Abstract
Purpose of review
Renal ischemia-reperfusion injury (IRI) is a common cause of acute kidney injury (AKI). Alterations in renal medullary blood flow (MBF) contribute to the pathogenesis of renal IRI. Here we review recent insights into the mechanisms of altered MBF in the pathogenesis of IRI.
Recent findings
Although cortical blood flow fully recovers following 30–45 minutes of bilateral IRI, recent studies have indicated that there is a prolonged secondary fall in MBF that is associated with a long term decline in renal function. Recent findings indicate that angiopoeitin-1, atrial natriuretic peptide, heme oxygenase-1, and the gasotransmitters, carbon monoxide and hydrogen sulfide, may limit the severity of IRI by preserving MBF. Additional studies have also suggested a role for cytochrome P450 derived 20-HETE in the post-ischemic fall in MBF.
Summary
Impaired MBF contributes to the pathogenesis of renal IRI. Measurement of renal MBF provides valuable insight into the underlying mechanisms of many renoprotective pathways. Identification of molecules that preserve renal MBF in IRI may lead to new therapies for AKI.
Keywords: hemodynamics, kidney, renal medulla, acute kidney injury
INTRODUCTION
Acute kidney injury (AKI) is a common complication of acute illness and significantly increases morbidity, mortality, and resource utilization. (1, 2) Renal ischemia-reperfusion injury (IRI) is a common cause of AKI in many clinical settings. (3, 4) IRI results from multiple factors affecting both the renal tubular epithelium and renal microvasculature. (5–7) Ischemia markedly reduces intracellular ATP concentration and initiates cellular injury which is exacerbated upon reperfusion by an increase in oxidative stress and inflammation. (5, 6) Alterations in renal blood flow (RBF) following IRI can result from microvascular injury, impaired renal vascular reactivity, and as a consequence of impaired red blood cell trafficking in the peritubular capillaries due to the infiltration of inflammatory cells and increased parenchymal pressure. (5, 8–11) Indeed, sustained reductions in renal blood flow are seen following severe prolonged IRI (>45min) in numerous experimental studies and in patients with post-ischemic renal failure following kidney transplantation. (12–16). With shorter periods of ischemia and less severe IRI, whole kidney, cortical and inner medullary blood flow typically fully recovers following reperfusion, however, outer medullary blood flow remains compromised for prolonged periods.
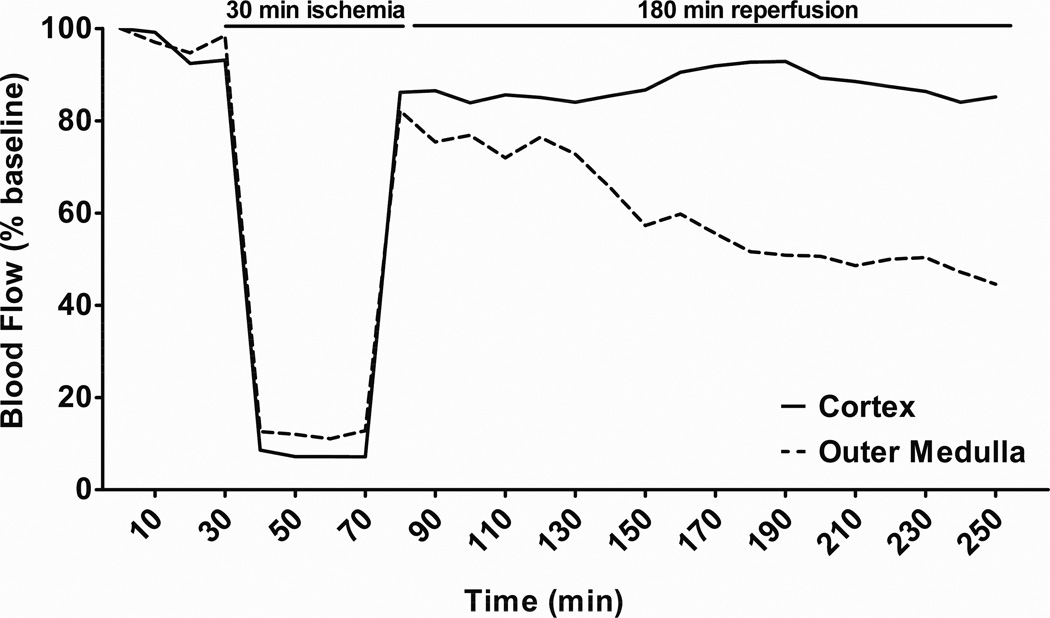
The post-ischemic fall in outer medullary blood flow (MBF) may be a critical event in extending renal tissue injury following reperfusion. (10, 17–19) Several studies in rat models of IRI highlight the role of prolonged post-ischemic impairment in MBF in the pathogenesis of IRI. Vetterlein et al. demonstrated a marked reduction in medullary plasma flow one hour after of reperfusion utilizing fluorescent tracers. (10) Using laser-Doppler flowmetry (LDF), Olof et al. and Conesa et al. observed marked and sustained reductions in outer MBF following reperfusion. (17, 18) As illustrated in figure 1, we recently found that while cortical blood flow returns to baseline levels, there is a persistent impairment in outer MBF upon reperfusion in rats exposed to 30 minutes of renal ischemia. (19) The prolonged reductions in outer MBF can exacerbate tubular epithelial cell injury, particularly in the S3 segment of the proximal tubule which subsists in the hypoxic microenvironment of the renal outer medulla. (6, 7, 20) Given the importance of these hemodynamic changes in the pathogenesis of IRI, the identification of mediators that contribute to the reduction in renal outer MBF and identification of compounds that can prevent this deficit is a logical step in the development of new therapies for AKI. (21) In this review we will highlight findings of recent studies (summarized in Table 1) that shed new light on the mechanisms of altered MBF following IRI.
Figure 1. Effect of renal ischemia-reperfusion injury on regional blood flow in the kidney.
Sprague-Dawley rats underwent 30 minutes renal ischemia and 180 minutes of reperfusion. Cortical blood flow (CBF) and outer medullary blood flow (MBF) were measured by laser-Doppler flowmetry. CBF and MBF decreased dramatically during the ischemic period and CBF rapidly recovered to baseline levels following reperfusion. In contrast, a transient improvement in MBF was seen immediately after reperfusion followed by a gradual decline to approximately 50% of baseline that was sustained for the 3 hour period of the experiment. Adapted from Regner et al. (19)
Table 1.
Summary of recent studies examining the regulation of renal MBF following IRI.
| Pathway | Renal IRI Model | Results | Reference |
|---|---|---|---|
| 20-HETE | 30 min bilateral warm ischemia in rats | 20-HETE analogue preserved post-ischemic MBF and mitigated renal injury | 19 |
| 45 min warm ischemia after uninephrectomy in rats | 20-HETE inhibitor improve post-ischemic MBF and mitigated renal injury | 27 | |
| HO-1 | 45 min of warm ischemia in rats | Induction of HO-1 decreased the post-ischemic fall in MBF and severity of renal dysfunction | 34 |
| H2S | 45 min bilateral warm ischemia in rats | Endogenous and exogenous H2S decreased renal injury and improved renal function | 45 |
| Porcine model of 25 min of warm ischemia and 18 hrs cold storage | H2S infusion improved total RBF and renal function | 46 | |
| CO | Porcine model of 10 min renal ischemia and 16 hrs of cold storage | Administration of CO releasing molecule-3 (CORM-3) preserved renal function and improved total RBF | 50 |
| ANP | Porcine model of 10 min warm ischemia and 18 hrs cold storage | ANP treatment improved MBF and renal function and reduced tubular injury | 52 |
| Ang1 | 22 min of bilateral warm ischemia in mice | Adenovirus overexpression of COMP-Ang1 improved post-ischemic MBF and renal function | 56 |
IRI, ischemia-reperfusion injury; MBF, medullary blood flow; min, minute; 20-HETE, 20-hydroxyeicostetraenoic acid; HO-1, heme oxygenase-1; H2S, hydrogen sulfide; hrs, hours; RBF, renal blood flow; CO, carbon monoxide; ANP; atrial natriuretic peptide; Ang1, angiopoietin-1; COMP-Ang1, cartilage oligomeric matrix protein-Ang1.
20-HETE
Arachidonic acid (AA) is released from membrane phospholipids in the renal outer medulla in response to ischemia and can be metabolized to 20-hydroxyeicosatetraenoic acid (20–HETE) by cytochrome p450 ω-hydroxylase. (22, 23) In the kidney, 20-HETE modulates vascular tone, renal blood flow, and tubular sodium transport. (23) 20-HETE increases vascular responsiveness to vasoconstrictors and would be expected to reduce tissue blood flow and exacerbate IRI. Indeed, 20-HETE has been shown to promote ischemic injury in the heart and brain. (24, 25) However, in the kidney 20-HETE was shown to increase MBF in a dose dependent manner in rats. (26) Two recent studies have addressed the effect of 20-HETE on renal IRI and the post-ischemic fall in MBF in rats.
Regner et al. demonstrated that inhibition of 20-HETE synthesis with HET0016 exacerbated renal IRI and that systemic administration of a stable 20-HETE analog, 5,14–20-HEDGE, mitigated renal IRI and prevented the post-ischemic decrease in renal outer MBF in rats. (19) 5,14–20-HEDGE also inhibited sodium transport and enhanced natriuresis in normal rats. These authors concluded that the 20-HETE analog mitigated IRI by preventing post-ischemic medullary hypoxia through a concurrent increase in renal MBF and a decrease in tubular transport and oxygen demand. (19) In a subsequent study, Hoff et al. demonstrated a ten-fold increase in 20-HETE levels in rat kidneys exposed to 45 minutes of ischemia. (27) They reported that bolus administration of the 20-HETE synthesis inhibitor, HET0016 or a 20-HETE antagonist, 6,15–20-HEDE, directly into the renal artery of a uninephrectomized rat attenuated the severity of renal IRI. 6,15–20-HEDE significantly improved medullary oxygenation and hastened the recovery of renal outer MBF. (27) However, 6,15–20-HEDE treatment did not fully return outer MBF to baseline levels, suggesting that the salutary effect of this compound on medullary oxygenation occurred via changes in oxygen utilization.
The reason for the divergent results remain to be determined but could be due to differences in the experimental design of these studies. (28) Regner et al. administered 5,14–20-HEDGE subcutaneously at a high dose that produced elevated blood levels for several hours whereas Hoff et al. administered a much smaller dose of 6,15–20-HEDE by bolus injection directly into the renal artery that likely produced high first-pass blood levels but much lower systemic levels following redistribution. (19, 27, 28) In addition, Regner et al. used a model of 30 minutes bilateral ischemia, whereas Hoff et al. performed 45 minutes warm ischemia on the remaining kidney following uninephrectomy. (19, 27, 28) This experimental difference appears to be critical in interpreting the divergent hemodynamic findings in these two studies since uninephrectomy has been shown to rapidly alter renal hemodynamics by increasing both cortical and medullary blood flow in the remaining kidney. (29) These studies clearly indicate a role for 20-HETE in the regulation of medullary blood flow and oxygenation following IRI, but further studies are needed to clarify the therapeutic potential of 20-HETE agonists and/or antagonists in renal IRI and to determine their mechanism of action.
HEME OXYGENASE-1
Heme oxygenase-1 (HO-1) converts heme into biliverdin and carbon monoxide while simultaneously releasing iron. (30) HO-1 is induced in the kidney following a number cellular stressors and plays a protective role in animal models of AKI, including IRI. (30) HO-1 is highly expressed in the renal medulla and inhibitors of HO-1 significantly decrease renal MBF. (31) In a porcine model of warm renal ischemia, HO-1 mRNA expression was positively correlated with total RBF during reperfusion. (32) In contrast, in HO-1 knockout mice exposed to 15 minutes of renal ischemia and 4 hour reperfusion there was a significant reduction in GFR but no change in total RBF compared to control mice. (33) Salom et al. reported that induction of HO-1 with CoCl2 decreased the severity of renal dysfunction in rats exposed to 45 minutes of renal ischemia. (34) Induction of HO-1 significantly decreased the post-ischemic fall in MBF as measured by LDF. This beneficial effect associated with a decrease in peroxynitrite formation during the ischemic period suggesting a role for reduced NO scavenging by reactive oxygen species rather than increased formation of carbon monoxide. (34) However, CoCl2 is also a potent inducer of hypoxia inducible factor-1 alpha which alters the expression of many genes that may contribute to the renoprotective effect of CoCl2. (35) Thus, further investigation is needed to identify the mediator of protective effect of HO-1 or CoCl2 in renal IRI and whether the mechanism is due in part, to preservation of renal MBF upon reperfusion.
GASOTRANSMITTERS
Gasotransmitters are gaseous molecules that modulate a variety of intracellular signaling pathways. (36) Nitric oxide (NO) is the best characterized of these paracrine factors and plays a key role in the regulation of renal medullary perfusion by promoting dilatation of vasa recta capillaries and antagonizing the effects of vasoconstrictors. (37–39) From this standpoint, NO would be expected to have a beneficial effect in renal IRI. However, NO produced in response to ischemia can combine with superoxide to produce peroxynitrite leading to an increase in oxidative stress and exacerbation of renal injury. (6, 40) Therefore, the impact of NO in renal IRI is dependent upon a number of factors including the temporal and spatial distribution of NO production and the type of nitric oxide synthase (NOS) activated following ischemia. The role of NO and NOS in the pathogenesis of renal IRI and AKI is very complex and has be extensively reviewed elsewhere. (41–44)}
In contrast to NO, much less is known about the role of the other gasotransmitters, hydrogen sulfide (H2S) and carbon monoxide (CO), in the control of MBF and the pathogenesis of renal IRI. H2S is enzymatically synthesized from L-cysteine or L-homocysteine and has been shown to activate ATP-sensitive potassium channels (KATP) in numerous cell types. (45) H2S is produced in the kidney through substrate dependent metabolism of L-cysteine and infusion of H2S donors into the renal artery of rats increases total RBF, GFR, and urine sodium excretion. (46) Tripatara et al. demonstrated that IRI increases the production of H2S in the kidney and that endogenous and exogenous H2S decreased the severity of renal dysfunction following IRI in rats. (47). Hosgood et al. subsequently demonstrated that H2S infusion improved total RBF and renal function in a porcine model of 25 minutes of warm ischemia and 18 hours of cold storage. (48) Whether the protective effect of H2S in renal IRI is mediated by an improvement in MBF remains to be studied. However, one would expect to find that H2S should produce a medullary vasodilation since KATPchannels are expressed by pericytes in the vasa recta. (45)
CO is produced from the metabolism of heme by heme oxygenase. The protective effect of HO-1 in models of renal injury appears to be mediated, in part, through the vasodilator actions of CO. (30, 49) Furthermore, the effect of HO-1 on MBF may be a result of CO mediated activation of guanylate cyclase. (31) Notably, CO donor compounds protected against renal IRI in mice, even in the presence of a heme oxygenase inhibitor, indicating that CO-mediated protection from IRI may be independent of HO-1 activity. (50) The influence of CO on renal hemodyamics following IRI has been explored in various models of IRI. In a rat model of kidney transplantation, exposure of recipient rats to inhaled CO (250 ppm) significantly decreased tubular injury and inflammation. This was associated with a significant increase in renal cortical blood flow upon reperfusion. (51) Hosgood et al. demonstrated that administration of the carbon monoxide releasing molecule, CORM-3 preserved renal function and improved total RBF in a porcine model of 10 minutes of warm renal ischemia and 16 hours of cold storage. (52) However, it remains to be determined whether the protective effect of CO in renal IRI is mediated by preservation of post-ischemic MBF.
ATRIAL NATRIURETIC PEPTIDE
Atrial natriuretic peptide (ANP) is secreted by cardiomyocytes following volume expansion and elevations in end diastolic filling pressure. Infusion of ANP increases GFR and MBF in rats. (53) Chujo et al. recently reported that MBF was significantly higher following IRI in rats treated with ANP as compared to vehicle treated controls. (54) This improvement in outer MBF in the ANP treated rats was associated with improvements in renal function and reduced tubular injury 24 and 48 hours following ischemia. (54) ANP, like NO, is a potent activator of guanylyl cyclase and suggests that strategies to increase cGMP, like NO, ANP or phosphodiesterase 5 inhibitors, might be useful in the treatment of ischemic AKI. Recent clinical trials using ANP for treatment or prevention of AKI have had mixed results depending on the treatment strategy and patient population. In this regard, it appears that low dose ANP therapy may decrease the need for renal replacement therapy in patients with post-operative AKI. (55)
ANGIOPOIETIN-1
Angiopoietin-1 (Ang1) is a key regulator of vascular development during embryogenesis and contributes to the vascular adaptation to injury and stress. (56) Cartilage oligomeric matrix protein-Ang1 (COMP-Ang1) is an engineered form of Ang1 with improved solubility and potency compared to native Ang1. (57) Jung et al. recently reported on the effects of adenovirus overexpression of COMP-Ang1 on renal injury and renal hemodynamics in mice exposed to 22 minutes of bilateral renal ischemia. (58) In comparison to vehicle treated controls, renal function in the COMP-Ang1 overexpressing mice was significantly improved 1, 2, and 3 days following reperfusion. Renal MBF was significantly higher in the COMP-Ang1 overexpressing mice at 2 hours and 2 days following ischemia. Most notably, COMP-Ang1 had long term benefits and was associated with a decrease in renal tubulointerstitial fibrosis 30 days after IRI. (58)
LONG-TERM EFFECTS OF IRI ON RENAL MBF
Recovery of renal function following AKI in humans is often incomplete and many patients develop long term complications. (59, 60) Several experimental studies have highlighted the importance of renal microvascular injury in the pathogenesis of chronic kidney disease and hypertension following IRI. Basile et al. evaluated the long-term effects of bilateral renal ischemia in rats. (61) They reported that renal function and tubular morphology fully recovered to control levels within 2 weeks. However, the rats developed proteinuria and tubulointerstitial fibrosis several months after the initial injury. (61) Notably, these abnormalities were preceded by a 30 to 50% reduction in the density of vasa recta capillaries in the outer medulla of the kidney as early as 4 weeks after ischemia. (61) In a subsequent study, this group demonstrated that IRI in rats leads to decreased GFR and RBF, impaired autoregulation of MBF, relative renal medullary hypoxia and sodium sensitive hypertension 5 weeks after ischemia. (62) These findings highlight the importance of injury to the vasa recta capillaries and the fall in MBF during recovery from ischemic injury and may explain the increased risk for the development of microalbuminuria, hypertension, and chronic kidney disease in patients with AKI. (59, 60, 63)
CONCLUSION
Sustained alterations in RBF are well described following renal IRI. This perturbation in RBF is primarily due to regional hypoperfusion in the renal outer medulla. Identification and characterization of mediators that can mitigate microvascular injury and preserve MBF following IRI may lead to therapies for AKI. Assessment of MBF is essential in determining the mechanism of action of putative renoprotective agents in IRI.
SUMMARY.
Renal ischemia-reperfusion injury is a common cause of acute kidney injury.
Ischemic renal injury is associated with prolonged reductions in renal medullary blood flow following reperfusion.
Reduced medullary blood flow promotes tubular necrosis and fibrosis in the outer medulla.
Nitric oxide, carbon monoxide, atrial natriuretic peptide and eicosanoids help preserve medullary blood flow and reduce ischemic renal injury.
ACKNOWLEDGEMENTS
This work was supported in part by grants HL36279 (RJR), HL29587 (RJR), and DK090123 (KRR) from the National Institutes of Health and a grant from the Clinical Translational Science Institute of Southeast Wisconsin (KRR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES AND RECOMMENDED READING
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006;2:364–377. doi: 10.1038/ncpneph0218. [DOI] [PubMed] [Google Scholar]
- 3.Lameire N, Vanholder R. Pathophysiologic Features and Prevention of Human and Experimental Acute Tubular Necrosis. J Am Soc Nephrol. 2001;12:S20–S32. [PubMed] [Google Scholar]
- 4.Lamiere The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006;2:364–377. doi: 10.1038/ncpneph0218. [DOI] [PubMed] [Google Scholar]
- 5.Bonventre JV, Weinberg JM. Recent Advances in the Pathophysiology of Ischemic Acute Renal Failure. J Am Soc Nephrol. 2003;14:2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 6.Devarajan P. Update on Mechanisms of Ischemic Acute Kidney Injury. J Am Soc Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 7.Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol. 2003;284:F608–F627. doi: 10.1152/ajprenal.00284.2002. [DOI] [PubMed] [Google Scholar]
- 8.Herrler T, Tischer A, Meyer A, Feiler S, et al. The intrinsic renal compartment syndrome: new perspectives in kidney transplantation. Transplantation. 89:40–46. doi: 10.1097/TP.0b013e3181c40aba. [DOI] [PubMed] [Google Scholar]
- 9.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 10.Vetterlein F, Petho A, Schmidt G. Distribution of capillary blood flow in rat kidney during postischemic renal failure. Am J Physiol. 1986;251:H510–H519. doi: 10.1152/ajpheart.1986.251.3.H510. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Tada T, Brodsky SV, Tanaka H, et al. Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am J Physiol Renal Physiol. 2002;282:F1150–F1155. doi: 10.1152/ajprenal.00310.2001. [DOI] [PubMed] [Google Scholar]
- 12.Cristol JP, Thiemermann C, Mitchell JA, Walder C, et al. Support of renal blood flow after ischaemic-reperfusion injury by endogenous formation of nitric oxide and of cyclo-oxygenase vasodilator metabolites. Br J Pharmacol. 1993;109:188–194. doi: 10.1111/j.1476-5381.1993.tb13552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cristol JP, Thiemermann C, Guerin MC, Torreilles J, et al. L-Arginine infusion after ischaemia-reperfusion of rat kidney enhances lipid peroxidation. J Lipid Mediat Cell Signal. 1996;13:9–17. doi: 10.1016/0929-7855(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 14.Mizutani A, Okajima K, Uchiba M, Noguchi T. Activated protein C reduces ischemia/reperfusion-induced renal injury in rats by inhibiting leukocyte activation. Blood. 2000;95:3781–3787. [PubMed] [Google Scholar]
- 15.Nijveldt RJ, Prins HA, van Kemenade FJ, Teerlink T, et al. Low Arginine Plasma Levels do not Aggravate Renal Blood Flow after Experimental Renal Ischaemia/reperfusion. European Journal of Vascular and Endovascular Surgery. 2001;22:232–239. doi: 10.1053/ejvs.2001.1444. [DOI] [PubMed] [Google Scholar]
- 16.Ramaswamy D, Corrigan G, Polhemus C, Boothroyd D, et al. Maintenance and recovery stages of postischemic acute renal failure in humans. Am J Physiol Renal Physiol. 2002;282:F271–F280. doi: 10.1152/ajprenal.0068.2001. [DOI] [PubMed] [Google Scholar]
- 17.Conesa EL, Valero F, Nadal JC, Fenoy FJ, et al. N-acetyl-L-cysteine improves renal medullary hypoperfusion in acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2001;281:R730–R737. doi: 10.1152/ajpregu.2001.281.3.R730. [DOI] [PubMed] [Google Scholar]
- 18.Olof P, Hellberg A, Kallskog O, Wolgast M. Red cell trapping and postischemic renal blood flow. Differences between the cortex, outer and inner medulla. Kidney Int. 1991;40:625–631. doi: 10.1038/ki.1991.254. [DOI] [PubMed] [Google Scholar]
- 19.Regner KR, Zuk A, Van Why SK, Shames BD, et al. Protective effect of 20-HETE analogues in experimental renal ischemia reperfusion injury. Kidney Int. 2009;75:511–517. doi: 10.1038/ki.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brezis M, Rosen S. Hypoxia of the Renal Medulla -- Its Implications for Disease. N Engl J Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 21.Tumlin JA. Impaired blood flow in acute kidney injury: pathophysiology and potential efficacy of intrarenal vasodilator therapy. Curr Opin Crit Care. 2009;15:514–519. doi: 10.1097/MCC.0b013e328332f6f9. [DOI] [PubMed] [Google Scholar]
- 22.Chien KR, Han A, Sen A, Buja LM, et al. Accumulation of unesterified arachidonic acid in ischemic canine myocardium. Relationship to a phosphatidylcholine deacylation- reacylation cycle and the depletion of membrane phospholipids. Circ Res. 1984;54:313–322. doi: 10.1161/01.res.54.3.313. [DOI] [PubMed] [Google Scholar]
- 23.Roman RJ. P-450 Metabolites of Arachidonic Acid in the Control of Cardiovascular Function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 24.Nithipatikom K, Gross ER, Endsley MP, Moore JM, et al. Inhibition of Cytochrome P450{omega}-Hydroxylase: A Novel Endogenous Cardioprotective Pathway. Circ Res. 2004;95:e65–e71. doi: 10.1161/01.RES.0000146277.62128.6f. [DOI] [PubMed] [Google Scholar]
- 25.Renic M, Klaus JA, Omura T, Kawashima N, et al. Effect of 20-HETE inhibition on infarct volume and cerebral blood flow after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2009;29:629–639. doi: 10.1038/jcbfm.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oyekan AO. Differential Effects of 20-Hydroxyeicosatetraenoic Acid on Intrarenal Blood Flow in the Rat. J Pharmacol Exp Ther. 2005;313:1289–1295. doi: 10.1124/jpet.104.080218. [DOI] [PubMed] [Google Scholar]
- 27. Hoff U, Lukitsch I, Chaykovska L, Ladwig M, et al. Inhibition of 20-HETE synthesis and action protects the kidney from ischemia/reperfusion injury. Kidney Int. 79:57–65. doi: 10.1038/ki.2010.377. * This study indicates that inhibitors of 20-HETE may be useful to prevent renal injury following transplantation
- 28. Roman RJ, Akbulut T, Park F, Regner KR. 20-HETE in acute kidney injury. Kidney Int. 79:10–13. doi: 10.1038/ki.2010.396. * This commentary highlights the recent controversy regarding the role of 20-HETE in renal IRI.
- 29.Young LS, Regan MC, Sweeney P, Barry KM, et al. Changes in regional renal blood flow after unilateral nephrectomy using the techniques of autoradiography and microautoradiography. J Urol. 1998;160:926–931. doi: 10.1016/S0022-5347(01)62834-9. [DOI] [PubMed] [Google Scholar]
- 30.Nath KA. Heme oxygenase-1: A provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70:432–443. doi: 10.1038/sj.ki.5001565. [DOI] [PubMed] [Google Scholar]
- 31.Zou AP, Billington H, Su N, Cowley AW., Jr Expression and actions of heme oxygenase in the renal medulla of rats. Hypertension. 2000;35:342–347. doi: 10.1161/01.hyp.35.1.342. [DOI] [PubMed] [Google Scholar]
- 32.Waller HL, Harper SJ, Hosgood SA, Bagul A, et al. Differential expression of cytoprotective and apoptotic genes in an ischaemia-reperfusion isolated organ perfusion model of the transplanted kidney. Transpl Int. 2007;20:625–631. doi: 10.1111/j.1432-2277.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- 33.Tracz MJ, Juncos JP, Croatt AJ, Ackerman AW, et al. Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int. 2007;72:1073–1080. doi: 10.1038/sj.ki.5002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salom MG, Ceron SN, Rodriguez F, Lopez B, et al. Heme oxygenase-1 induction improves ischemic renal failure: role of nitric oxide and peroxynitrite. Am J Physiol Heart Circ Physiol. 2007;293:H3542–H3549. doi: 10.1152/ajpheart.00977.2007. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto M, Makino Y, Tanaka T, Tanaka H, et al. Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol. 2003;14:1825–1832. doi: 10.1097/01.asn.0000074239.22357.06. [DOI] [PubMed] [Google Scholar]
- 36.Mustafa AK, Gadalla MM, Snyder SH. Signaling by Gasotransmitters. Sci Signal. 2009;2 doi: 10.1126/scisignal.268re2. re2-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowley AW, Mori T, Mattson D, Zou A-P. Role of renal NO production in the regulation of medullary blood flow. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2003;284:R1355–R1369. doi: 10.1152/ajpregu.00701.2002. [DOI] [PubMed] [Google Scholar]
- 38. O'Connor PM, Cowley AW., Jr Modulation of pressure-natriuresis by renal medullary reactive oxygen species and nitric oxide. Curr Hypertens Rep. 2010;12:86–92. doi: 10.1007/s11906-010-0094-6. * This review highlights the complex relationship role of nitric oxide and reactive oxygen species in the control of renal medullary hemodynamics.
- 39.Pallone TL, Mattson DL. Role of nitric oxide in regulation of the renal medulla in normal and hypertensive kidneys. Curr Opin Nephrol Hypertens. 2002;11:93–98. doi: 10.1097/00041552-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459:923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 41.Legrand M, Mik EG, Johannes T, Payen D, et al. Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol Med. 2008;14:502–516. doi: 10.2119/2008-00006.Legrand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goligorsky MS, Brodsky SV, Noiri E. NO bioavailability, endothelial dysfunction, and acute renal failure: new insights into pathophysiology. Seminars in Nephrology. 2004;24:316–323. doi: 10.1016/j.semnephrol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Goligorsky MS, Brodsky SV, Noiri E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int. 2002;61:855–861. doi: 10.1046/j.1523-1755.2002.00233.x. [DOI] [PubMed] [Google Scholar]
- 44.Heemskerk S, Masereeuw R, Russel FG, Pickkers P. Selective iNOS inhibition for the treatment of sepsis-induced acute kidney injury. Nat Rev Nephrol. 2009;5:629–640. doi: 10.1038/nrneph.2009.155. [DOI] [PubMed] [Google Scholar]
- 45. Beltowski J. Hypoxia in the renal medulla: implications for hydrogen sulfide signaling. J Pharmacol Exp Ther. 334:358–363. doi: 10.1124/jpet.110.166637. * This study reveals that the renal medulla produces hydrogen sulfide and it may serve as a buffer against the development IRI.
- 46.Xia M, Chen L, Muh RW, Li P-L, et al. Production and Actions of Hydrogen Sulfide, a Novel Gaseous Bioactive Substance, in the Kidneys. Journal of Pharmacology and Experimental Therapeutics. 2009;329:1056–1062. doi: 10.1124/jpet.108.149963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tripatara P, Patel NSA, Brancaleone V, Renshaw D, et al. Characterisation of cystathionine gamma-lyase/hydrogen sulphide pathway in ischaemia/reperfusion injury of the mouse kidney: An in vivo study. European Journal of Pharmacology. 2009;606:205–209. doi: 10.1016/j.ejphar.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 48.Hosgood SA, Nicholson ML. Hydrogen sulphide ameliorates ischaemia-reperfusion injury in an experimental model of non-heart-beating donor kidney transplantation. Br J Surg. 97:202–209. doi: 10.1002/bjs.6856. [DOI] [PubMed] [Google Scholar]
- 49.Ferenbach DA, Kluth DC, Hughes J. Hemeoxygenase-1 and Renal Ischaemia-Reperfusion Injury. Nephron Experimental Nephrology. 115:e33–e37. doi: 10.1159/000313828. [DOI] [PubMed] [Google Scholar]
- 50.Vera T, Henegar JR, Drummond HA, Rimoldi JM, et al. Protective effect of carbon monoxide-releasing compounds in ischemia-induced acute renal failure. J Am Soc Nephrol. 2005;16:950–958. doi: 10.1681/ASN.2004090736. [DOI] [PubMed] [Google Scholar]
- 51.Neto JS, Nakao A, Kimizuka K, Romanosky AJ, et al. Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. Am J Physiol Renal Physiol. 2004;287:F979–F989. doi: 10.1152/ajprenal.00158.2004. [DOI] [PubMed] [Google Scholar]
- 52.Hosgood SA, Bagul A, Kaushik M, Rimoldi J, et al. Application of nitric oxide and carbon monoxide in a model of renal preservation. Br J Surg. 2008;95:1060–1067. doi: 10.1002/bjs.6174. [DOI] [PubMed] [Google Scholar]
- 53.Kiberd BA, Larson TS, Robertson CR, Jamison RL. Effect of atrial natriuretic peptide on vasa recta blood flow in the rat. American Journal of Physiology - Renal Physiology. 1987;252:F1112–F1117. doi: 10.1152/ajprenal.1987.252.6.F1112. [DOI] [PubMed] [Google Scholar]
- 54. Chujo K, Ueno M, Asaga T, Sakamoto H, et al. Atrial natriuretic peptide enhances recovery from ischemia/reperfusion-induced renal injury in rats. J Biosci Bioeng. 109:526–530. doi: 10.1016/j.jbiosc.2009.11.021. ** This study demonstrates that atrial natiuretic peptide enhances post-ischemic medullary blood flow and protects against renal IRI in rats.
- 55.Nigwekar SU, Navaneethan SD, Parikh CR, Hix JK. Atrial natriuretic peptide for preventing and treating acute kidney injury. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD006028.pub2. CD006028. [DOI] [PubMed] [Google Scholar]
- 56.Jeansson M, Gawlik A, Anderson G, Li C, et al. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest. 121:2278–2289. doi: 10.1172/JCI46322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, et al. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci U S A. 2004;101:5547–5552. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jung YJ, Kim DH, Lee AS, Lee S, et al. Peritubular capillary preservation with COMP-angiopoietin-1 decreases ischemia-reperfusion-induced acute kidney injury. Am J Physiol Renal Physiol. 2009;297:F952–F960. doi: 10.1152/ajprenal.00064.2009. [DOI] [PubMed] [Google Scholar]
- 59.Coca SG, Yusuf B, Shlipak MG, Garg AX, et al. Long-term Risk of Mortality and Other Adverse Outcomes After Acute Kidney Injury: A Systematic Review and Meta-analysis. American Journal of Kidney Diseases. 2009;53:961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Triverio P-A, Martin P-Y, Romand J, Pugin J, et al. Long-term prognosis after acute kidney injury requiring renal replacement therapy. Nephrol Dial Transplant. 2009;24:2186–2189. doi: 10.1093/ndt/gfp072. [DOI] [PubMed] [Google Scholar]
- 61.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 62.Pechman KR, De Miguel C, Lund H, Leonard EC, et al. Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1358–R1363. doi: 10.1152/ajpregu.91022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, et al. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006;69:184–189. doi: 10.1038/sj.ki.5000032. [DOI] [PubMed] [Google Scholar]