Abstract
Class 3 secreted semaphorins (Sema3A to 3F) participate in many aspects of axon guidance through holoreceptor complexes that include Neuropilin-1 (Npn-1) or Neuropilin-2 and one of the four class A plexin proteins. However, unlike other Sema3 family proteins, Sema3E directly binds to Plexin-D1 without neuropilins. Its biological function was first explored in intersomitic vessel formation and since its initial discovery, Sema3E-Plexin-D1 signaling has been found to participate in the many biological systems in addition to vascular development, via seemingly different mode of actions. For example, temporal and spatial control of ligand vs. receptor results in two different mechanisms governing vascular patterning. Interactions with other transmembrane proteins such as neuropilin or VEGFR2 results in different axonal behaviors. Ligand receptor localization on pre- vs. post-synaptic neurons is used to control different types of synapse formation. Perhaps different downstream effectors will also result in different functional outcomes. Given the limited number of ligands and receptors in the genome and their multifunctional nature, we expect that more modes of action will be discovered in the future. In this review, we highlight current advances on the mechanisms of how Sema3E-Plexin-D1 interaction shapes the networks of multiple biological systems, in particular the vascular and nervous systems.
Keywords: Semaphorin3E, Plexin-D1, Semaphorins, Plexins, Neural development, Vascular, development, Guidance, Signaling
<General introduction>
The semaphorins are a large family of axon guidance cues that consists of both secreted and membrane-bound proteins. There are seven class 3 secreted semaphorins (Sema3A-3G)[1-3]. In contrast to their membrane-associated semaphorin cousins, most vertebrate class 3 semaphorins are known to bind neuropilins and form holoreceptor complex with plexins. The exception to this rule is Sema3E, which binds its receptor Plexin-D1 directly and independently of the neuropilins [4].
Like other Sema3s, Sema3E contains Sema, PSI (plexin-semaphorin-integrin), and Ig (immunoglobulin) domains and a basic C terminus tail [5]. Plexin-D1 is a relatively new member of the Plexin family, which is made up of A, B, C and D subfamilies[6]. Like all plexin family members, Plexin-D1 contains a sema-domain, three Met-related sequences (MRS), three glycine/proline-rich motifs, a single transmembrane domain, and two highly conserved intracellular domains known together as the SEX-plexin domain. Plexin-D1 differs from other plexins in the third MRS motif, which contains only six of the eight conserved cysteines normally encountered in a MRS. Semaphorins and plexins can interact via their sema domains [6] [7]. Recent structural work has revealed that binding of each homodimer arrangement of semaphorins and plexins forms a heterodimer complex that then elicits conformational change in the complex. This structural alteration transmits signals to the intracellular domain of Plexins [8]. Semaphorin signaling has largely been studied in vitro in the context of axon guidance, and proteins found to be downstream of the ligand-receptor interaction include small GTPases, cyclic nucleotides, and kinases [3] [9]. Several recent in vivo studies have elegantly demonstrated that specific downstream effectors mediate specific aspect of semaphorin-mediated neuronal function, suggesting that unique pathways exist to control different semaphorin mediated effects [10]. As a relative novel ligand-receptor pair, so far little is known about Sema3E-Plexin-D1, signaling especially in in vivo settings.
The mechanisms of Sema3E and Plexin-D1 in shaping vascular topology
Plexin-D1 is dynamically expressed in endothelial cells of the entire body during early development, indicating an important role in vascular network formation. Plexin-D1 mRNA can be detected as early as E9.5 in the blood vessels of developing mouse embryos and continues to be expressed in endothelial cells during embryogenesis until it is down-regulated shortly before birth [7]. Both Plexin-D1 morphant zebrafish and Plexin-D1 knockout mice exhibit severe intersomitic vessel defects [4, 11, 12]. In addition, Plexin-D1 is expressed in the endocardium and Plexin-D1 knockout mice have failed septation of the cardiac outflow tracts, leading mice to be born with defects of the aortic arch arteries. The ligands that mediate Plexin-D1’s effect on cardiac function are Sema3A and Sema3C, which act through Plexin-D1/neuropilin complexes [12]. However, the ligand that mediates Plexin-D1 function in endothelial cells is Sema3E and, surprisingly, Sema3E binds directly to Plexin-D1 independently of neuropilins [4]. It was first studied in the context of intersomitic vessel formation, where Sema3E is expressed in the caudal region of each developing somite, whereas Plexin-D1 is expressed in the intersomitic blood vessels adjacent to the somite boundary on the rostral region of each somite (Fig. 1A). Sema3E acts as a repulsive cue to restrict vessel growth and branching in the intersomitic space, as ectopic Sema3E overexpression in chick embryos inhibits vessel growth [4]. Conversely, in both Sema3E and Plexin-D1 knockout mice, the intersomitic vessels are no longer excluded from the normally Sema3E-expressing caudal region of the somite and extended ectopically throughout the somites, resulting in exuberant growth and a loss of the normal segmented pattern [4] (Fig. 1A). In addition to its role in intersomitic vessel patterning, recent work has also demonstrated the similar repulsive function of Sema3E in the initial formation of the dorsal aorta. Sema3E secreted from the notochord and lateral plate mesoderm are required for the formation of avascular regions that coordinate to sculpt the mouse dorsal aorta. In sema3E knockout embryos, a branched aortic plexus develops abnormally with a markedly narrowed avascular midline [13].
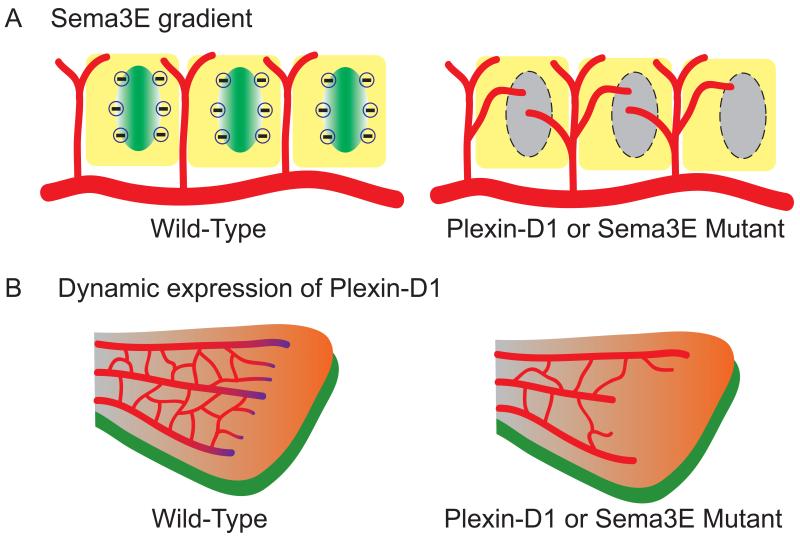
Fig. 1. Temporal and spatial control of ligand vs. receptor results in two different mechanisms governing vascular patterning.
(A) The repulsive gradient generated by Sema3E in the mouse somite determines the proper patterning of Plexin-D1-expressing intersomitic vessels [4]. During intersomitic vessel (red) development in the mouse embryo, Sema3E (gradient in green) is expressed in the caudal region of each somite (yellow), whereas Plexin-D1 is expressed in the adjacent intersomitic vessels (red) on the rostral region of each somite. The repulsive cues generated by the Sema3E gradient restrict vessel growth and branching in the intersomitic space. Mice lacking Sema3E or Plexin-D1 lose the repulsive gradient signals (grey oval), thereby allowing blood vessels to encroach on somites and display exuberant blood vessel growth in the entire somite and a loss of the normal segmented pattern.
(B) In the retina, dynamic regulation of Plexin-D1 level instead of a Sema3E gradient is crucial to establish properly patterned retinal vasculature [15, 16]. In contrast to Sema3E gradient in the intersomitic vessels, in the retinal vasculature (red), Plexin-D1 is selectively expressed in endothelial cells (purple gradient) at the front of sprouting blood vessels in response to the VEGF gradient (orange), whereas Sema3E (green) is evenly expressed in RGCs underneath the retinal vasculature. The dynamic regulation of Plexin-D1 by VEGF in the sprouting front cells modulates the ratio between tip and stalk cells via VEGF-induced feedback mechanism to ensure balanced vascular network formation. Therefore, loss of dynamic Plexin-D1 regulation in the Plexin-D1 or Sema3E mutant shows less-branched and uneven front vasculature (right side of B).
Plexin-D1 morphant zebrafish exhibit similar defects in intersomitic vessel patterning, the ligand mediating this effect has been suggested to be Sema3A [11]. In addition, Sema3E, Plexin-D1’s partner in mammals, is expressed in the vessels and not somites in the zebrafish trunk and acts primarily through Plexin-B2, not Plexin-D1 to control the timing of angioblast sprouting [14]. This suggests that Sema3E plays a different role in vascular development in the two species, whereas its receptor, Plexin-D1 seems to transduce similar repellent signals to establish proper intersomitic vasculature in both mice and fish.
The repulsive gradient generated by Sema3E in the mouse somite determines the proper patterning of Plexin-D1-expressing intersomitic vessels (Fig 1A). Given Plexin-D1’s widespread expression in the embryonic vasculature, is it possible that this simple repellent action of Sema3E can generate all of the diverse vascular patterns in the body? A different mode of action of Sema3E-Plexin-D1 signaling was recently discovered in the mouse retinal vasculature. In contrast to intersomitic vessel patterning in which Sema3E is expressed in a caudal-to-rostral gradient in the somite [4], in the retina, Sema3E is expressed uniformly by retinal ganglion cells (RGCs) and does not appear to form a gradient along the central-to-peripheral axis [15]. However, Plexin-D1 is dynamically expressed only in the actively sprouting vessels in the mouse retina in a VEGF-dependent manner [15] [16]. In the retina, dynamic regulation of Plexin-D1 level instead of a Sema3E gradient is crucial to establish properly patterned retinal vasculature. These findings demonstrate that different cellular distribution of Sema3E and Plexin-D1 can regulate the formation of different vascular topologies (Fig 1B). Likewise, the vascular patterning of retinas lacking Sema3E or Plexin-D1 showed completely different phenotypes from the mutant intersomitic vasculature. In the absence of Sema3E-Plexin-D1 signaling, exuberant abnormal vessels grow in the entire somite whereas in the mouse retina, uneven and decreased vessel sprouting was observed [15] [16].
When traditional axon guidance cues were initially discovered as a new class of molecules to regulate vascular patterning, it is unclear whether it has any connections with the well known VEGF pathway. Until recently, the intimate interaction between Plexin-D1 signaling and VEGF signaling was revealed by several studies in both mouse and zebrafish to shape the vascular network [15] [16] [17] [18]. In the developing retinal vasculature, Plexin-D1 is selectively expressed in endothelial cells at the front of actively sprouting blood vessels and Sema3E is expressed in RGCs. VEGF can directly control the dynamic expression of Plexin-D1 [15] [16] and Sema3E-Plexin-D1 signaling in turn negatively regulates VEGF-induced Dll4-Notch signaling, which is a key lateral inhibition pathway that controls the ratio of endothelial tip cells and stalk cells [15]. Sema3E-Plexin-D1 gain of function suppresses Dll4 expression, whereas loss of function of Sema3E-Plexin-D1 increases Dll4 expression and Notch activity, and subsequently decreases tip/stalk cell ratio. As a result, a lack of Sema3E or Plexin-D1 in mice leads to a disruption of vascular patterning in retinal vasculature, generating an uneven growing front and a less-branched network [15]. On the other hand, Plexin-D1 expression is tightly regulated by the VEGF gradient. This topographical difference of Sema3E and Plexin-D1 expression in the vasculature of different tissues enables the creation of unique vascular structures. What could be the biological meaning of the negative feedback mechanism of Sema3E-Plexin-D1 signaling? It has been suggested that the VEGF-Notch feedback and dynamic tip and stalk cell position-shuffling promotes reiterative sprouting and branching and thus robust network formation during angiogenesis [19]. Because Sema3E-Plexin-D1 signaling negatively regulates VEGF signaling, it will be interesting to know whether Sema3E-Plexin-D1 is actively involved in regulating the timing of the tip/stalk cell switching during angiogenesis (Fig. 2A).
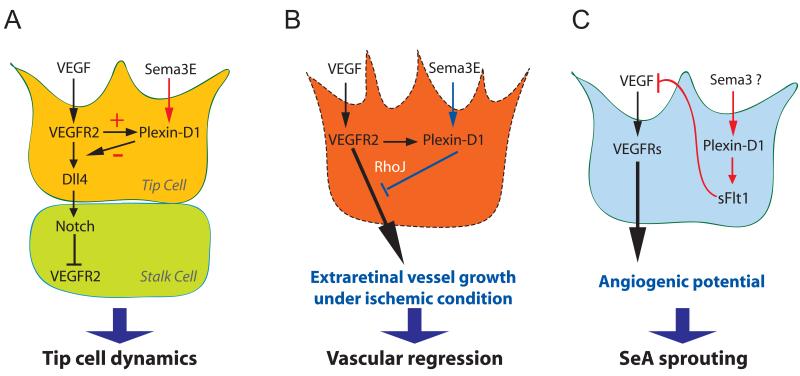
Fig. 2. Sema3E-Plexin-D1 signaling in regulating vascular patterning via its interaction with VEGF signaling.
(A) Tip cell dynamics are regulated by Sema3E-Plexin-D1 modulating Dll4-Notch signaling via a VEGF-induced negative feedback mechanism [15]. VEGF directly controls the expression of Plexin-D1 selectively in endothelial cells at the front of actively sprouting blood vessels. In turn, Sema3E-Plexin-D1 signaling inhibits the activity of VEGF-induced Dll4-Notch signaling pathway, which controls the balance between tip and stalk cells, thereby the retinal vascular network topology.
(B) Sema3E-Plexin-D1 signaling contributes to proper vasculature formation after pathological condition such as ischemic retinopathy [16]. Sema3E-Plexin-D1 signaling counteracts VEGF-mediated signaling and the small GTPase RhoJ might be involved in modulating the two pathways. Under the ischemic retinopathy condition, abnormal extraretinal vascular projections are suppressed by enhancing Sema3E-Plexin-D1 signaling. (C) Plexin-D1 signaling induces soluble flt-1 expression to negatively control VEGF signaling in zebra fish truck [17]. During zebrafish segmental artery development, Plexin-D1-mediated signaling limits aorta’s angiogenic potential by inhibiting VEGF signaling via soluble flt-1. The specific member of Sema3 subfamily that mediates this event in the zebrafish is yet to be identified.
In pathological angiogenesis, cross-talk between Sema3E-Pleixn-D1 is achieved through the activation of the small GTPase RhoJ, which has been shown to inhibit endothelial filopodia. In contrast to the VEGF-induced activation of Cdc42, which plays a crucial role in filopodia protrusion, Sema3E-induced activation of RhoJ mediates endothelial filopodia retraction. RhoJ and Cdc42 are inactivated by VEGF and Sema3E, respectively, suggesting that VEGF and Sema3E signals inversely activate or inactivate Cdc42 and RhoJ. In a mouse model of ischemic retinopathy, prominent expression of endothelial Plexin-D1 and RhoJ was limited to the abnormal extraretinal vessels. By targeting Plexin-D1, intravitreal injection of Sema3E protein selectively suppressed the disoriented outgrowth of extraretinal vessels, leading to the regeneration of normal vasculature in ischemic retina [16] (Fig 2B).
In zebrafish, soluble Flt1 (sFlt1) mediates the cross-talk between VEGF and Sema3E-Plexin-D1 signaling. In the zebrafish trunk, loss of Plexin-D1 induces the sprouting of ectopic segmental arteries and Plexin-D1 signaling suppresses the VEGF-induced angiogenic potential of the aorta. Moreover, Plexin-D1 antagonizes VEGF signaling by promoting the expression of sFlt1, a Flk1/VEGFR2-decoy, in endothelial cells [11] [17] (Fig. 2C). Studies in zebrafish suggest that the interplay between Plexin-D1 and VEGFR seems to be evolutionarily conserved during vascular development, but through different biological mechanisms. Therefore, it is interesting to find out whether Plexin-D1 functions to regulate VEGFR signaling via enhancing sFlt1 expression in mammalian vascular development. Because it has already been shown that elimination of Sema3E in zebrafish does not lead to an identical phenotype to that seen in mammals [14], another Sema3 or other types of ligands may associate with Plexin-D1 in fish. This result clearly demonstrates the existence of both evolutional similarity and difference across species.
The mechanisms of Sema3E and Plexin-D1 in wiring the nervous system
Plexin-D1 expression in the nervous system is observed as early as E12.5 and its expression is broadly expanded in the central nervous system (CNS) as well as the peripheral nervous system (PNS) in mouse [7]. Interestingly, in contrast to the vascular system where Plexin-D1 expression is down-regulated at later embryonic stages, Plexin-D1 level is maintained late into embryonic development as well as early in postnatal growth in the mouse, suggesting that Plexin-D1 might be involved in various aspects of neuronal wiring [7] [20].
Sema3E-Plexin-D1 has a dual function in axonal growth depending on the presence of neuropilins (Fig. 3A). For certain subpopulations of corticofugal and striatonigral neurons that express Plexin-D1 but not Npn-1, Sema3E acts as a repellent. In contrast, in subiculo-mammillary neurons, the presence of receptor complexes of Neuropilin-1 in addition to Plexin-D1 switches the Sema3E signal from repulsion to attraction and/or stimulation of axonal growth [21]. Recently, the attractive role of Sema3E in the subiculo-mammillary neurons was also shown to be transduced by the VEGF-independent function of VEGFR2 in complexes with both Plexin-D1 and Npn1. In this situation, Sema3E binds to the Plexin-D1/Npn1/VEGFR2 receptor complex and VEGFR2 is the signal transducing subunit. This VEGFR2-mediated axon growth was shown to be mediated through the PI3K/Akt pathway [22]. These results suggest that only specific neuronal populations in which all three of these receptors are expressed can have an attractive response to Sema3E.
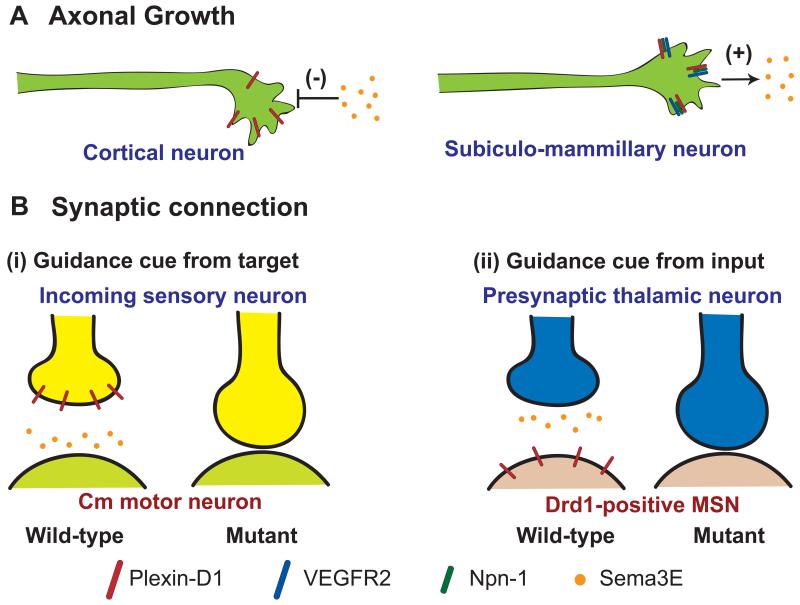
Fig. 3. Diverse functions of Sema3E-Plexin-D1 signaling in wiring the nervous system.
(A) Sema3E-Plexin-D1 signaling promotes or inhibits axonal growth depending on its association with other transmembrane proteins. In cortical neurons, Sema3E acts as a classical repulsive cue and inhibits neuronal growth solely through the Plexin-D1 receptor (Left) [21]. However, Sema3E plays as a positive regulator in the subiculo-mammillary neurons where Plexin-D1 forms complex with co-receptors, neuropilin-1 and VEGF receptor (Right) [21, 22]. (B) Sema3E-Plexin-D1 signaling determines different type of synapse specificity and formation depending on ligand receptor localization on pre- vs. post-synaptic neurons. Two different cases of synaptic recognition regulation by Sema3E-Plexin-D1 signaling have been reported. (B-i) Postsynaptic target releases Sema3E to control synapse specificity in spinal cord motor neuron circuitry [23]. In the spinal cord, incoming cutaneous maximus (CM) sensory neurons express Plexin-D1 and target CM motor neurons express repulsive cue, Sema3E, thus there is no monosynaptic CM sensory-motor connections. On the other hand, absence of Sema3E-Plexin-D1 signaling produces abnormal CM sensory-motor circuit. (B-ii) Incoming presynaptic neurons express Sema3E to control synapse specificity in pathway specific thalamostriatal circuitry [20]. In contrast to sensory-motor circuit in the spinal cord, Sema3E is expressed in incoming thalamic neurons in thalamostriatal projections whereas Plexin-D1 is expressed specifically in Drd1-positive direct pathway MSNs. Lack of Sema3E-Plexin-D1 signaling causes ectopic thalamostriatal synapse in direct pathway MSNs.
Sema3E-Plexin-D1 also has different mode of action in regulating synapse formation and specificity depending on the pre and post synaptic localization of the ligand and receptor (Fig. 3B). It has recently been uncovered that Sema3E-Plexin-D1 signaling is involved not only in axonal growth and guidance but also in synaptic recognition in the spinal cord and striatum. In the spinal cord, Sema3E-Plexin-D1 plays a role in the specificity of monosynaptic sensory-motor connections [23]. Motor neurons innervating triceps muscle receive monosynaptic inputs from Tri sensory afferents, whereas cutaneous maximus (CM) motor neurons lack monosynaptic inputs from CM sensory afferents. Sema3E is expressed by CM motor neurons but not Tri motor neurons whereas Plexin-D1 is expressed in CM proprioceptive sensory neurons and Tri proprioceptive sensory neurons. Absence of Sema3E-Plexin-D1 signaling causes aberrant monosynaptic sensory-motor connections between CM sensory and CM motor neurons. Ectopic expression of Sema3E in Tri motor neurons reduced monosynaptic connections between Tri afferents and Tri motor neurons [23] [24]. Therefore, in the spinal cord post-synaptic neurons releasing guidance cue, Sema3E, and repel incoming axons that express Plexin-D1 to prevent inappropriate synapse formation (Fig. 3Bi).
Compared to the synaptic recognition case in the spinal cord, a recent study in the basal ganglia demonstrated that reversed expression, where Sema3E is secreted by incoming thalamic axons and Plexin-D1 expressed by one subtype of postsynaptic neuron, could specify synaptic specificity [20] (Fig 3Bii). As the input nucleus of the basal ganglia, the striatum receives convergent excitatory inputs carrying motor, sensory, and cognitive information from the cortex and thalamus. Specific excitatory synaptic connections need to be formed between axons arising from these two areas and two functionally distinct but anatomically intermingled populations of targets, direct and indirect pathway striatal medium spiny neurons (MSNs). The molecular mechanism that generates specificity in this complex wiring diagram is not fully understood. Recent study found that Sema3E-Plexin-D1 signaling controls pathway-specific synapse formation in the thalamo-striatal pathway [20]. Sema3E is secreted by thalamostriatal axons and Plexin-D1 is selectively expressed only in direct pathway MSN. Genetic ablation of Plexin-D1 or Sema3E strengthens glutamatergic synaptogenesis onto direct pathway MSNs without affecting synapses onto indirect pathway MSNs. The increased synaptic strength is also accompanied by increased synaptic density, indicating that Sema3E-Plexin-D1 signaling normally restricts the number of these synapses. Collectively, these studies suggest that Sema3E-Plexin-D1 signaling determines synaptic recognition and specificity in multiple parts of the nervous system, but the molecular and cellular mechanism underlying proper circuit establishment in each case is not yet known.
The role of Sema3E and Plexin-D1 in the development of other systems
The inhibition of blood supply has long been thought to be an attractive therapeutic method for slowing or halting tumor growth. Therefore, the modulation of vessel growth toward tumors using Sema3E-Plexin-D1 signaling is tempting for therapeutic purpose. In contrast to the developing vessels in which Sema3E acts as an anti-angiogenic factor through Plexin-D1, Sema3E-Plexin-D1 signaling in tumors and tumor microenvironments has two distinct roles depending on the ligand isoforms involved [25] [26]. Two different functional activities of Sema3E are exerted depending on Sema3E’s proteolytic cleavage by furin-like pro-protein-convertases [27]. The Sema3E proteolytic fragment p61 is the active and predominant form and promotes invasiveness and metastasis mediated by the trans-activation of erythroblastic leukemia viral oncogene homolog 2 (ErbB2)-epidermal growth factor receptor (EGFR) oncogenic tyrosine kinase receptors [25]. Recently, a point-mutated Sema3E isoform resistant to furin-mediated cleavage has been shown to have anti-angiogenic activity as well as anti-invasive/metastatic activity by competing with the endogenous p61 isoform [26]. So far, it is not clear whether different Sema3E isoforms play significant biological functions during normal development, and this will be an interesting question for future studies. In addition, it will be important to discover if different Sema3E isoforms are able to execute diverse functions through Plexin-D1 alone, or if co-receptors such as ErbB2 are required.
In addition to the vascular and nervous system, Sema3E-Plexin-D1 signaling has been intensively studied in the immune system. Plexin-D1 is highly expressed in the CD4+CD8+ double positive thymocytes and maturing CD69+ thymocytes. Sema3E-Plexin-D1 signaling directs the migration of maturing thymocytes to the medulla by inhibiting CXCR4 and CCR9-mediated migration signals [28]. It has recently been revealed that Plexin-D1 is also expressed during B lymphocyte activation and enables these cells to respond to germinal center chemokines such as CXCL12, CXCL13 and CCL19 from the germinal center [29]. In dendritic cells, Plexin-D1 is a negative regulator of IL-12/IL-23p40 response [30].
Global Plexin-D1 deficiency causes axial skeletal patterning defects and endothelial-cell-specific (Tie2-Cre-driven) Plexin-D1 knockout mice show similar skeletal malformations [31] [32]. However, since this phenotype is associated with a severe reduction of the bone marrow microvasculature, the skeletal defects are most likely secondary to vascular abnormalities [32]. Plexin-D1 is also required for Sema3-mediated cleft formation in the developing submendibular gland [33]. The precise mechanisms underlying these biological functions are still wait to be elucidated.
Sema3E-Plexin-D1 mediated signaling mechanisms
How Plexin-D1 transduce its intracellular signaling upon Sema3E bining to mediate the variety of biological functions is still largely unknown. Studies in cultured cells have shown that Plexin-D1 itself displays R-Ras GAP activity to inhibit migration of Cos-7 cells, and these actions specifically require the small GTPase Rnd2. In addition, Rnd2 was found to bind to Plexin-D1 in cortical neurons, and Sema3E-Plexin-D1-induced inhibition of axon outgrowth of cortical neurons required Rnd2 and downregulation of R-Ras activity [34].
In endothelial cells, Sema3E acts on Plexin-D1, thus initiating an anti-angiogenic signaling pathway that results in the retraction of filopodia in endothelial tip cells. Sema3E induces the rapid disassembly of integrin-mediated adhesive structures, thereby inhibiting endothelial cell adhesion to the extracellular matrix. This process requires the activation of the small GTPase Arf6 (ADP-ribosylation factor 6), which regulates intracellular trafficking of b1 integrin (Sakurai A, et al, 2011 MCB). It has recently been reported that GEP100 (guanine nucleotide exchange protein 100)/Brag2, a guanine nucleotide exchange factor for Arf6, mediates Sema3E-induced Arf6 activation in endothelial cells through phosphoinositide signaling [35]. The mechanism of negative regulation of Sema3E-Plexin-D1 on VEGF-induced angiogenesis, as shown in retinal vascular development [15] [16] has also been examined in human endothelial umbilical vein endothelial cells (HUVECs) and the antagonizing effect was found to be mediated by the inhibition of the ERK and Akt signaling pathways [36]. In the retinal vasculature, it has been shown that Sema3E-Plexin-D1 signaling down-regulates VEGF-induced Dll4-Notch signaling [15]. However, it is not fully understood whether Sema3E-Plexin-D1 signaling directly influences DLL4-Notch or controls it indirectly via other VEGFR signaling pathways. In addition, another study has suggested that the small GTPase RhoJ might be a mediator between Sema3E-Plexin-D1 and VEGF-VEGFR signaling through the regulation of GDP/GTP binding to RhoJ [16]. In this study, Sema3E-Plexin-D1 signaling through RhoJ was found to be a therapeutic target for treating abnormal vasculature in ischemic retinas. In addition to the retina model, Sema3E-Plexin-D1 was found to negatively regulate postnatal angiogenesis in a hindlimb ischemia model and in diabetic mice. Interestingly, Sema3E expression is upregulated by p53 in the pathological condition, but its molecular mechanism and correlation with VEGF signaling has yet been studied [36]. Given the diversity of Sema3E-Plexin-D1 function in different cell types (neurons vs. endothelial cells) and different biological process (vascular patterning, axonal growth, and synapse formation), unique downstream signaling pathways are most likely used for different functions.
Summary and Future Directions
The seemingly simple ligand-receptor interaction between Sema3E and Plexin-D1 has already been shown to be vital for a variety of functions in different cell types and systems. Different mechanism of action are used to control unique functions via this common ligand-receptor. For example, temporal and spatial control of ligand vs. receptor results in two different mechanisms governing vascular patterning. Interactions with other transmembrane proteins such as neuropilin or VEGFR2 results in different axonal behaviors. Ligand receptor localization on pre- vs. post-synaptic neurons is used to control different types of synapse formation. Perhaps different downstream effectors will also result in different functional outcomes. Given the limited number of ligands and receptors in the genome and their multifunctional nature, we expect that more modes of action will be discovered in the future. The variety of mechanisms used by Sema3E and Plexin-D1 mediate their biological functions set a perfect example of how a limited number of genes can generate such complicated vascular and neuronal networks.
As shown in many previous studies, Sema3E-Plexin-D1 signaling seems to have diverse roles in designing multiple systems during development. How does Sema3E-Plexin-D1 signaling coordinate between multiple systems, in particular two adjacent structures such as neurovascular units? Specifically, since Sema3E is guidance cue secreted from specific tissues, how does Sema3E action controlled in situations where Plexin-D1 is expressed on multiple adjacent tissues, for example in both the nervous and vascular systems? During development, temporal and spatial expression and activation of guidance cues is critical to coordinate each system. Therefore, to understand the coordination mechanism, further study about how Sema3E and Plexin-D1 expression is regulated at the transcriptional, translational, and post-translational levels will be crucial.
Another complicated issue that remains unresolved is the signaling cascade downstream of Sema3E-Plexin-D1 binding. For example, it has shown that the small GTPase Rnd2 is downstream of Plexin-D1 in cortical neurons, whereas other small GTPases such as Arf6 and RhoJ are known players in endothelial cells. Although a few studies have uncovered downstream molecules and their functions have been linked to cytoskeletal changes, it is not yet clear whether the identified molecules are common to all pathways downstream of Plexin-D1 or if downstream signal molecules can be specified in each system. Moreover, how Sema3E-Plexin-D1 signaling interacts with other signaling pathways is an open question. In the retinal vasculature as well as in subiculo-mammillary neurons, Sema3E-Plexin-D1 signaling is known to be interwoven with VEGF-VEGFR2 signaling. In addition, Sema3E-Plexin-D1 signaling inhibits VEGF-induced ERK and Akt activation, but it is still unclear how Plexin-D1 is specifically linked with multiple VEGFR2 signaling pathways.
To date, the study of Sema3E-Plexin-D1 signaling has mainly been focused on the developmental stage. Considering their significant expression in adult tissues, it is thought that Sema3E-Plexin-D1 signaling might play some essential roles in adult processes such as vessel regeneration and rewiring of synapses. Indeed, the therapeutic potential of Sema3E as an anti-angiogenic factor has been proven in tumor growth and ischemic abnormal vessel development. In addition to vascular disease, since Sema3E-Plexin-D1 signaling is involved in establishing the wiring of many circuits, as exemplified by the spinal cord and basal ganglia, it has tremendous potential as a therapeutic for many neurological disorders. Thus, further study about the molecular mechanism of Sema3E-Plexin-D1 signaling could lead to future therapeutic breakthroughs.
Highlights.
Sema3E can bind and signal directly with Plexin-D1, independently of neuropilins
Sema3E-Plexin-D1 regulates multiple aspects of neural and vascular development
Sema3E-Plexin-D1 controls developmental processes via different mechanisms of actions
Different downstream effectors will also result in different functional outcomes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kolodkin AL, Matthes DJ, O’Connor TP, Patel NH, Admon A, Bentley D, Goodman CS. Fasciclin IV: sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron. 1992;9:831–845. doi: 10.1016/0896-6273(92)90237-8. [DOI] [PubMed] [Google Scholar]
- [2].Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- [3].Pasterkamp RJ, Kolodkin AL. Semaphorin junction: making tracks toward neural connectivity. Current opinion in neurobiology. 2003;13:79–89. doi: 10.1016/s0959-4388(03)00003-5. [DOI] [PubMed] [Google Scholar]
- [4].Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- [5].Eichmann A, Makinen T, Alitalo K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes & development. 2005;19:1013–1021. doi: 10.1101/gad.1305405. [DOI] [PubMed] [Google Scholar]
- [6].Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- [7].van der Zwaag B, Hellemons AJ, Leenders WP, Burbach JP, Brunner HG, Padberg GW, Van Bokhoven H. PLEXIN-D1, a novel plexin family member, is expressed in vascular endothelium and the central nervous system during mouse embryogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2002;225:336–343. doi: 10.1002/dvdy.10159. [DOI] [PubMed] [Google Scholar]
- [8].Nogi T, Yasui N, Mihara E, Matsunaga Y, Noda M, Yamashita N, Toyofuku T, Uchiyama S, Goshima Y, Kumanogoh A, Takagi J. Structural basis for semaphorin signalling through the plexin receptor. Nature. 2010;467:1123–1127. doi: 10.1038/nature09473. [DOI] [PubMed] [Google Scholar]
- [9].Gelfand MV, Hong S, Gu C. Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning. Trends in cell biology. 2009;19:99110. doi: 10.1016/j.tcb.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Riccomagno MM, Hurtado A, Wang H, Macopson JG, Griner EM, Betz A, Brose N, Kazanietz MG, Kolodkin AL. The RacGAP beta2-Chimaerin selectively mediates axonal pruning in the hippocampus. Cell. 2012;149:1594–1606. doi: 10.1016/j.cell.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Van NP, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-plexin signaling guides patterning of the developing vasculature. Developmental cell. 2004;7:117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- [12].Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Developmental cell. 2004;7:107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- [13].Meadows SM, Fletcher PJ, Moran C, Xu K, Neufeld G, Chauvet S, Mann F, Krieg PA, Cleaver O. Integration of repulsive guidance cues generates avascular zones that shape mammalian blood vessels. Circulation research. 2012;110:34–46. doi: 10.1161/CIRCRESAHA.111.249847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lamont RE, Lamont EJ, Childs SJ. Antagonistic interactions among Plexins regulate the timing of intersegmental vessel formation. Developmental biology. 2009;331:199209. doi: 10.1016/j.ydbio.2009.04.037. [DOI] [PubMed] [Google Scholar]
- [15].Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C. Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes & development. 2011;25:1399–1411. doi: 10.1101/gad.2042011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S, Uemura A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. The Journal of clinical investigation. 2011;121:1974–1985. doi: 10.1172/JCI44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zygmunt T, Gay CM, Blondelle J, Singh MK, Flaherty KM, Means PC, Herwig L, Krudewig A, Belting HG, Affolter M, Epstein JA, Torres-Vazquez J. Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1. Developmental cell. 2011;21:301–314. doi: 10.1016/j.devcel.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tamagnone L, Mazzone M. Semaphorin signals on the road of endothelial tip cells. Developmental cell. 2011;21:189–190. doi: 10.1016/j.devcel.2011.07.017. [DOI] [PubMed] [Google Scholar]
- [19].Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nature cell biology. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- [20].Ding JB, Oh WJ, Sabatini BL, Gu C. Semaphorin 3E-Plexin-D1 signaling controls pathway-specific synapse formation in the striatum. Nature neuroscience. 2012;15:215–223. doi: 10.1038/nn.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chauvet S, Cohen S, Yoshida Y, Fekrane L, Livet J, Gayet O, Segu L, Buhot MC, Jessell TM, Henderson CE, Mann F. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron. 2007;56:807–822. doi: 10.1016/j.neuron.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bellon A, Luchino J, Haigh K, Rougon G, Haigh J, Chauvet S, Mann F. VEGFR2 (KDR/Flk1) signaling mediates axon growth in response to semaphorin 3E in the developing brain. Neuron. 2010;66:205–219. doi: 10.1016/j.neuron.2010.04.006. [DOI] [PubMed] [Google Scholar]
- [23].Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;459:842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yoshida Y. Semaphorin signaling in vertebrate neural circuit assembly. Frontiers in molecular neuroscience. 2012;5:71. doi: 10.3389/fnmol.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Casazza A, Finisguerra V, Capparuccia L, Camperi A, Swiercz JM, Rizzolio S, Rolny C, Christensen C, Bertotti A, Sarotto I, Risio M, Trusolino L, Weitz J, Schneider M, Mazzone M, Comoglio PM, Tamagnone L. Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. The Journal of clinical investigation. 2010;120:2684–2698. doi: 10.1172/JCI42118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Casazza A, Kigel B, Maione F, Capparuccia L, Kessler O, Giraudo E, Mazzone M, Neufeld G, Tamagnone L. Tumour growth inhibition and anti-metastatic activity of a mutated furin-resistant Semaphorin 3E isoform. EMBO molecular medicine. 2012;4:234–250. doi: 10.1002/emmm.201100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Christensen C, Ambartsumian N, Gilestro G, Thomsen B, Comoglio P, Tamagnone L, Guldberg P, Lukanidin E. Proteolytic processing converts the repelling signal Sema3E into an inducer of invasive growth and lung metastasis. Cancer research. 2005;65:6167–6177. doi: 10.1158/0008-5472.CAN-04-4309. [DOI] [PubMed] [Google Scholar]
- [28].Choi YI, Duke-Cohan JS, Ahmed WB, Handley MA, Mann F, Epstein JA, Clayton LK, Reinherz EL. PlexinD1 glycoprotein controls migration of positively selected thymocytes into the medulla. Immunity. 2008;29:888–898. doi: 10.1016/j.immuni.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Holl EK, O’Connor BP, Holl TM, Roney KE, Zimmermann AG, Jha S, Kelsoe G, Ting JP. Plexin-D1 is a novel regulator of germinal centers and humoral immune responses. J Immunol. 2011;186:5603–5611. doi: 10.4049/jimmunol.1003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Holl EK, Roney KE, Allen IC, Steinbach E, Arthur JC, Buntzman A, Plevy S, Frelinger J, Ting JP. Plexin-B2 and Plexin-D1 in dendritic cells: expression and IL-12/IL-23p40 production. PloS one. 2012;7:e43333. doi: 10.1371/journal.pone.0043333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kanda T, Yoshida Y, Izu Y, Nifuji A, Ezura Y, Nakashima K, Noda M. PlexinD1 deficiency induces defects in axial skeletal morphogenesis. Journal of cellular biochemistry. 2007;101:1329–1337. doi: 10.1002/jcb.21306. [DOI] [PubMed] [Google Scholar]
- [32].Zhang Y, Singh MK, Degenhardt KR, Lu MM, Bennett J, Yoshida Y, Epstein JA. Tie2Cre-mediated inactivation of plexinD1 results in congenital heart, vascular and skeletal defects. Developmental biology. 2009;325:82–93. doi: 10.1016/j.ydbio.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chung L, Yang TL, Huang HR, Hsu SM, Cheng HJ, Huang PH. Semaphorin signaling facilitates cleft formation in the developing salivary gland. Development. 2007;134:2935–2945. doi: 10.1242/dev.005066. [DOI] [PubMed] [Google Scholar]
- [34].Uesugi K, Oinuma I, Katoh H, Negishi M. Different requirement for Rnd GTPases of R-Ras GAP activity of Plexin-C1 and Plexin-D1. The Journal of biological chemistry. 2009;284:6743–6751. doi: 10.1074/jbc.M805213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sakurai A, Jian X, Lee CJ, Manavski Y, Chavakis E, Donaldson J, Randazzo PA, Gutkind JS. Phosphatidylinositol-4-phosphate 5-kinase and GEP100/Brag2 protein mediate antiangiogenic signaling by semaphorin 3E-plexin-D1 through Arf6 protein. The Journal of biological chemistry. 2011;286:34335–34345. doi: 10.1074/jbc.M111.259499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moriya J, Minamino T, Tateno K, Okada S, Uemura A, Shimizu I, Yokoyama M, Nojima A, Okada M, Koga H, Komuro I. Inhibition of semaphorin as a novel strategy for therapeutic angiogenesis. Circulation research. 2010;106:391–398. doi: 10.1161/CIRCRESAHA.109.210815. [DOI] [PubMed] [Google Scholar]