Abstract
Olfactory dysfunction is common in Parkinson’s disease (PD) and has been attributed to early deposition of α-synuclein pathology in olfactory areas. The pathophysiology of olfactory dysfunction in PD, however, remains poorly understood. Changes in odor identification suggest in part impairment in odor memory, possibly due to hippocampal dysfunction. Olfactory dysfunction occurs also in Alzheimer’s disease (AD) and increases with severity of dementia. Cholinergic degeneration is not only a feature of AD but can also occur in PD, at least in a subset of patients with cognitive changes. We reported previously that impaired odor identification in early PD is more closely correlated with hippocampal dopaminergic than nigrostriatal dopaminergic denervation. Results of our multi-tracer PET studies show that odor identification deficits in PD are best predicted by cholinergic denervation and to a lesser extent by dopaminergic denervation. These results suggest that olfactory dysfunction in PD may have multiple components including hippocampal dysfunction secondary to cholinergic and dopaminergic denervations. Olfactory dysfunction in PD may be most marked in subjects at risk of incipient dementia and may reflect the transition of PD toward a stage with more heterogeneous multi-system neurodegenerations. Our preliminary imaging data do not support a significant contribution of amyloidopathy or serotoninergic denervation to abnormal olfactory functions in PD, at least in the absence of dementia. We outline how progressive changes in olfaction may be used as a biomarker of cholinergic denervation and cognitive decline in PD patients. We will discuss also the utility of olfactory testing as an early screening test for neurodegeneration.
Keywords: Acetylcholine, cognitive, dopamine, Parkinson’s disease, olfaction, positron emission tomography
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder presenting with motor and non-motor symptoms. Olfactory dysfunction is a frequent and early non-motor feature of PD with deficits described in odor identification, discrimination, threshold detection, and recognition memory (Doty 2012). Simple detection of odors is believed to be dependent on the peripheral olfactory system, while identification and discrimination requires higher-order cognitive control and entails activation of the central olfactory structures (Hedner et al. 2010, Lotsch et al. 2008). Recent findings have shown associations of odor identification test scores with a wide range of other non-motor symptoms of PD, such as cognition (Bohnen et al. 2010), sleep disturbances, mood (Berendse et al. 2011), and neuropsychiatric manifestations (Morley et al. 2011). This suggests at least partial overlap between the pathophysiologies of olfactory dysfunction and these other non-motor symptoms in PD. Furthermore, olfactory impairment does not respond well to antiparkinsonian medications and does not have a linear relationship with stage, progression, or duration of motor disease in PD (Doty 2012, Meusel et al. 2010).
Olfactory dysfunction in PD has been attributed to early pathological deposition of -synuclein pathology in primary and secondary olfactory centers (Braak et al. 2003, Pearce et al. 1995) but comorbid Alzheimer’s disease (AD) type changes may also play a role (Kovacs 2004, Mundinano et al. 2011). Olfactory impairment in PD may also be related to impaired adult neurogenesis, including the subgranular layer of the dentate gyrus of the hippocampus (Hoglinger et al. 2004).
Multiple neurotransmitters are involved in olfactory neurotransmission: dopamine, acetylcholine, norepinephrine and serotonin (Benarroch 2010). Lewy bodies and/or AD pathology can also be deposited in subcortical nuclei of neuromodulator projection systems. For example, pathological deposits in the nucleus basalis of Meynert have been associated with degeneration of limbic and ascending cortical cholinergic projections (Jellinger 1991). Similarly, pathology of the raphe nucleus has been associated with serotoninergic degeneration and locus ceruleus pathology can be associated with noradrenergic changes in PD (Jellinger 1991). Furthermore, mesencephalic Lewy body pathology in the ventral tegmental area (VTA) in PD can be associated with degeneration of limbic and ascending mesofrontal cortical dopaminergic projections (Braak et al. 2001, Jellinger 1991). Deficiency of catecholamines, especially in the mesolimbic projection area, has been proposed as another underlying cause of hyposmia in PD (Korten and Meulstee 1980). It is plausible that disease-related changes in olfactory neurotransmitter functions may contribute to olfactory impairment in PD (Doty 2012).
Positron emission tomography (PET) or single photon emission computed tomography (SPECT) are imaging techniques that allow in vivo assessment of neurochemical changes that can be related to performance on olfactory tests. We will discuss the current literature of dopaminergic and cholinergic imaging and olfactory impairment and also present preliminary findings of correlations between serotoninergic and -amyloid (Aβ) imaging findings and olfactory performance in PD.
Dopaminergic imaging
We and other researchers have previously reported an association between olfactory dysfunction and the degree of nigrostriatal dopaminergic denervation in PD (Table 1). We found a modest but significant relationship between performance on the University of Pennsylvania Odor Identification Test (UPSIT) and dopamine transporter (DAT) activity of the caudate nucleus. Correlations were higher for ventral compared to dorsal striatal DAT activity (Bohnen et al. 2007). The caudate nucleus may play a role in the discrimination of odor quality but does not appear to have primary involvement in olfactory processing (Savic 2002). The reported association between deficits in olfactory performance and abnormalities in nigrostriatal dopaminergic binding is likely not caused exclusively by dopamine neuron degeneration. This is also illustrated by the fact that patients with MPTP-induced toxic Parkinsonism who have nigrostriatal dopaminergic denervation were found to have a normal sense of smell (Doty et al. 1992).
Table 1.
Dopaminergic SPECT and PET and olfaction correlation studies in PD. It is possible that relative differences in olfactory and regional striatal dopaminergic correlations between SPECT and PET studies in part may reflect differences in spatial resolution between these imaging techniques or pharmacological differences between ligands.
| Radioligand | Findings |
|---|---|
| [123I]- -CIT DAT SPECT (Lehrner et al. 1995) |
No significant correlation between striatal DAT binding and olfactory (threshold, memory, identification) functions. |
| [99mTc] -TRODAT SPECT (Siderowf et al. 2005) |
Higher UPSIT scores had strong correlation with striatal DAT binding that were higher in the putamen compared to the caudate nucleus. |
| [11C]β-CFT DAT PET (Bohnen et al. 2007) | Higher UPSIT scores had better correlation with ventral compared to dorsal striatal DAT activity. |
| [11C]β-CFT DAT PET (Bohnen et al. 2008) | UPSIT scores had best correlation with hippocampal compared to neostriatal DAT activity. |
| [123I]-FP-CIT DAT SPECT (Deeb et al. 2010) |
Moderate correlation between striatal DAT and UPSIT scores but no differences between affected versus less affected hemispheres. No correlation between striatal DAT and taste thresholds. |
| [123I]-FP-CIT DAT SPECT (Berendse et al. 2011) |
UPSIT scores had modest correlation with striatal (putaminal and caudate nucleus) DAT uptake, slightly better for the least denervated hemisphere. |
Odor identification requires recognizing or naming the odor, a learned response, and raises the possibility of altered function of structures, such as the hippocampus, involved in higher order cognitive or memory processing (Bohnen et al. 2008). We explored also the relationship between mesolimbic DAT activity and odor identifications in PD. Twenty-nine PD patients underwent olfactory testing using the UPSIT and [11C]β-CFT DAT PET. Lower UPSIT scores in PD were more robustly correlated with hippocampal rather than amygdala, ventral or dorsal striatal dopamine denervation as shown by DAT binding (Bohnen et al. 2008). These findings suggest that dopaminergic denervation of the hippocampus may be a component of the pathophysiology of hyposmia in PD. The VTA predominantly supplies dopaminergic innervation to the mesocortical forebrain (Bjorklund and Dunnett 2007). In our study, we found a stronger association between abnormal odor identification and hippocampal rather than nigrostriatal DAT, which could suggest that abnormal odor identification in PD is more related to dopaminergic degeneration of the VTA than a pure nigrostriatal mechanism.
Cholinergic imaging
The cholinergic system modulates several aspects of central olfactory functions (Doty 2012). The role of the cholinergic system in olfaction deserves close examination since olfactory dysfunction occurs not only in PD but also in AD (Doty et al. 1987). We tested the hypothesis that olfactory deficits would correlate with central cholinergic denervation and that effects would be more prominent in the limbic system, especially the hippocampus (Bohnen et al. 2010). We performed [11C]PMP acetylcholinesterase (AChE) and [11C]DTBZ vesicular monoamine transporter type 2 (VMAT2) PET and UPSIT testing in 58 PD patients without dementia. No subjects were on anti-cholinergic or anti-AChE drugs. We found that UPSIT scores correlated positively with AChE activity in the hippocampal formation, amygdala, and neocortex. Striatal VMAT2 activity correlated positively with UPSIT scores. Regression analysis demonstrated a significant regressor effect for limbic AChE activity, borderline for striatal VMAT2 activity, but not significant for cortical AChE activity. We also found that odor identification scores correlated positively with scores on cognitive measures of episodic verbal learning (Bohnen et al. 2010).
These findings show that in PD, cholinergic denervation of the limbic system is a more robust determinant of deficits in odor identification than nigrostriatal dopaminergic denervation alone. The correlation between limbic AChE activity and memory performance was relatively modest suggesting that our findings cannot be simply explained on the basis of memory deficits. These findings indicate that cholinergic denervation, especially of the limbic system, including the hippocampus, may contribute to the pathophysiology of olfactory dysfunction in PD.
Serotoninergic imaging
Serotonin may play an important role in olfactory function as lesioning of the serotoninergic fibers in the olfactory bulb causes glomerular atrophy and olfactory disturbances in the rat (Moriizumi et al. 1994). Significant presynaptic serotoninergic reductions occur in PD without evidence of major depression (Albin et al. 2008). We have preliminary data of 41 patients with PD without dementia (mean age 65.1 6.6) who underwent [11C]DASB serotonin transporter (SERT) PET and UPSIT. No subjects were on antidepressant drugs. There were no significant correlations between UPSIT scores and SERT binding in the raphe nucleus (r=−0.18, p=0.26), amygdala (r=−0.13, p=0.42), hippocampus (r=−0.22, p=0.16), striatum (r=−0.05, p=0.78), or neocortex (r=−0.02, p=0.89). These preliminary findings suggest no major role of regional cerebral, including limbic, serotoninergic denervation and deficits in odor identification in PD without dementia.
Aβ imaging
Although PD is pathologically characterized by the presence of intraneuronal Lewy body inclusion, AD pathology including Aβ deposition can also occur (Mundinano et al. 2011). We have preliminary data of 22 patients with PD with mild cognitive impairment but without significant dementia (mean age 69.7 6.7) who underwent A brain PET imaging with Pittsburgh compound B ([11C]PIB) and UPSIT. A subset of 16 of these patients also underwent AChE PET using the [11C]PMP ligand. Analysis yielded a robust correlation between UPSIT scores and cortical AChE activity (r=0.74, p<0.001) but not with cortical [11C]PIB retention (r=0.006, p=0.98). Cortical [11C]PIB binding showed no significant correlation with cortical PMP activity (r=0.085, p=0.75) in the 16 patients subset. The lack of a correlation between cortical AChE activity and [11C]PIB binding suggests that cholinergic denervation and Aβ deposition are concurrent but relatively independent pathophysiologic processes in the context of PD with mild cognitive impairment. These preliminary findings suggest no major role for cortical Aβ depositions in odor identification functions in PD with mild cognitive impairment.
Discussion
Odor identification requires higher-level cognitive processing as it involves odor recognition and memory retrieval. These processes probably involve the hippocampus and entorhinal-perirhinal cortex, orbitofrontal, insular and inferior frontal cortex in order to produce a correct identification of the odor name (Wang et al. 2005). In our PD studies, we found a stronger association between abnormal odor identification and hippocampal rather than nigrostriatal DAT activity, which suggests that olfactory dysfunction is more related to dopaminergic degeneration of the VTA than the substantia nigra (Bohnen et al. 2008).
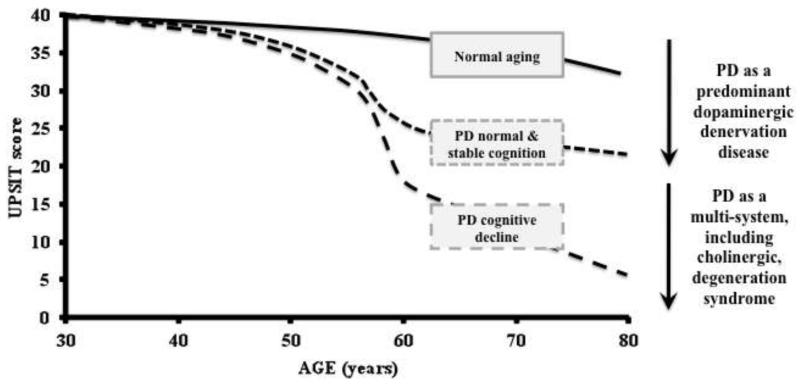
Results of our combined multi-tracer dopaminergic and cholinergic PET studies showed that odor identification deficits in PD are better explained by cholinergic mesolimbic, especially hippocampal, denervation than by nigrostriatal dopaminergic denervation. Cholinergic denervation is heterogeneous with reduced AChE activity in about one third of PD patients without dementia (Bohnen et al. 2012). Worsening olfactory functions in advancing PD may be associated with cholinergic neuron degeneration and associated cognitive dysfunction. For example, a recent 3-year prospective cohort study found that the presence of severe olfactory dysfunction was a prodromal symptom of subsequent dementia development in PD (Baba et al. 2012). We propose a simple model of longitudinal changes in olfactory function where patients who remain cognitively in the normal range have relative stable odor identification scores over time. In contrast, PD patients with progressive cognitive decline, who likely have progressive cholinergic denervation, may manifest progressive olfactory loss (Figure 1). Alternatively, greater deficits in odor identification may only serve as a marker to identify a subset of PD subjects at risk for clinically significant cognitive impairment, including future dementia risk. It should be noted, however, that the relationship between progressive changes in olfaction and cognition in PD remains to be studied.
Figure 1.
Proposed model of longitudinal changes in UPSIT scores over time in normal aging, PD without and with incipient cognitive impairment. PD subjects without cognitive decline will have an early floor effect and subsequent stability. This may represent PD as a Lewy body disease with predominant dopaminergic denervation. In contrast, PD subjects with cognitive decline will exhibit progressive olfactory impairment that may parallel the development of PD as a multisystem, including cholinergic, degeneration syndrome.
A weakness of our olfactory studies is that they are only based on tests of odor identification and are cross-sectional in nature. Further research needs to be done not only to correlate odor identification but also odor detection threshold, memory and discrimination in both cross-sectional and prospective cohort study designs. Furthermore, we found no significant correlations between UPSIT scores and serotoninergic denervation or increased Aβ-amyloid binding in PD (without significant dementia) in our preliminary analyses requiring further study of these mechanisms in olfactory dysfunction in PD.
Olfactory dysfunction is now recognized as a common early or presymptomatic manifestation of PD and olfactory testing has been suggested as a component of presymptomatic testing in at-risk individuals. For example, a Dutch study found that a subset of hyposmic relatives of patients with PD who also had abnormal striatal dopaminergic activity on DAT SPECT subsequently developed clinical parkinsonism within several years of follow-up (Berendse et al. 2001). Haehner et al. found that 7% of subjects with idiopathic hyposmia developed PD during a 4-year follow-up period on neurological examination (Haehner et al. 2007). A recent large-scale example of a screening study is the Parkinson At-Risk Syndrome Study, which studies the association between impaired olfaction and other prodromal features of PD (Siderowf et al. 2012). Testing of olfactory functions in 4,999 subjects with no neurological diagnosis found 669 subjects with hyposmia. Notably, the relative prevalence of hyposmia was about twice as high in the subgroup who had a combination of 4 or more non-motor features commonly seen in PD, such as constipation, dream enactment behavior, anxiety or depression. We found that about 40-50% of community-dwelling elderly with low UPSIT also have low striatal DAT activity (Wong et al. 2010) raising the possibility of incidental Lewy body disease or prodromal PD (Ross et al. 2006). Therefore, smell tests could possibly be used for screening of neurodegeneration. A requirement of a screening test, however, is to have high sensitivity and specificity. For olfactory screening testing in older adults, high sensitivity but low specificity is expected as olfactory impairments do not only occur in PD but also in AD or other neurodegenerations (Doty 2012). Therefore, a multi-tiered screening approach will be needed where inexpensive tests with high sensitivity, like a smell identification test, are used to detect elderly at risk of PD or AD and more expensive tests of higher specificity, like PET or SPECT scans, may be used to identify the underlying neurodegenerative process more specifically. A clinical combination of olfactory dysfunction and REM sleep behavior disorder may more specifically indicate an -synucleinopathy, such as PD (Stiasny-Kolster et al. 2005).
We conclude that olfactory dysfunction has multiple components including hippocampal dysfunction secondary to cholinergic and dopaminergic denervations that may parallel the heterogeneity of the multi-system neurodegeneration syndrome of PD. Olfactory dysfunction may be most marked in subjects at risk of incipient dementia and may reflect the transition of PD toward a stage with more prominent non-dopaminergic degenerations.
Acknowledgements
This work was supported by the Department of Veterans Affairs (I01 RX000317); the Michael J. Fox Foundation; and the National Institutes of Health (grant numbers P01 NS019608, NS015655 and RO1 NS070856).
Abbreviations
- Aβ
-amyloid
- AChE
acetylcholinesterase
- AD
Alzheimer’s disease
- DAT
dopamine transporter
- PD
Parkinson’s disease
- PET
positron emission tomography
- SERT
serotonin transporter
- SPECT
single photon computed emission tomography
- UPSIT
University of Pennsylvania smell identification test
- VMAT2
vesicular monoamine transporter type 2
- VTA
ventral tegmental area.
Footnotes
Ethical standards
All subjects in our studies gave their informed consent prior to their inclusion in the studies, which have been approved by the ethic committees in accordance with the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Albin RL, Koeppe RA, Bohnen NI, et al. Spared caudal brainstem SERT binding in early Parkinson’s disease. J Cereb Blood Flow Metab. 2008;28:441–4. doi: 10.1038/sj.jcbfm.9600599. [DOI] [PubMed] [Google Scholar]
- Baba T, Kikuchi A, Hirayama K, et al. Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson’s disease: a 3 year longitudinal study. Brain. 2012;135:161–9. doi: 10.1093/brain/awr321. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Olfactory system: functional organization and involvement in neurodegenerative disease. Neurology. 2010;75:1104–9. doi: 10.1212/WNL.0b013e3181f3db84. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Booij J, Francot CM, et al. Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann Neurol. 2001;50:34–41. doi: 10.1002/ana.1049. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Roos DS, Raijmakers P, et al. Motor and non-motor correlates of olfactory dysfunction in Parkinson’s disease. J Neurol Sci. 2011;310:21–4. doi: 10.1016/j.jns.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Gedela S, Herath P, et al. Selective hyposmia in Parkinson disease: Association with hippocampal dopamine activity. Neurosci Lett. 2008;447:12–6. doi: 10.1016/j.neulet.2008.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Gedela S, Kuwabara H, et al. Selective hyposmia and nigrostriatal dopaminergic denervation in Parkinson’s disease. J Neurol. 2007;254:84–90. doi: 10.1007/s00415-006-0284-y. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Mueller MLTM, Kotagal V, et al. Heterogeneity of cholinergic denervation in Parkinson disease. J Cereb Blood Flow Metab. 2012;32:1609–17. doi: 10.1038/jcbfm.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Muller MLTM, Kotagal V, et al. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson disease. Brain. 2010;133:1747–54. doi: 10.1093/brain/awq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak E, Sandmann-Keil D, Rub U, et al. alpha-synuclein immunopositive Parkinson’s disease-related inclusion bodies in lower brain stem nuclei. Acta Neuropathol. 2001;101:195–201. doi: 10.1007/s004010000247. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Deeb J, Shah M, Muhammed N, et al. A basic smell test is as sensitive as a dopamine transporter scan: comparison of olfaction, taste and DaTSCAN in the diagnosis of Parkinson’s disease. QJM. 2010;103:941–52. doi: 10.1093/qjmed/hcq142. [DOI] [PubMed] [Google Scholar]
- Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012;8:329–39. doi: 10.1038/nrneurol.2012.80. [DOI] [PubMed] [Google Scholar]
- Doty RL, Reyes PF, Gregor T. Presence of both odor identification and detection deficits in Alzheimer’s disease. Brain Res Bull. 1987;18:597–600. doi: 10.1016/0361-9230(87)90129-8. [DOI] [PubMed] [Google Scholar]
- Doty RL, Singh A, Tetrud J, et al. Lack of major olfactory dysfunction in MPTP-induced parkinsonism. Ann Neurol. 1992;32:97–100. doi: 10.1002/ana.410320116. [DOI] [PubMed] [Google Scholar]
- Haehner A, Hummel T, Hummel C, et al. Olfactory loss may be a first sign of idiopathic Parkinson’s disease. Mov Disord. 2007;22:839–42. doi: 10.1002/mds.21413. [DOI] [PubMed] [Google Scholar]
- Hedner M, Larsson M, Arnold N, et al. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol. 2010;32:1062–7. doi: 10.1080/13803391003683070. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–35. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol. 1991;14:153–97. doi: 10.1007/BF03159935. [DOI] [PubMed] [Google Scholar]
- Korten JJ, Meulstee J. Olfactory disturbances in Parkinsonism. Clin Neurol Neurosurg. 1980;82:113–8. doi: 10.1016/0303-8467(80)90005-0. [DOI] [PubMed] [Google Scholar]
- Kovacs T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res Rev. 2004;3:215–32. doi: 10.1016/j.arr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Lehrner J, Brucke T, Kryspin-Exner I, et al. Impaired olfactory function in Parkinson’s disease. Lancet. 1995;345:1054–5. doi: 10.1016/s0140-6736(95)90797-1. [DOI] [PubMed] [Google Scholar]
- Lotsch J, Reichmann H, Hummel T. Different odor tests contribute differently to the evaluation of olfactory loss. Chem Senses. 2008;33:17–21. doi: 10.1093/chemse/bjm058. [DOI] [PubMed] [Google Scholar]
- Meusel T, Westermann B, Fuhr P, et al. The course of olfactory deficits in patients with Parkinson’s disease--a study based on psychophysical and electrophysiological measures. Neurosci Lett. 2010;486:166–70. doi: 10.1016/j.neulet.2010.09.044. [DOI] [PubMed] [Google Scholar]
- Moriizumi T, Tsukatani T, Sakashita H, et al. Olfactory disturbance induced by deafferentation of serotonergic fibers in the olfactory bulb. Neuroscience. 1994;61:733–8. doi: 10.1016/0306-4522(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Morley JF, Weintraub D, Mamikonyan E, et al. Olfactory dysfunction is associated with neuropsychiatric manifestations in Parkinson’s disease. Mov Disord. 2011;26:2051–7. doi: 10.1002/mds.23792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundinano IC, Caballero MC, Ordonez C, et al. Increased dopaminergic cells and protein aggregates in the olfactory bulb of patients with neurodegenerative disorders. Acta Neuropathol. 2011;122:61–74. doi: 10.1007/s00401-011-0830-2. [DOI] [PubMed] [Google Scholar]
- Pearce RK, Hawkes CH, Daniel SE. The anterior olfactory nucleus in Parkinson’s disease. Mov Disord. 1995;10:283–7. doi: 10.1002/mds.870100309. [DOI] [PubMed] [Google Scholar]
- Ross GW, Abbott RD, Petrovitch H, et al. Association of olfactory dysfunction with incidental Lewy bodies. Mov Disord. 2006;21:2062–7. doi: 10.1002/mds.21076. [DOI] [PubMed] [Google Scholar]
- Savic I. Imaging of brain activation by odorants in humans. Curr Opin Neurobiol. 2002;12:455–61. doi: 10.1016/s0959-4388(02)00346-x. [DOI] [PubMed] [Google Scholar]
- Siderowf A, Jennings D, Eberly S, et al. Impaired olfaction and other prodromal features in the Parkinson At-Risk Syndrome Study. Mov Disord. 2012;27:406–12. doi: 10.1002/mds.24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siderowf A, Newberg A, Chou KL, et al. [99mTc]TRODAT-1 SPECT imaging correlates with odor identification in early Parkinson disease. Neurology. 2005;64:1716–20. doi: 10.1212/01.WNL.0000161874.52302.5D. [DOI] [PubMed] [Google Scholar]
- Stiasny-Kolster K, Doerr Y, Moller JC, et al. Combination of ‘idiopathic’ REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain. 2005;128:126–37. doi: 10.1093/brain/awh322. [DOI] [PubMed] [Google Scholar]
- Wang J, Eslinger PJ, Smith MB, et al. Functional magnetic resonance imaging study of human olfaction and normal aging. J Gerontol A Biol Sci Med Sci. 2005;60:510–4. doi: 10.1093/gerona/60.4.510. [DOI] [PubMed] [Google Scholar]
- Wong KK, Muller ML, Kuwabara H, et al. Olfactory loss and nigrostriatal dopaminergic denervation in the elderly. Neurosci Lett. 2010;484:163–7. doi: 10.1016/j.neulet.2010.08.037. [DOI] [PubMed] [Google Scholar]