
The first structure of a CD1 molecule (mouse CD1d) revealed a classical MHC-like architecture with a platform domain composed of two alpha helices supported by a beta sheet, yet instead of a shallow groove adapted to binding peptides, two major “tunnels” were found that extended deep into the platform domain (Brigl and Brenner, 2004). These features explained CD1d’s ability to bind lipids, and suggested that, despite being monomorphic, these molecules could present a diverse array of lipids varying in their head groups. The nomenclature of the tunnels (also referred to as pockets) was derived from that of classical MHC peptide binding pockets, and thus in CD1d they were named A’ and F’, based on their location in the CD1d molecule. Further structural studies of human CD1d revealed high conservation between the mouse and human version of this molecule, however structures of the human Group 1 isoforms, CD1a and CD1b, revealed that while the overall topology of these molecules were conserved, that sequence variation between the isoforms translated into quite different tunnel structures and demonstrated that in humans, the isoforms had become specialized to present particular, albeit overlapping, classes of lipid antigens to T cells (Brigl and Brenner, 2004). The structures of CD1a and CD1b differ in the size and length of their lipid antigen pockets; CD1a has a very short A’ pocket which was proposed to be a “molecular ruler” for the antigens it could present, whereas CD1b had a network of pocket and tunnel structures that explained its ability to present lipids with very long hydrocarbon chains. The structure of CD1c in complex with a mycoketide (Scharf et al., 2010) revealed several unique features of this isoform; specifically, CD1c has a unique portal (termed the D’ portal due to its proximity to the D’ pocket in classical MHC molecules) which likely allows for diversity in the length of hydrocarbon chains bound in the A’ pocket; indeed, MPM is bound in this pocket. Furthermore, CD1c’s F’ pocket, which is typically closed to solvent in other CD1 isoforms, is open, and has a deep groove-like structure that is reminiscent of the peptide binding grooves of classical MHC molecules. In this F’ groove we found a dodecameric fatty acid chain which was likely derived from the insect cells used to produce the protein. This suggests this F’ groove can bind a diverse array of ligands, and provides us with a model by which to test whether other ligands, such as the peptide moiety of a lipopeptide, can also be bound in this feature.
Future Perspectives
The diverse structural features of the Group 1 CD1 molecules provides a compelling rational for why the mouse is an inadequate model for understanding how lipid presentation in humans functions to defend against microbial disease, or how these molecules may contribute to autoimmunity. However, recent “humanized” mouse lines that express these Group 1 CD1 molecules may be pivotal in understanding how the diverse αβ T cell repertoire can recognize antigens presented in the context of these molecules (Felio et al., 2009; Lockridge et al., 2011). Furthermore, efforts to characterize the repertoire of T cells that respond to CD1c, either presenting endogenous or exogenous lipid antigens, will provide an important link to CD1c’s role in human immunity. Already it has been shown that CD1c can be recognized both by αβ and γδ T cells; the αβ T cell repertoire appears to express diverse TCRs while the γδ T cell repertoire appears to express a restricted Vδ repertoire (Vδ1). It is unclear if CD1c restricted αβ and γδ T cells perform coordinated functions; through better characterization and understanding of their tissue restriction, effector functions and lipid specificity we will begin to build a roadmap of how the Group 1 CD1 molecules function in human immunity. The diverse tissue expression and broad antigen presentation capabilities of the Group 1 CD1 molecules may also underlie an important surveillance mechanism both for pathogens and host homeostasis; current efforts to define a cellular “lipidome” and how this varies across tissues, is modified through microbial infection (i.e. viruses, bacteria and yeast/fungi) and changes during cellular transformation, will provide key information on the antigenic repertoire of these molecules. Manipulation of the lipids presented by these molecules may also provide a key therapeutic for human disease, with potential applications for auto-immunity, metabolic disorders, cancer and microbial infection.
Figure.
The structure of the Group 1 CD1 molecule, CD1c, is shown in magenta in ribbon (top) and surface (bottom). CD1c is presenting the mycobacterial lipid derived from M. tuberculosis, mannosyl-β1-phosphomycoketide (MPM), shown in yellow. Apparent in the structure was also a C12 spacer hydrocarbon chain, shown in pale green. The chemical structures of these lipids are shown at the bottom. CD1c has the canonical A’ and F’ pockets, however CD1c has a modified structure, including a unique exit portal (D/E portal) and an open F’ pocket resembling a groove-like structure. These modifications allow CD1c to present a unique repertoire of lipid antigens such as mycoketides and lipopeptides.
Highlights.
CD1 molecules present lipid antigens to T cells; we discuss the role of CD1c in human immunity.
CD1c’s unique structural modifications are discussed, enabling the presentation of diverse lipid-based antigens.
Future studies include characterizing the T cell repertoire reactive to CD1c, both from the αβ and γδ T cell lineages.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- Felio K, Nguyen H, Dascher CC, Choi HJ, Li S, Zimmer MI, Colmone A, Moody DB, Brenner MB, Wang CR. CD1-restricted adaptive immune responses to Mycobacteria in human group 1 CD1 transgenic mice. J Exp Med. 2009;206:2497–2509. doi: 10.1084/jem.20090898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig NA, Guan Z, Li D, McMichael A, Raetz CR, Xu XN. Identification of self-lipids presented by CD1c and CD1d proteins. J Biol Chem. 2011;286:37692–37701. doi: 10.1074/jbc.M111.267948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockridge JL, Chen X, Zhou Y, Rajesh D, Roenneburg DA, Hegde S, Gerdts S, Cheng TY, Anderson RJ, Painter GF, et al. Analysis of the CD1 antigen presenting system in humanized SCID mice. PLoS One. 2011;6:e21701. doi: 10.1371/journal.pone.0021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf L, Li NS, Hawk AJ, Garzon D, Zhang T, Fox LM, Kazen AR, Shah S, Haddadian EJ, Gumperz JE, et al. The 2.5 a structure of CD1c in complex with a mycobacterial lipid reveals an open groove ideally suited for diverse antigen presentation. Immunity. 2010;33:853–862. doi: 10.1016/j.immuni.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rhijn I, Young DC, De Jong A, Vazquez J, Cheng TY, Talekar R, Barral DC, Leon L, Brenner MB, Katz JT, et al. CD1c bypasses lysosomes to present a lipopeptide antigen with 12 amino acids. J Exp Med. 2009;206:1409–1422. doi: 10.1084/jem.20082480. [DOI] [PMC free article] [PubMed] [Google Scholar]