Abstract
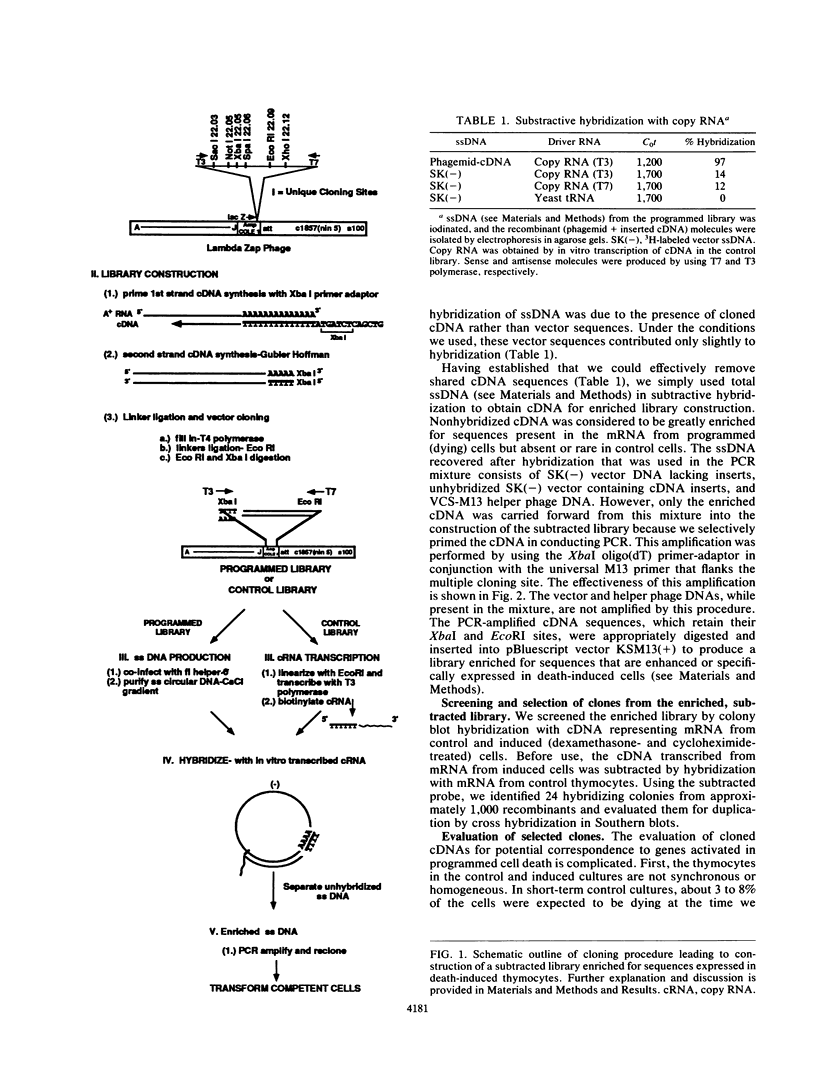
Programmed cell death is an essential cellular process that occurs in epithelial turnover, neural development, and regulation of cell populations of the immune system. Thymocytes undergo programmed cell death in response to several inductive stimuli, including exposure to glucocorticoids or radiation. This program can be blocked by inhibitors of RNA or protein synthesis; this implies that new proteins are required to execute the death programs. To search for possible death-associated mRNAs, we directionally cloned cDNA representing mRNA from control and dexamethasone-treated thymocytes. These libraries were used to produce ample amounts of DNA and RNA used in subtractive hybridization for the removal of sequences present in both control and induced cells. The remaining unhybridized sequences were selectively amplified by polymerase chain reaction and cloned to produce a library enriched for sequences expressed in death-induced cells. From this library we isolated cDNAs of death-associated mRNAs. One of these mRNAs, RP-8, appears within 1 h after exposure to gamma radiation, and a second mRNA, RP-2, is observed within 2 h. Both of these mRNAs accumulate during a period when a reference mRNA, actin, is declining. RP-2 and RP-8 are no longer detectable after 6 h postinduction, when apoptosis and mRNA degradation are evident in the culture. Sequence analysis of RP-8 cDNA indicates the presence of a zinc finger domain suggestive of a possible DNA regulatory role for the RP-8 protein. cDNA sequence results on RP-2 classify the corresponding protein as an integral membrane protein. We conclude that RP-2 and RP-8 are death-associated mRNAs that should be functionally evaluated in the context of the death process. As previously suggested, it may be that a family of "death genes" is activated by various stimuli depending on the type of cell, in a manner somewhat analogous to the induction of heat shock (stress) protein genes.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buttyan R., Olsson C. A., Pintar J., Chang C., Bandyk M., Ng P. Y., Sawczuk I. S. Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol. 1989 Aug;9(8):3473–3481. doi: 10.1128/mcb.9.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikaraishi D. M., Deeb S. S., Sueoka N. Sequence complexity of nuclear RNAs in adult rat tissues. Cell. 1978 Jan;13(1):111–120. doi: 10.1016/0092-8674(78)90142-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cidlowski J. A. Glucocorticoids stimulate ribonucleic acid degradation in isolated rat thymic lymphocytes in vitro. Endocrinology. 1982 Jul;111(1):184–190. doi: 10.1210/endo-111-1-184. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Compton M. M., Cidlowski J. A. Identification of a glucocorticoid-induced nuclease in thymocytes. A potential "lysis gene" product. J Biol Chem. 1987 Jun 15;262(17):8288–8292. [PubMed] [Google Scholar]
- Duguid J. R., Rohwer R. G., Seed B. Isolation of cDNAs of scrapie-modulated RNAs by subtractive hybridization of a cDNA library. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5738–5742. doi: 10.1073/pnas.85.15.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Fan C. M., Maniatis T. A DNA-binding protein containing two widely separated zinc finger motifs that recognize the same DNA sequence. Genes Dev. 1990 Jan;4(1):29–42. doi: 10.1101/gad.4.1.29. [DOI] [PubMed] [Google Scholar]
- Fasano L., Röder L., Coré N., Alexandre E., Vola C., Jacq B., Kerridge S. The gene teashirt is required for the development of Drosophila embryonic trunk segments and encodes a protein with widely spaced zinc finger motifs. Cell. 1991 Jan 11;64(1):63–79. doi: 10.1016/0092-8674(91)90209-h. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Harrigan M. T., Baughman G., Campbell N. F., Bourgeois S. Isolation and characterization of glucocorticoid- and cyclic AMP-induced genes in T lymphocytes. Mol Cell Biol. 1989 Aug;9(8):3438–3446. doi: 10.1128/mcb.9.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. The calcium dependent endonuclease activity of isolated nuclear preparations. Relationships between its occurrence and the occurrence of other classes of enzymes found in nuclear preparations. Biochem Biophys Res Commun. 1973 May 15;52(2):475–481. doi: 10.1016/0006-291x(73)90736-5. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- Palazzolo M. J., Meyerowitz E. M. A family of lambda phage cDNA cloning vectors, lambda SWAJ, allowing the amplification of RNA sequences. Gene. 1987;52(2-3):197–206. doi: 10.1016/0378-1119(87)90046-1. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G., Giarre M., Farah J., Gausz J., Spierer A., Spierer P. Dependence of position-effect variegation in Drosophila on dose of a gene encoding an unusual zinc-finger protein. Nature. 1990 Mar 15;344(6263):219–223. doi: 10.1038/344219a0. [DOI] [PubMed] [Google Scholar]
- Schneider C., King R. M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988 Sep 9;54(6):787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Sellins K. S., Cohen J. J. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987 Nov 15;139(10):3199–3206. [PubMed] [Google Scholar]
- Sive H. L., St John T. A simple subtractive hybridization technique employing photoactivatable biotin and phenol extraction. Nucleic Acids Res. 1988 Nov 25;16(22):10937–10937. doi: 10.1093/nar/16.22.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N., Bell P. A. Glucocorticoid-induced cell-size changes and nuclear fragility in rat thymocytes. Mol Cell Endocrinol. 1981 Apr;22(1):71–84. doi: 10.1016/0303-7207(81)90103-9. [DOI] [PubMed] [Google Scholar]
- Travis G. H., Sutcliffe J. G. Phenol emulsion-enhanced DNA-driven subtractive cDNA cloning: isolation of low-abundance monkey cortex-specific mRNAs. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1696–1700. doi: 10.1073/pnas.85.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadewitz A. G., Lockshin R. A. Programmed cell death: dying cells synthesize a co-ordinated, unique set of proteins in two different episodes of cell death. FEBS Lett. 1988 Dec 5;241(1-2):19–23. doi: 10.1016/0014-5793(88)81022-6. [DOI] [PubMed] [Google Scholar]
- Welcher A. A., Torres A. R., Ward D. C. Selective enrichment of specific DNA, cDNA and RNA sequences using biotinylated probes, avidin and copper-chelate agarose. Nucleic Acids Res. 1986 Dec 22;14(24):10027–10044. doi: 10.1093/nar/14.24.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G. Hormone-induced cell death. Purification ad properties of thymocytes undergoing apoptosis after glucocorticoid treatment. Am J Pathol. 1982 Oct;109(1):78–87. [PMC free article] [PubMed] [Google Scholar]
- Yuan J. Y., Horvitz H. R. The Caenorhabditis elegans genes ced-3 and ced-4 act cell autonomously to cause programmed cell death. Dev Biol. 1990 Mar;138(1):33–41. doi: 10.1016/0012-1606(90)90174-h. [DOI] [PubMed] [Google Scholar]






